Abstract
Please cite this paper as: Broor et al. (2011) Emergence of 2009A/H1N1 cases in a tertiary care hospital in New Delhi, India. Influenza and Other Respiratory Viruses 5(6), e552–e557.
Objective To determine virologic and epidemiologic characteristics of pandemic (H1N1) 2009 at All India Institute of Medical Sciences (AIIMS) a tertiary care hospital in New Delhi, India.
Methods Nasal and throat swabs from patients with febrile acute respiratory illness (FARI) from August to December 2009 (n = 1401) were tested for 2009A/H1N1 and seasonal influenza A viruses by real‐time RT‐PCR.
Results Of 1401 samples tested, 475 (33·9%) were positive for influenza A, of these majority (412; 87%) were 2009A/H1N1, whereas the remaining 63 (13%) were seasonal influenza A (49 were A/H3 and 14 were A/H1). While co‐circulation of 2009A/H1N1 and A/H3 was observed in August–September, subsequent months had exclusive pandemic influenza activity (October–December 2009). Pandemic 2009A/H1N1 emergence did not follow typical seasonal influenza seasonality in New Delhi, which normally peaks in July–August, but instead showed bimodal peaks in weeks 39 and 48 in 2009. The percent of specimens testing positive for 2009A/H1N1 influenza virus was found to be highest in >5‐ to 18‐year age group (41·2%; OR = 2·3; CI = 1·6–3·2; P = 0·00).
Conclusions Taken together, our data provide high prevalence of pandemic 2009A/H1N1 in urban New Delhi with bimodal peaks in weeks 39 and 48 and highest risk group being the children of school‐going age (aged >5–18).
Keywords: Emergence, India, New Delhi, pandemic H1N1
Introduction
Influenza is a prevalent viral infection that can cause severe or fatal disease; together with documented annual outbreaks of epidemic or pandemic proportions, control of influenza has become a major public health challenge. 1 Of even greater concern is the ability of influenza A viruses to undergo natural genetic changes that could result in a virus capable of rapid spread in the human population, as has been observed with recent 2009A/H1N1 pandemic. 1 , 2 A novel influenza A (H1N1) virus emerged in mid‐April 2009 spread rapidly among humans worldwide, and a pandemic was declared by the World Health Organization on June 11, 2009. 1 , 2 The 2009A/H1N1 virus has a unique combination of gene segments: NA and M gene segments in this lineage are from Eurasian swine lineage; HA, NP and NS gene segments are from the classical swine lineage; PB2 and PA gene segments are from the North American swine triple reassortant lineage, originally of avian origin; and the PB1 gene segment is also from the swine triple reassortant lineage originally of human origin. 3
Since the emergence of pandemic (2009A/H1N1) influenza in April 2009 worldwide, more than 214 countries have reported laboratory‐confirmed cases of pandemic (2009A/H1N1). 1 The first imported case of 2009A/H1N1 in India was detected on May 16, 2009, at Hyderabad airport, and the virus soon spread to almost all major cities in India. 4 The initial cases of pandemic H1N1 influenza in India were seen in travelers from other countries; however, thereafter, the virus became entrenched in the cities and communities, and indigenous transmission was observed. 4 Emergence of pandemic influenza resulted in surge of influenza testing, and 46142 of 203 165 (22·8%) persons tested for 2009A/H1N1 in India in various Government‐of‐India facilities till December 27, 2010, were found to be positive for 2009A/H1N1 influenza virus. 4 In the present study, we report the prevalence and trend of emergence of 2009A/H1N1 virus in febrile acute respiratory illness (FARI) cases seen at a tertiary care hospital in New Delhi from August 3 to January 3, 2010 (week 32–53), and compare it along with the influenza seasonality data over past 3 years (2007–2009). We also describe the transition in New Delhi from influenza cases caused primarily by seasonal influenza to cases caused exclusively by 2009A/H1N1 by the end of 2009.
