Abstract
Objectives
While increasing attention is paid to the rising prevalence of chronic diseases in Africa, there is little focus on chronic kidney disease (CKD). This systematic review assesses CKD burden among the general population and high-risk groups on the entire African continent.
Design, setting and participants
We searched Medline and PubMed databases for articles published between 1 January 1995 and 7 April 2017 by sensitive search strategies focusing on CKD surveys at the community level and high-risk groups. In total, 7918 references were evaluated, of which 7766 articles were excluded because they did not meet the inclusion criteria. Thus, 152 studies were included in the final analysis.
Outcome measurement
The prevalence of CKD in each study group was expressed as a range and pooled prevalence rate of CKD was calculated as a point estimate and 95% CI. No meta-analysis was done. Data were presented for different populations.
Results
In the community-level studies, based on available medium-quality and high-quality studies, the prevalence of CKD ranged from 2% to 41% (pooled prevalence: 10.1%; 95% CI 9.8% to 10.5%). The prevalence of CKD in the high-risk groups ranged from 1% to 46% (pooled prevalence: 5.6%; 95% CI 5.4% to 5.8%) in patients with HIV (based on available medium-quality and high-quality studies), 11%–90% (pooled prevalence: 24.7%; 95% CI 23.6% to 25.7%) in patients with diabetes (based on all available studies which are of low quality except four of medium quality) and 13%–51% (pooled prevalence: 34.5%; 95 % CI 34.04% to 36%) in patients with hypertension (based on all available studies which are of low quality except two of medium quality).
Conclusion
In Africa, CKD is a public health problem, mainly attributed to high-risk conditions as hypertension and diabetes. The poor data quality restricts the validity of the findings and draws the attention to the importance of designing future robust studies.
Keywords: CKD, Africa, systematic review
Strengths and limitations of this study.
This systematic review assessed the chronic kidney disease (CKD) burden among the general population and high-risk groups on the entire African continent based on studies that covered all of Africa from 1 January 1995 until 7 April 2017.
The quality of the included articles was assessed based on standard criteria dealing with clinical trials, diagnostic studies and observational studies. The articles were assessed based on the population sampling and precision, sampling technique, response rate and exclusion rate.
No meta-analysis was conducted in this review due to the huge discrepancy in the definition used to identify CKD, the methods of creatinine measurement, urine protein assessment and in the quality of the reporting.
There is paucity of information about CKD prevalence in age and gender groups, which affects the accuracy of the pooled prevalence estimated from each group.
The prevalence of CKD reported in this review should be interpreted with caution due to the low quality of the majoirty of studies in Africa, the bias introduced from the heterogeneity between studies, analytical and methodological issues, sample size, and study population selection.
Introduction
Chronic kidney disease (CKD) is an emerging global public health problem.1 The disease is a component of a new epidemic of chronic conditions that replaced malnutrition and infection as leading causes of mortality during the 20th century.2 Age-standardised death rates due to CKD have increased during the last 23 years. CKD has shifted from the 36th cause of death in 1990 to the 19th cause in 2013.3 The worldwide increase in CKD and kidney failure—necessitating renal replacement therapy—and the high rate of cardiovascular mortality and morbidity attributable to CKD are poised to reach epidemic proportions over the next decade. CKD complications represent a considerable burden on global healthcare resources and only a small number of countries have sufficiently robust economies to meet the challenge posed by this disease. Socioeconomic differences in health exist and individuals of lower socioeconomic status (SES) have a higher risk for mortality and morbidity compared with those of higher SES.4 A change in the global approach to CKD from the treatment of end stage renal disease (ESRD) to intensive primary and secondary prevention is therefore considered an absolute public health priority.5
Africa is the second largest continent in the world, with a population of over 1 billion; 961.5 million people live in sub-Saharan Africa and 195 million in Northern Africa.6 Africa now faces the dual challenge of infectious illnesses and chronic diseases. Africa’s chronic disease burden is secondary to various factors, including increased life expectancy, changing lifestyle practices, poverty, urbanisation and globalisation.7 The World Health Assembly advocated the Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013–2020. One of its targets is to reduce premature mortality from chronic diseases by 25% in 2025. These actions have the potential to make a significant impact on the burden of CKD.8 Unfortunately, CKD problem remains underestimated on the entire continent due to lack of epidemiological information from different African countries. There exists only a single systematic review conducted in sub-Saharan Africa, which concluded that CKD is a prevalent and potentially escalating disease across sub-Saharan Africa, with both communicable and non-communicable risk factors.9 Strategies aimed at managing CKD epidemics in Africa critically depend on a reliable assessment of the burden of the problem and the establishment of affordable early detection programmes. Previous studies reported the prevalence of CKD among the general population or the specific prevalence of this condition in diseases that are recognised as drivers of renal damage (eg, diabetes mellitus). These estimates have varied across studies due to differences in the methods of glomerular filtration rate (GFR) measurement, background risk (general population vs high-risk groups) or demographic characteristics (eg, age, gender).10
With this background in mind, this review aimed to increase the systematic information on the burden of CKD in the general population and high-risk groups of the entire African continent and provide an estimate of the prevalence of CKD in different regions of Africa.