Material and methods
Case definition
Febrile acute respiratory illness is defined as sudden onset of fever ≥37·8°C (100°F) or history of sudden onset of fever and new or worsening cough or shortness of breath or sore throat or rhinorrhea. This definition includes influenza‐like illness (ILI).
Study patients
Patients (n = 1401) who met the criteria of FARI and were referred to virology laboratory for influenza testing from August 3, 2009 (week 32), to January 3, 2010 (week 53), from outpatient clinics (OPD; n = 1155) and hospitalized inpatient (IPD; n = 246) cases at All India Institute of Medical Sciences (AIIMS), a tertiary care hospital in New Delhi, were included in the study. The cases were referred based on the clinical judgment of the treating physician. AIIMS is a tertiary care hospital; however, during pandemic, many patients were attending AIIMS OPD owing to the availability of better healthcare facilities. During the pandemic, majority of patients with suspected influenza etiology were referred for testing owing to increased awareness of pandemic influenza, among both general population and physicians. Although 2009A/H1N1 was first reported in Delhi on June 7, testing for 2009A/H1N1 began at AIIMS from August 3, 2009, and the first case positive for 2009A/H1N1 was detected on August 5, 2009. Ethical clearance for the study was not required as samples were referred to the laboratory for diagnostic purposes as a public health response to mitigate the pandemic.
Sentinel ILI surveillance was established at AIIMS in December 2004 and is conducted at two sites i.e., Employee’s Health Clinic (EHS) at AIIMS, which is attended by all AIIMS employees and their families, and pediatric OPD at Comprehensive Rural Health Services Project (CHRSP) at Ballabgarh. Study physicians visited AIIMS clinic four times a week and the Ballabgarh clinic twice a week, and at least 5–10 nasopharyngeal samples were randomly collected/week and tested for influenza viruses. A total of 2630 samples collected from patients with ILI presenting at EHS OPD at AIIMS or Pediatric OPD at Ballabgarh as part of influenza surveillance in years 2007–2009 [2007 (n = 714), 2008 (n = 834) and 2009 (n = 1082)] were included for understanding the seasonality and trends of influenza in preceding years.
Laboratory diagnosis
Combined throat and nasal swabs from patients with FARI/ILI were collected in viral transport medium and transported to virology laboratory on ice within 4 hours. All referred samples from FARI cases from AIIMS OPD and IPD were tested by real‐time RT‐PCR for the detection of influenza A viruses including 2009A/H1N1 using the Centers for Disease Control and Prevention protocol. 5 All seasonal influenza‐A‐positive samples were further subtyped using primers and probes for A/H1 and A/H3. 5 The results of viral testing were communicated to the clinician within 24–48 hours to help them make decision for antiviral therapy.
Virus isolation and identification
Samples from sentinel surveillance sites collected from 2007 to August 2009 were tested for influenza viruses (A and B) by virus isolation in Madin–Darby Canine Kidney (MDCK) cells followed by HA and HI for virus identification and subtyping. Virus isolation was carried out in MDCK cells cultured in growth medium [DMEM supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (0·25 μg/ml)] and incubated at 37°C with 5% CO2. Respiratory samples were inoculated into confluent monolayers of MDCK cells in the presence of viral growth medium (DMEM without serum with 2 μg/ml TPCK‐treated trypsin). Cells were observed for 7 days and harvested when cytopathic effect was evident. Supernatants from all flasks were subjected to hemagglutination (HA) test using standard method protocol described by WHO. Subtype identification of HA‐positive isolates was performed by hemagglutination inhibition (HI) test by standard methods.
A confirmed case was one having ILI clinical definition and yielding an isolate of influenza in MDCK cell line. However, to test whether any sample may have been missed owing to the emergence of pandemic 2009A/H1N1, all sentinel surveillance samples from March to August 2009 were retested by real‐time RT‐PCR for influenza A, and none of the sample was found positive for 2009A/H1N1. Since August 2009, all sentinel surveillance samples have continued to be tested by real‐time RT‐PCR for the detection of influenza viruses including 2009A/H1N1.
Sequencing of HA1 gene of selected 2009A/H1N1‐positive specimens was carried out using standard methodologies, and phylogenetic analysis was carried out using New Delhi sequences compared to other sequences in the GenBank. 3
Statistical analysis
Statistical analysis was conducted using stata 11 software (StataCorp LP, College Station, TX, USA). Fisher’s exact test was performed for statistical analysis, and differences were considered significant if P < 0·05. Odds ratio with Pearson’s chi‐squared test was used owing to multiple attribute data set (qualitative) to show the significant age group, keeping 0–5 years as the comparison age group.
Results
Prevalence of seasonal influenza A and 2009A/H1N1 influenza
Of the 1401 referred FARI samples from AIIMS from weeks 32 to 53, in 2009, 475 (33·9%) were positive for influenza A. Further subtype analysis of 475 positive influenza A specimens revealed that 412 (86·7%) were 2009A/H1N1, whereas 63 (13·2%) were seasonal influenza A. Among seasonal influenza A positives, H3N2 was detected as the predominant subtype (49/63, 78%) followed by H1N1 (14/63, 22%). Of 412 cases that were 2009A/H1N1 positive, 240 were men and 172 were women (M/F ratio 1·4: 1).
To understand the emergence of 2009A/H1N1 in New Delhi, weekly trends of seasonal and pandemic (2009A/H1N1) influenza were analyzed among FARI cases presenting at AIIMS, New Delhi. It was observed that there was co‐circulation of both seasonal A and pandemic (2009A/H1N1) influenza between weeks 32 and 37; by week 38, prevalence of seasonal influenza began to decline followed by the emergence of two peaks of 2009A/H1N1, one at week 39 and another at week 48 (Figure 1). During the peak at week 39 and 48, >100 samples were tested with >40% positivity for 2009A/H1N1 at each of the week. Interestingly, not a single sample was positive for seasonal influenza A after week 41 (with the exception of one case each in week 49 and 50), suggesting that 2009A/H1N1 almost totally replaced seasonal influenza viruses during remainder of 2009.
Figure 1.
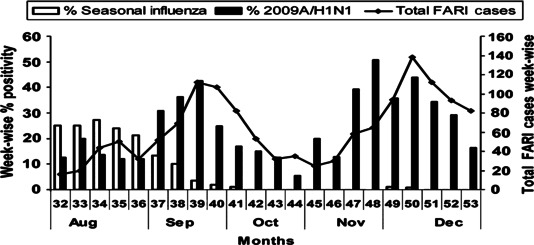
Percent of specimens testing positive for seasonal and 2009A/H1N1 at a tertiary care hospital, All India Institute of Medical Sciences, New Delhi, India, from August to December 2009. Weekwise prevalence of seasonal (open box), 2009A/H1N1 (filled box) with distribution of febrile acute respiratory illness cases (Line drawing with closed diamonds) from Week 32 (August) to week 53 (December 2009).
Seasonality trends of influenza in 2007, 2008, and 2009
To better understand the seasonality of influenza in New Delhi, the monthwise percentage of seasonal influenza positivity was plotted over the total number of samples positive for seasonal influenza in the respective years from 2007 to 2009. (The monthwise percent positivity of 2009A/H1N1 was calculated against the total number positive for pandemic 2009A/H1N1 virus in 2009) (Figure 2). Testing for seasonal influenza in preceding 3 years (n = 2630) revealed positivity of 7·7% (55/714) in 2007 and 6·7% (56/834) in 2008 and revealed an increase in seasonal influenza positivity to 11% (120/1082) in 2009. Data on seasonality revealed that New Delhi has a major peak of influenza activity in the months of July and August (Figure 2), which coincides with rainy season, with minor peaks in December, February, or March. Further, overall influenza positivity rate ranged from 6 to 8% in 2007–2008 to >26% in 2009 (seasonal influenza 11% and pandemic influenza 15%). Two important observations were made concerning influenza infections in 2009. First, it was observed that although overall prevalence of seasonal influenza was somewhat higher than those in the previous years but the peak of seasonal influenza was still seen in the months of July and August (Figure 2), the increase (26%) in total influenza positivity in 2009 was likely due to the appearance of pandemic influenza and also to the sampling bias as an increased number of ILI cases presenting to the sentinel surveillance sites owing to the introduction of novel 2009A/H1N1 in the naïve population were being tested for the presence of influenza. Second, the pandemic virus (2009A/H1N1) unlike seasonal influenza became the predominant virus from September to December (Figure 2). As observed in previous years, little to no seasonal influenza activity was observed in October–December of 2009; however, 2009A/H1N1 continued to prevail for the remainder of 2009. The FARI surveillance has continued since, and a second peak of 2009A/H1N1 was observed in August 2010 (data not shown).