Materials and methods
Data source and search strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.11 A systematic literature search was performed in the PubMed and Ovid Medline databases by two authors (DB and SA) to identify articles reporting epidemiology data on CKD in the adult population in any geographical area of the African continent. This employed focused, highly sensitive search strategies (online supplementary table 1). The search covered the time frame from 1 January 1995 to 7 April 2017. Papers without language and study design restrictions were located and screened. References from relevant studies were screened for supplementary articles.
bmjopen-2016-015069supp001.pdf (80.9KB, pdf)
Study selection and data extraction
Titles and abstracts were screened independently by two authors (SA and GD), who discarded studies that were not relevant to the topic. Case reports, reviews, editorials, letters and studies focusing on African–Americans not living on the African continent, conducted entirely among children, or dealing with acute kidney injury or kidney transplantation were excluded. Two authors (SA and ED) independently assessed the retrieved abstracts and the full texts of these studies to determine eligibility according to the inclusion criteria. Disagreements were resolved through discussion and consensus, or through consultation with a third reviewer (DB), who solved these differences based on study judgements. Furthermore, screening of reference lists of all of the retrieved studies was conducted to check for relevant articles, and a supplementary scan of the reference lists of the systematic reviews was performed to identify any additional studies. Data were extracted from full-text articles and registered using a specifically designed form. These data included study design, geographical area, sample size, the definition of CKD used, prevalence of CKD, age, gender, GFR measurement, type of creatinine assay, proteinuria, the method of outcome assessment, and associated comorbidities such as diabetes mellitus and hypertension. Data extraction was performed by one reviewer (SA) and independently verified by another reviewer (DB).
Data extraction and analysis
Studies were categorised according to the reference population as follows: (1) studies dealing with the general population and (2) studies focusing on particular diseases such as diabetes, hypertension, lupus and HIV, or settings, for example, hospital-based surveys and occupational studies.
Information on the assessment of kidney function was collected, including the equation adopted for GFR estimation (Cockroft-Gault (CG), Modification of Diet in Renal Disease (MDRD), Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI)), the type of creatinine assay (Jaffe, standardised or unknown), and the type of proteinuria or albuminuria assay used (semiquantitative assessment by urinary strips or quantitative in urine samples or 24-hour collection). When the study included two or three GFR equations, we defined the CKD prevalence based on the CKD-EPI equation whenever this information was provided. Otherwise, we considered the MDRD equation and lastly the CG equation. In the case of ethnicity correction,12–14 we included the equation that corrected for ethnicity. Information on the definition of CKD used in each study was also included (either the internationally accepted definition as Kidney Disease Outcome Quality Initiative (KDOQI), or other ways of defining CKD).
Quality assessment
Two independent authors (SA and DB) appraised each article independently and assessed its quality based on standard criteria described into details in previous methodology reviews dealing with clinical trials,15 diagnostic studies16 and observational studies.17 The articles were assessed based on the subject sampling and precision, sampling technique, response rate, method of assessment of kidney function and exclusion rate.
Statistical analyses
The principal demographic and clinical data for each study were summarised as the mean and SD or as absolute number and percentage, as appropriate. The age range in each study was also recorded. The range of the CKD prevalence for each study group was reported. The pooled prevalence rate of CKD was expressed as a point estimate and 95% CI. The prevalence from each study was weighed by the sample size, then the pooled prevalence was categorised by the African region. The inter-rater agreement for inclusion and quality assessment was determined using Cohen’s kappa (κ) coefficient.18 The percentage of the different causes of CKD was weighed by the sample size of each study done among patients with CKD. Then we simply summed the number of patients for each aetiological factor and divided it by the total sample size from the whole included studies. No meta-analysis was conducted in this study. Data were appropriately presented for different populations (general population and patients with CKD). Patients’ data were stratified by the type of underlying condition, that is, hypertension, diabetes mellitus, HIV or systemic lupus erythematosus. All calculations were conducted using SPSS for Windows V.21.