Figure 2.
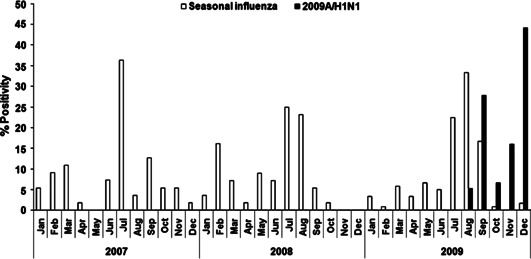
Monthly trends of seasonal influenza positivity (open box) or pandemic 2009A/H1N1 (Closed box) in years 2007, 2008, and 2009 in New Delhi, India. Co‐circulation of seasonal and 2009A/H1N1 was observed in weeks 32–41, with peak of 2009A/H1N1 in weeks 39 and 48.
Prevalence of 2009A/H1N1 and seasonal influenza A among hospitalized and outpatient clinics at AIIMS
Of 1401 referred FARI samples tested, 246 were from inpatient department (IPD) and 1155 were from outpatient department (OPD) (Table 1). Overall influenza positivity was higher in OPD cases (410/1155; 35·5%), when compared to IPD cases (65/246; 26·4%) (OR = 1·53; CI = 1·2–2·12; P < 0·01). Further subtype distribution revealed that of 246 IPD cases, 52 (21·1%) were 2009A/H1N1 positive and 13 (5·3%) were seasonal influenza A positive. In contrast, of 1155 OPD cases, 360 (31·2%) were 2009A/H1N1 positive and 50 (4·3%) were seasonal influenza A positive. Further, the positivity of 2009A/H1N1 was significantly higher in patients presenting to OPD clinics (31·2%) than in IPD patients (21·1%) (OR = 1·69; CI = 1·2–2·4; P < 0·01) (Table 1). For seasonal influenza, there was no difference in the positivity between OPD and IPD patients (P = 0·50) (Table 1).
Table 1.
Influenza positivity among cases enrolled as hospitalized cases (IPD) or outpatient (OPD) at All India Institute of Medical Sciences (AIIMS)
| Age (years) | Total febrile acute respiratory illness cases | AIIMS Hospitalized IPD | AIIMS OPD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total no. | Influenza pos (%) | Seasonal Flu no (%) | Pandemic H1N1 pos (%) | Total no. | Influenza pos (%) | Seasonal pos (%) | Pandemic H1N1 pos (%) | ||
| 0–5 | 262 | 65 | 17 (26·1) | 4 (6·1) | 13 (20·0) | 197 | 51 (25·8) | 3 (1·5) | 48 (24·3) |
| >5–18 | 451 | 71 | 18 (25·3) | 2 (2·8) | 16 (22·5) | 380 | 183 (48·1) | 13 (3·4) | 170 (44·7) |
| >18–45 | 587 | 81 | 26 (32·1) | 6 (7·4) | 20 (24·7) | 506 | 165 (32·6) | 28 (5·5) | 137 (27·1) |
| >45 | 101 | 29 | 4 (13·8) | 1 (3·4) | 3 (10·3) | 72 | 11 (15·3) | 6 (8·3) | 5 (6·9) |
| Total | 1401 | 246 | 65 (26·4)* | 13 (5·3) | 52 (21·1)** | 1155 | 410 (35·5)* | 50 (4·3) | 360 (31·2)** |
*P value for total influenza positivity between inpatient department (IPD) verses outpatient department (OPD) cases P < 0·01 (OR = 1·53 CI = 1·12–2·12).
**P value for total 2009A/ H1N1 positivity between OPD verses IPD cases P < 0·01 (OR = 1·69; CI = 1·20–2·40).
Age distribution of influenza A during pandemic year
Overall influenza positivity was highest among children between >5 and 18 years of age (201/451; 44·5%) (Table 2). More importantly, 2009A/H1N1 was also detected most frequently among patients aged >5–18 years (41·2%; OR = 2·3; CI = 1·6–3·2; P = 000), followed by those in >18–45 years of age (26·7%) (Table 2). Of the 412 positive 2009A/H1N1 cases, 52 (12·6%) required hospitalization.
Table 2.
Distribution of FARI cases, seasonal influenza positives and 2009A/H1N1 positives by age groups from August 2009 to January 3 2010
| Age (years) | Total FARI cases | Influenza positive (%)* | Seasonal Flu no (%) | Pandemic H1N1 pos (%) | Odds ratio** | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| 0–5 | 262 | 68(25·9) | 7 (2·6) | 61 (23·3) | – | 1·00 | – |
| >5–18 | 451 | 201(44·5) | 15 (3·3) | 186 (41·2) | 2·31 | 1·64–3·25 | 0·000 |
| >18–45 | 587 | 191(32·5) | 34 (5·8) | 157 (26·7) | 1·20 | 0·85–1·68 | 0·286 |
| >45–60 | 101 | 15 (14·9) | 7 (6·9) | 8 (7·9) | 0·38 | 0·17–0·86 | 0·020 |
| Total | 1401 | 475 | 63 | 412 | – |
*Patients positive for seasonal or pandemic H1N1 influenza infection confirmed by real‐time reverse transcription‐PCR.
**Odds ratio, 95% confidence interval, and P value were calculated for different age groups for pandemic 2009A/H1N1 in comparison with total febrile acute respiratory illness (FARI) cases.
Phylogenetic and evolutionary analysis of selected 2009A/H1N1 strains was carried out on the basis of HA1 nucleotide sequences. All New Delhi viruses were genetically closely related to A/California/07/2009 and were related to other strains characterized from India (data not shown).
Discussion
Influenza A virus was detected in 33·9% of respiratory samples submitted to AIIMS Laboratory from August 3, 2009, to January 3, 2010, which is somewhat higher than the overall percent of specimens testing positive in other parts of India during the same period 4 and may be related to differences in sampling strategy at different locations. For instance, prevalence of 2009A/H1N1 was reported to be 18% in Pune in western India 6 and 10% in eastern India. 7 During the initial pandemic phase (weeks 32–36), we observed a higher prevalence of seasonal influenza A (64·5%) than that of 2009A/H1N1 (35·5%) among influenza positive samples. This is unlike in some cities in Mexico and United States where, in earlier pandemic period, >90% of the positive samples were attributable to 2009A/H1N1 influenza. 1 , 2 The trend changed by week 37 in New Delhi, India, when 2009A/H1N1 became predominant (85·3% in weeks 37–40 and >99% thereafter). These observations are similar to situations in other parts of the world (Europe, Mexico, Australia, and New Zealand) where 2009A/H1N1 completely replaced seasonal influenza, 1 , 8 , 9 , 10 although continued transmission of both seasonal and pandemic influenza has been observed in both western and eastern parts of India. 6 , 7 Likewise, while a distinct bimodal pattern of peak activity was observed in weeks 39 and 48 in New Delhi, the eastern and western parts of India observed single peak of 2009A/H1N1. 6 , 7 In the current study, after initial introduction of 2009A/H1N1, a sustained transmission of the virus through the 53rd week of the year 2009 was observed. The FARI surveillance has continued since, and a second peak of 2009A/H1N1 was observed in August 2010. The prevalence of 2009A/H1N1 peaked 6–7 weeks after initial virus detection, and during this period, cases attributable to 2009A/H1N1 were almost 15 times greater than seasonal influenza. Similar trends of higher pandemic virus positivity were observed in other studies as well. 11