Results
Search results
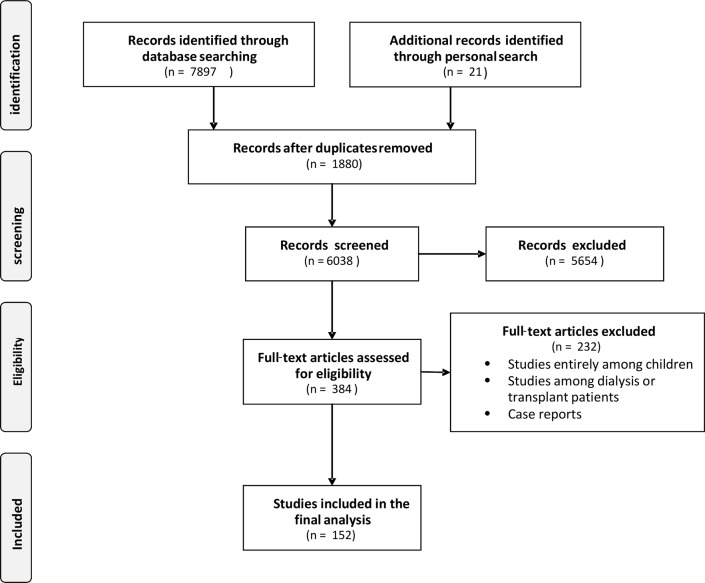
The flow diagram of the selection process is depicted in figure 1. In total, 7897 potentially relevant references were initially retrieved. Twenty-one additional citations were found through a personal search. By screening titles and abstracts, a total of 7534 citations were excluded because of search overlap, dealing with the wrong population (African–American, acute kidney injury (AKI), cancer or post-transplant patients) or not providing actual data on CKD. Review articles, case reports, editorials or letters were also excluded. Among the 384 studies selected for full-text examination, 232 were excluded because they dealt with a population different from that specifically targeted in this systematic review, such as paediatric populations (122 studies), transplant patients (n=44) or others (n=46) (eg, Africans living in non-African countries), or because only narrative data were provided (n=20). A total of 152 articles were therefore reviewed in detail and included in the analysis. The main characteristics of these studies are summarised in table 1. The inter-rater agreement for inclusion wa-s κ=0.90 and for the quality assessment was κ=0.85.
Figure 1.
Flow diagram of the study selection.
Table 1.
Characteristics of the study population included in the analysis
| Study population | Studies (n) | Study characteristics |
| General population | 29 | n=30 169, age ranging from 12 to 95 years; 48% male |
| Patients with diabetes | 18 | n=9082, age ranging from 14 to 90 years; 43% male |
| Patients with hypertension | 9 | n=4123, age ranging from 19 to 90 years; 43% male |
| Patients with HIV | 42 | n=67 432, age ranging from 13 to 74 years; 36% male |
| Occupational group | 2 | n=153, age ranging from 22 to 59 years; one study only enrolled women and the other principally enrolled men |
| Family practice patients | 7 | n=3250, age ranging from 20 to 74 years; 44% male |
| Patients with lupus | 1 | n=43, age ranging from 16 to 55 years; 7% male |
| Rheumatoid arthritis | 1 | n=233, age ranging from 40 to 70 years; 17.2% male |
| Sickle cell anaemia | 1 | n=194, age ranging from 12 to 40 years; 43.3% male |
| Patients with chronic kidney disease | 42 | n=34 236, age ranging from 12 to 90 years; 58% male |
Study characteristics
Among the 152 studies reviewed, 29 were general population studies (table 2). One hundred and twenty-three studies focused on selected groups, of which 42 included patients with HIV (table 3), 18 studied patients with diabetes (table 4), 9 included hypertensive subjects (table 5) and 12 were conducted in other populations (table 6), including one study in patients with lupus,19 one study in patients with rheumatoid arthritis,20 one study among patients with sickle cell anaemia,21 two in specific occupational settings (silica exposure22 and exposure to the nephrotoxic hair-dye, paraphenylenediamine23) and seven studies in family practice24–26 or hospital-based27–30 surveys. Forty-two studies were conducted among patients with CKD (online supplementary table 2).31–72
Table 2.
Studies on CKD among the general population
| Study ID | Year, country, region | Location | N | Population characteristics | Definition of CKD | Method of outcome assessment | Type of creatinine assay | Proteinuria | CKD prevalence | Quality assessment |
| Abdelsatir169 | 2013, Sudan, Northeast | All village inhabitants | 389 | Age (years): 41±15 Male gender: 16.2 % Hypertension: 39.6 %; DM: 17 % BMI category (kg/m 2) < 18 : 6.2 % 18 – 24.9 : 65.8 % 25 – 29.9 : 20.2 % = 30 : 7.8 % |
Not identified, personal history | Personal history | Not mentioned | Not measured | Total prevalence (as reported): 6.40% | Low |
| Fatiu73 | 2011, Nigeria, West | Market population | 286 | Age (years): 49.5±5.7 Male gender: 9.8% Hypertension: 37.7% BMI (kg/m2): 26.76±5.28 <20: 7.4% 20–25: 33.4% >25: 59% |
Proteinuria =+1 | Midstream urine sample was tested by urinary strip | Not measured | 29.70% | Total prevalence (based on proteinuria prevalence): 29.7% | Medium |
| Traore74 | 1998, Mali, West | All household population of the villages | 1098 | Age (years): 30±12 Male gender: 52% |
Proteinuria =+1 | Microhaematuria and proteinuria by urinary strip | Not measured | 40.80% | Total prevalence (based on proteinuria prevalence): 40.80% | Medium |
| Matsha12 | 2013, South Africa, South | Bellville town inhabitants | 1202 | Age (years): 52.9±14.8 Male gender: 24.7% SBP: 125±20 DBP: 76±13 DM: 26.4% BMI: 29.9±7.2 |
eGFR <60 mL/min | Four variables: MDRD, CG, CKD-EPI | Standardised creatinine assay | Not measured | Prevalence of stages 3–5: 7.4% (based on CKD-EPI with ethnicity correction) | Medium |
| Seck97 | 2014, Senegal, West | Two-stage cluster sampling of urban and rural inhabitants of Saint-Louis | 1037 | Age (years): 48.0±16.9 Male gender: 40% Hypertension: 39.1% DM: 12.7% BMI: 26.3±6.8 kg/m2 |
KDOQI | Albuminuria by urinary strips; positive samples were confirmed by 24-hour albuminuria, eGFR by 186 MDRD | 5.3% albuminuria >1 g/L | Total prevalence: 6.1% | High | |
| Pruijm116 | 2008, Seychelles, East |
A random sex-stratified and age-stratified sample inhabitants of Seychelles | 1255 | Age (years): range, 25–64 Male gender: 46% |
KDOQI | Quantitative microalbuminuria by ACR, eGFR using MDRD | Not mentioned | 11.4% microalbuminuria, 0.7% macroalbuminuria | Total prevalence: 15.3% Prevalence of stages 3–4 CKD: 3.2% |
High |
| Sumaili98 | 2009, Congo, Central | Multistage sampling of residents of Kinshasa | 500 | Age (years): 38.6±14.4 Male gender: 41% Hypertension: 27.6% DM: 11.7% BMI category (kg/m2) 25–29.9: 20.3% =30: 14.9% |
KDOQI | Proteinuria by urinary strip and 24-hour proteinuria, eGFR by CG and 175 MDRD | Kinetic Jaffe and IDMS-calibrated | 18% proteinuria by dipstick 5% (=300 mg/day) |
Total prevalence MDRD: 12.4% CG: 19% Prevalence by stage (MDRD) Stage 1: 2% Stage 2: 2.4% Stage 3: 7.8% Stage 4: 0% Stage 5: 0.2% |
High |
| Matsha159 | 2014, South Africa, South | All residents of Cape Town | 320 | Age (years): mean, 56.4 (95% CI 55.1 to 57.6) Male gender: 22% SBP: 124.7 (95% CI 122.8 to 126.7) mm Hg DBP: 75.5 (95% CI 74.2 to 76.7) mm Hg BMI: 31.9 (95% CI 31.2 to 32.7) kg/m2 Mean eGFR at baseline: 68.6±16.7 mL/min/1.73 m2 |