Analysis of influenza activity since 2007 revealed that New Delhi (situated in North India) has a major peak of influenza activity during the rainy season in July–August, followed by minor peak in winter season (December–February or March). Our findings of seasonal peaks in influenza virus activity in New Delhi (July–August), India, are consistent with data reported from other parts of India, as well as surrounding Southeast Asian countries where peak influenza activities coincides with rainy seasons. 12 , 13 , 14 Unlike typical seasonal influenza peaks, which occur in July–August in New Delhi, 2009A/H1N1 peaked from September to December 2009. These observations are consistent with other data worldwide where pandemic H1N1 did not follow the usual seasonal influenza patterns. 1 , 7 , 15 In contrast, the peak activity of pandemic influenza virus in western and eastern India coincided with seasonal influenza peak observed during rainy season. Further, the circulating strain of 2009A/H1N1 in New Delhi grouped with clade 7 of pandemic 2009A/H1N1 influenza viruses, as are majority of other Indian strains. 16
Comparison of 2009A/H1N1 positivity revealed a significantly higher prevalence among OPD cases, when compared to IPD cases at AIIMS. Such difference in positivity may, in part, be due to late presentation of IPD cases at this tertiary care hospital. Thus, the severity of 2009A/H1N1 in New Delhi was milder compared to that observed in Pune, India. 6 In addition, the severity of pandemic influenza varied considerably worldwide. 1 , 6 , 8
The second important aspect of our study is the observation of the highest rate of 2009A/H1N1 positivity among children in the age group >5–18 years, which could be due to high exposure rates among school‐aged children and/or a lack of herd immunity against the novel triple assorted 2009A/H1N1. The rates of 2009A/H1N1 have varied between various age groups in different parts of the world. 7 , 8 , 17 , 18 For instance, in Japan, 88·5% of cases of 2009A/H1N1 were reported in persons <20 years of age, 17 whereas in United Kingdom, children under 16 were more susceptible to infection in the households. 18 The lowest positive rate for pandemic influenza was observed in the >60‐year age group, suggesting that persons in this group may have previously been exposed, through infection or immunization, to a genetically and antigenically closely related influenza A (H1N1). 19 Close monitoring of the cases is needed to determine patterns of hospitalization, intensive care utilization, and fatality.
Our study has several limitations. The influenza surveillance during the pandemic phase from August till December 2009 was expanded to include all FARI cases referred at AIIMS, whereas sentinel surveillance is carried out with systemic sample collection from an employee health clinic at the AIIMS, New Delhi, and a pediatric OPD at rural Ballabgarh throughout the year. Thus, a clear sample bias exists during the pandemic period, which may explain high percent positivity for pandemic 2009A/H1N1 in the tertiary care hospital at AIIMS. We have presented only 3 years of surveillance data available, and additional years of surveillance data are needed to confirm the observed influenza seasonality trends. Although our data demonstrate that the influenza virus is a significant cause of ILI/FARI among outpatients/inpatients seeking care at government facilities in New Delhi, our findings might be an underestimate as our sampled population was not representative of the general population owing to limited sentinel sites included in the study. Continued surveillance and understanding of seasonality would provide useful information that may improve preparedness plans for future pandemics.
Conflict of Interest
No conflict of interest to declare.
Acknowledgements
We acknowledge Dr H Kaur for helping in implementation of study and S Dhakad and Y. Singh for technical support. We also acknowledge the partial financial support from Indian Council of Medical Research and the Centers for Disease Control and Prevention, Atlanta, USA, and CDC reagent program for providing diagnostic kits and positive controls.
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the Center for Disease Control and Prevention.
References
- 1. Novel Swine‐Origin Influenza A (H1N1) Virus Investigation Team , Dawood FS, Jain S, Finelli L et al. Emergence of a novel swine origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605–15. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization – Pandemic (H1N1) . 2009. Update 81. 30 December 2009. Available at: http://www.who.int/csr/don/2009_12_30/en/index.html (Accessed 26 August 2010).