eGFR<60 mL/min/ 1.73 m2 | eGFR: 186 MDRD (four variables) | Not mentioned | Not measured | Total prevalence: 28.9% Prevalence by categories eGFR >90 mL/min/1.73 m2: 9.4% eGFR60 90 mL/min/1.73 m2: 58.7% eGFR30 60 mL/min/1.73 m2: 28.1% eGFR <30 mL/min/1.73 m2: 0.9% |
Medium |
| Sumaili75 | 2008, Congo, Central | All residents of Kinshasa | 3018 | Age (years): 44.3±15.3 Male gender: 59% Hypertension: 18% DM: 4% |
Proteinuria =+1 | Proteinuria by urinary strip | Not assessed | 17.1% | Total prevalence (based on proteinuria prevalence): 17.1% Prevalence by age 12–21 years: 8.7% 22–31 years: 11.4% 32–41 years: 18.6% 42–51 years: 18.2% 52–61 years: 18.9% 62–71 years: 22.4% =72 years: 19.7% |
High |
| Egbi76 | 2014, Nigeria, West | All civil servants in Bayelsa | 179 | Age (years): 45.2±10.3 Male gender: 53.1% SBP: 128.5±17.5 mm Hg DBP: 81.8±13.2 mm Hg |
eGFR <60 mL/min/1.73 m2 and/or presence of proteinuria of at least +1 on dipstick urinalysis | Proteinuria by urinary strip, eGFR by CG equation standardised for body surface area | Kinetic Jaffe | 5.6% | Total prevalence: 7.8% Prevalence by stage Stage 1:3.4% Stage 2: 2.2% Stage 3: 2.2% None in stage 4 or 5 |
Low |
| Oluyombo105 | 2013, Nigeria, West | Multistage sampling of households of Ilie | 454 | Age (years): 45.8±19.0 Male gender: 43% Hypertension: 20.4% DM: 0.6% |
eGFR <60 mL/min and/or macroalbuminuria (ACR >300 mg/g or dipstick proteinuria) | Proteinuria by urinary strip, negative cases were estimated for albumin-to-creatinine ratio, eGFR by 186 MDRD | Kinetic Jaffe | Macroalbuminuria in 8.9% | Total prevalence: 18.8% Prevalence by stage Stage 1: 2.4% Stage 2: 4.1% Stage 3: 11.8% Stage 4: 0.5% |
High |
| Eastwood13 | 2010, Ghana, West | Inhabitants of 12 villages | 944 | Age (years): 54.7±11.2 Male gender: 38% SBP: 125.5±26.0 mm Hg DBP: 74.4 13.6 mm Hg DM: 4% BMI: 21.1±4.2 kg/m2 |
KDOQI | 175 MDRD, CG, CKD-EPI | Kinetic Jaffe and calibrated IDMS | Total prevalence (based on CKD-EPI and ethnicity correction): 1.7% MDRD: 1.6% (7.2 % without ethnicity correction) CKD-EPI: 1.7% (4.7% without ethnicity correction) CG: 21.0% |
High | |
| Gouda117 | 2011, Egypt, North | Community based in Al-Buhayrah governorate | 417 | Age (years): 39.12±14.29 Male gender: 43.2% Hypertension: 25.20% DM: 10.6% BMI: 29.96±6.18 kg/m2 |
eGFR <60 mL/min/1.73 m2 | Quantitative assessment of urinary ACR, eGFR by 175 MDRD | IDMS-calibrated | 10.6% microalbuminuria | Total prevalence: 18% Prevalence by age 18–29 years: 0.8% 30–44 years: 6.1% 45–60 years: 19.6% >60 years: 40% Prevalence by gender Female: 9.6% Male: 12% |
Medium |
| Ayodele77 | 2011, Nigeria, West | People at a major trade centre, the public servant secretariat and the state broadcasting station | 586 | Age (years): 42.4±11.2 Male gender: 61.4% Hypertension: 16.4% DM: 3.8% BMI: 25.9±5.4 kg/m2 |
Proteinuria =+1 | Proteinuria by urinary strip | Not assessed | 2.50% | Total prevalence (based on proteinuria): 2.50% Prevalence by gender Female: 1.7% Male: 3% |
Medium |
| Abu-Aisha78 | 2009, Sudan, East | Pilot survey of police housing complex | 273 | Age (years): 34.3±12 Male gender: 49.1% Hypertension: 27% DM: 5.1% |
eGFR <60 mL/min/1.73 m2 and/or proteinuria | Proteinuria by urinary strip, 175 MDRD, CG | Not mentioned | 5.30% | Total prevalence (MDRD): 7.7% (11% by CG) Prevalence by stage Stage 1 or 2: 4.7% Stage 3: 2.6% Stage 4: 0% Stage 5: 0.4% |