- 3. Garten RJ, Davis CT, Russell CA et al. Antigenic and genetic characteristics of swine‐origin 2009 A (H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ministry of Health and Family Welfare, Government of India. Press Releases. Update on Swine Flu Situation as on 30th April 2009 (PIB 30‐4‐2009). Available at: http://pib.nic.in/release/release.asp?relid=48583 (Accessed 26 August 2010).
- 5. World Health Organization . CDC protocol of realtime RTPCR for influenza A (H1N1). Geneva. 2009. Available at http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf (Accessed 20 July 2009).
- 6. Mishra AC, Chadha MS, Choudhary ML, Potdar VA. Pandemic Influenza (H1N1) 2009 is associated with severe disease in India. PLoS ONE 2010; 5:e10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mukherjee A, Roy T, Agarwal SA et al. Prevalance and epidemiology of pandemic H1N1 strains in hospitals of eastern India. J Public Health Epidemiol 2010; 2:171–174. [Google Scholar]
- 8. Echevarría‐Zuno S, Mejía‐Aranguré JM, Mar‐Obeso AJ et al. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet 2009; 374:2072–9. [DOI] [PubMed] [Google Scholar]
- 9. Kelly H, Grant K. Interim analysis of pandemic influenza (H1N1) 2009 in Australia; Surveillance trends, age of infection and effectiveness of seasonal vaccination. Euro Surveill 2009; 14:pii. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention (CDC) . Surveillance for the 2009 Pandemic Influenza A (H1N1) virus and seasonal influenza viruses‐ New Zealand, 2009. Morb Mortal Wkly Rep 2009; 58:918–21. [PubMed] [Google Scholar]
- 11. Perez D, Sorrell E, Angel M et al. Fitness of pandemic H1N1 and seasonal influenza A viruses during co‐infection: evidence of competitive advantage of pandemic H1N1 influenza versus seasonal influenza. Version 2. PLoS Curr Influenza 2009; 1:RRN1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal AS, Sarkar M, Chakrabarti S et al. Comparative evaluation of real‐time PCR and conventional RT‐PCR during a 2 year surveillance for influenza and respiratory syncytial virus among children with acute respiratory infections in Kolkata, India, reveals a distinct seasonality of infection. J Med Microbiol 2009; 58:1616–1622. [DOI] [PubMed] [Google Scholar]
- 13. Rao BL, Yeolekar LR, Kadam SS et al. Influenza surveillance in Pune, India, 2003. Southeast Asian J Trop Med Public Health 2005; 36:906–909. [PubMed] [Google Scholar]
- 14. Park AW, Glass K. Dynamic patterns of avian and human influenza in east and southeast Asia. Lancet Infect Dis 2007; 7:543–548. [DOI] [PubMed] [Google Scholar]
- 15. Leo Y‐S, Lye DC, Barkham T, Krishnan P, Seow E, Chow A. Pandemic (H1N1) 2009 surveillance and prevalence of seasonal influenza, Singapore. Emerg Infect Dis 2010; 16:103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Potdar VA, Chadha MS, Jadhav SM, Mullick J, Cherian SS, Mishra AC. Genetic characterization of the Influenza A pandemic (H1N1) 2009 virus isolates from India. PLoS ONE 2010; 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamigaki T, Oshitani H. Epidemiological characteristic and low case fatality rate of pandemic (H1N1) 2009 in Japan. PLoS Curr Influenza 2009; 1:RRN1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghani AC, Baguelin M, Griffin JT et al. The early transmission dynamics of H1N1pdm influenza in the United Kingdom. PLoS Curr Influenza 2009; 2:RRN1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Centre for Disease Control and Prevention (CDC) . Serum cross‐reactive antibody response to novel Influenza A (H1N1) after vaccination with seasonal influenza vaccine. MMWR 2009; 58:521–4. [PubMed] [Google Scholar]