Medium |
| Gharbi106 | 2012, Morocco, North | Stratified random sampling of population in two towns | 10 524 | Age (years): range, 25–70 Male gender: 50% Hypertension: 16.7% |
eGFR <60 mL/ min/1.73 m2 or macroalbuminuria or dipstick abnormalities (proteinuria =++1 or haematuria =++1) or diabetes type 1 associated with microalbuminuria |
175 MDRD, microalbuminuria and proteinuria by urinary strip and ACR | Kinetic Jaffe and IDMS | Microalbuminuria (30–299 mg/L): 5.26% | Total prevalence 2.90% | High |
| Odenigbo153 | 2014, Nigeria, West | All attendees to lectures of the Ebreime Foundation for the elderly | 170 | Age (years): 68.1±7.7 Male gender: 67.1% |
eGFR <60 mL/min/1.73 m2 | 175 MDRD | IDMS-calibrated | Total prevalence: 43.50% (all cases were at stage 3) Prevalence by age =65 years: 49.1% >65 years: 40.7% Prevalence by gender Female: 64% Male: 33% |
Low | |
| Booysen155 | 2016, South Africa, South |
Participants from families of black African descent | 1221 | Age (years): 44.1±18.4 Male gender: 34.9% BMI (kg/m2): 29.5±8.0 Hypertension: 45% DM: 25.2% |
eGFR <60 mL/min/1.73 m2 | eGFR by CG, four variables MDRD, CKD-EPI | IDMS-calibrated | Not measured | Total prevalence: 6.3% | High |
| Kalyesubula90 | 2017, Uganda, East |
Community-based survey among all households of Wakiso District | 955 | Age (years): 31 (IQR: 24–42) Male gender: 33% BMI (kg/m2) categories Underweight: 5.5% Normal: 56.9% Overweight: 24.2% Obese: 13.4% Diabetics: 5.9% |
KDOQI | Proteinuria by dipstick and eGFR by CG, MDRD and CKD-EPI | Kinetic Jaffe | 0.3% | Total prevalence: 15.2% Prevalence by stage Stage 1: 6.2% Stage 2: 12.7% Stage 3: 2.4% Stage 4: 0% Stage 5: 0.1% |
High |
| Kaze91 | 2015, Cameroon, Central-West | Population of the Littoral region | 500 | Age (years): 45.3±13.2 Male gender: 53.4% BMI (kg/m2): 27.1±5.3 DM: 2.8% Hypertension: 12.2% |
Any albuminuria and/or eGFR<60 mL/min/1.73 m2 | Albuminuria by dipstick and eGFR by CG, MDRD, CKD-EPI | Kinetic Jaffe and IDMS | 7.2% | Total prevalence (CKD-EPI): 10% (14.2% by CG, 11% MDRD) Prevalence by gender Female: 9.8% Male: 10.1% |
High |
| Kaze112 | 2015, Cameroon, Central-West | Population of the Western region | 439 | Age (years): 47±16.1 Male gender: 42.1% Hypertension: 10.7% DM: 5.9% |
Albuminuria and/or eGFR <60 mL/min confirmed 3 months later | Albuminuria by dipstick and ACR and eGFR by CG, MDRD, CKD-EPI | Kinetic Jaffe and IDMS | 12.1% had albuminuria | Total prevalence (CKD-EPI): 27.6% (38.5% by CG, 27.3% MDRD) Prevalence by gender Female: 15.4% Male: 10.2% |
High |
| Laurence130 | 2016, South Africa, South | Teachers from public schools in in the urban area of the Metro South Education District | 489 | Age (years): 46.3±8.5 Male gender: 30% BMI (kg/m2) Male: 29.1±4.8 Female: 32.4.1±7 Hypertension: 48.5% DM: 10.1% |
Proteinuria =0.30 mg/mg or eGFR <60 mL/min/1.73 m2 | Proteinuria by PCR and eGFR using MDRD | Kinetic Jaffe | Not mentioned | Total prevalence: 10.4% Prevalence by gender Female: 10.9% Male: 9% |
Medium |
| Lunyera92 | 2016, Uganda, East | Urban residents of Kampala | 141 | Age (years): 64% in age group of 18–39 Male gender: 43% BMI (kg/m2): 25.9 (IQR 22.7–30.7) Hypertension: 38% Impaired fasting blood glucose: 13% |
Proteinuria as urine protein of =1+ on dipstick in the absence of haematuria and leucocyturia | Proteinuria by dipstick | Not measured | 13% | Total prevalence (based on proteinuria): 13% Prevalence by age 18–39 years: 16% 40–59 years: 4% =60 years: 0% Prevalence by gender Female: 11% Male: 15% |
Low |
| Mogueo131 | 2015, South Africa, South | Household residents of BellVille | 902 | Age (years): 55±15 Male gender: 23% BMI(kg/m2): 29.9±7.2 Hypertension: 49.8% Diabetes mellitus: 27.9% |
eGFR <60 mL/min/1.73 m2 or any nephropathy | Albuminuria by ACR and eGFR by MDRD and CKD-EPI | Kinetic Jaffe | 2.3% | Total prevalence (CKD-EPI): 21.7% (prevalence by MDRD: 29.7%) Prevalence by gender Female: 23.3% Male: 16.6% |
Medium |
| Peck148 | 2016, Tanzania, East | Stratified multistage sampling of adult population in Mwanza City, Geita and Kahama | 1043 | Age (years): 35.5±15.3 Male gender: 45.7% BMI (kg/m2) categories Underweight: 10.5% Normal: 71% Overweight: 11.8% Obese: 6.6% DM: 0.9% Hypertension: 17.3% |
eGFR <60 mL/min/1.73 m2 | eGFR by MDRD and CKD-EPI | Kinetic Jaffe | Not measured | Total prevalence (CKD-EPI): 7% Prevalence by age <25 years: 3.4% 25–34 years: 4.9% 35–44 years: 7.2% =45 years: 12.1% Prevalence by gender Female: 6% Male: 7.3% |
High |
| Stanifer132 | 2016, Tanzania, East | Stratified, cluster-designed, cross-sectional household | 481 | Age (years): 46.9±15.1 Male gender: 74.4% DM: 9.4% Hypertension: 31% |
Presence of albuminuria (=30 mg/dL; confirmed by repeat assessment) and/or a reduction in eGFR =60 mL/min/1.73 m2 |
Quantitative assessment of albuminuria and eGFR by MDRD and CKD-EPI | IDMS | 6.8% | Total prevalence: 11.9% | High |
| Stanifer133 | 2015, Tanzania, East | Randomly selected adults | 481 | Age (years): 45 (IQR 35–59) Male gender: 25.6% DM: 12.7% Hypertension: 28% |
eGFR <60 mL/min/1.73 m2 and/or persistent albuminuria | Quantitative assessment of albuminuria and eGFR by MDRD | IDMS | Not mentioned | Total prevalence: 7% Prevalence by age 18–39 years: 7.6% 40–59 years: 5.4% 60+ years: 7.7% Prevalence by gender Female: 6.2% Male: 7.9% |
High |
| Stanifer134 | 2016, Tanzania, East | Stratified, cluster-designed, cross-sectional survey | 606 | Age (years): 45.5±15.5 Male gender: 24.6% DM: 10.1% Hypertension: 23.7% |
Presence of albuminuria (=30 mg/dL confirmed by repeat assessment) and/or a once-measured eGFR =60 mL/min/1.73 m2 | Quantitative assessment of albuminuria and eGFR by MDRD | IDMS | Not mentioned | Total prevalence: 8% Prevalence by age 18–39 years: 6.4% 40–59 years: 9.3% 60+ years: 10.5% Prevalence by gender Female: 7.2% Male: 11.4% |
High |
| Wachukwu93 | 2015, Nigeria, West | Adult volunteers in a university | 259 | Age (years):28.3±9.7 Male gender: 52.1% SBP (mm Hg): 117.3±15.5 DBP (mm Hg): 75.7±11.7 |
eGFR <60 mL/min/1.73 m2 | Proteinuria by dipstick and eGFR by CG | Not mentioned | 12.4% | Total prevalence: 1.9% | Low |
ACR, albumin to creatinine ratio; BMI, body mass index; CG, Cockroft-Gault; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IDMS, isotope dilution mass spectrometry; KDOQI, Kidney Disease Outcome Quality Initiative; MDRD, Modification of Diet in Renal Disease; SBP, systolic blood pressure.
Table 3.
Studies on CKD among patients with HIV
| Author | Year, country, region | Location | N | Study group | Population characteristics | Definition of CKD | Methods of outcome assessment | Creatinine assay | Proteinuria | CKD prevalence | Quality assessment |
| Wkba142 | 2013, Ghana, West | ART clinic at the regional hospital | 442 | HIV (276 HAART-naïve patients 166 on HAART) |
Age (years): HAART-naïve (33.42±0.88), on HAART (36.91±0.77) Male gender: HAART-naïve (28.3%), on HAART (22.3%) |
eGFR <60 mL/min/1.73 m2 for >3 months | CG, 186 MDRD, CKD-EPI | Kinetic Jaffe | Not measured | Total prevalence (CKD-EPI): 10.2% HAART-naïve: 8.7% CG, 9.1% MDRD, 8.7% CKD-EPI On HAART: 14.5% CG, 12.6% MDRD, 12.6% CKD-EPI Prevalence by gender Female: HAART-naïve (7.5%), HAART (14%) Male: HAART-naïve (11.5%), HAART (8.1%) |
Low |
| Stöhr143 | 2011, Uganda, Zimbabwe, East and South | Three centres in Uganda and Zimbabwe | 3316 | HIV-infected patients initiating ART | Age (years): 36.8 (32–42.2) Male gender: 35% SBP: median: 110 (IQR: 100–120) mm Hg DBP: median: 70 (60–80) mm Hg BMI: 21.1 (19.1–23.6) kg/m2 |
eGFR <60 mL/min/1.73 m2 on ≥2 consecutive visits 80 days apart or confirmed 25% decrease if eGFR <60 mL/min/1.73 m2 at baseline | CG | Kinetic Jaffe | Not measured | Total prevalence: 7.2% | Medium |
| Stöhr144 | 2008, Uganda, Zimbabwe, East and South | Three centres in Uganda and Zimbabwe | 3316 | HIV-infected patients on ART | Age (years): 36.8 (32–42.2) Male gender: 35% SBP: median: 110 (IQR: 100–120) mm Hg DBP: median: 70 (60–80) mm Hg BMI categories <18.5 kg/m2: 18% 18.5 to <25 kg/m2: 66% 25 to <30 kg/m2: 12% ≥30 kg/m2: 4% |
eGFR <60 mL/min 1.73 m2 on ≥2 consecutive occasions >80 days apart or confirmed 25% decrease if eGFR <60 mL/min/1.73 m2 at baseline | 186 MDRD, CG | Kinetic Jaffe | Not measured | Total prevalence (MDRD): 3.1% CG: 7.4% |
Medium |
| Cailhol79 | 2011, Burundi, East | Outpatients HIV clinic | 300 | HIV-infected patients | Age (years): 40.1 (33–46.5) Male gender: 29.7% Hypertension: 2.7% DM: 2% BMI: median: 21.8 (19.3–24.2) kg/m2 |
KDOQI | Proteinuria by urinary strip, CG, 186 MDRD | Not mentioned | 6.10% | Total prevalence (MDRD): 45.7% CG: 46.5% Prevalence by stages (using MDRD) Stage 1: 30.2% Stage 2: 13.5% Stage 3: 2% Stages 4 and 5: no patients |
Medium |
| Masimango107 | 2014, Congo, Central | Outpatient HIV clinic | 235 | HIV-infected patients | Age (years): 40.0±10.7 Male gender: 27.8% Hypertension: 46.8% DM: 1.7% BMI: 22.3±3.8 kg/m2 |
Proteinuria ≥+1 by urinary strip or albuminuria ≥30 mg/dL | Proteinuria by urinary strip and ACR | Not measured | Proteinuria ≥+1: 41.3% | Total prevalence (based on proteinuria): 41.3% | Low |
| Reid145 | 2008, Uganda, Zimbabwe, East and South | Three centres in Uganda and Zimbabwe | 3316 | HIV-infected, ART-naïve adults with CD4+ cell counts of <200 cells/mm3 | Age (years): 36.8 (IQR: 32.0–42.2) Male gender: 35% SBP: median: 110 (IQR: 100–120) mm Hg DBP: median: 70 (IQR: 60–80) mm Hg BMI: median: 21.1 (IQR: 19.1–23.6) kg/m2 |
eGFR <60 mL/min 1.73 m2 on ≥2 consecutive occasions >80 days apart or confirmed 25% decrease if eGFR <60 mL/min/1.73 m2 at baseline | CG | Kinetic Jaffe | Not measured | Total prevalence: 7% | Medium |
| Fabian108 | 2009, South Africa, South | HIV outpatient clinic at Johannesburg Hospital | 578 | HIV-infected naïve ART patients | Age (years): 37 (range 16–70 years) Male gender: 38% DM: 4.6% among group with microalbuminuria |
Proteinuria ≥+1 by urinary strip or albuminuria ≥30 mg/dL | Proteinuria by urinary strip and PCR | Not measured | 43.7% had proteinuria | Total prevalence (based on proteinuria prevalence): 43.7% | Low |
| Lucas154 | 2010, Uganda, East | All consenting individuals residing in every household in 50 Rakai District communities | 1960 | 1202 HIV-infected patients and 664 HIV-negative age-matched and sex-matched controls | Age (years): HIV-negative: 28 (IQR: 24–35); HIV-positive: 30 (IQR: 25–36) Male gender: HIV-negative: 38.7%; HIV-positive: 36.4% |
eGFR <60 mL/min/1.73 m2 | MDRD | IDMS-calibrated | Not measured | Total prevalence among HIV-positive: 0.7% | Medium |
| Jao160 | 2011, sub-Saharan | Primary healthcare units | 2495 | HIV-infected patients before ART | Age (years): 30 (IQR: 27–35) Male gender: 30% BMI: 22.8 (IQR: 20.4–25.6) kg/m2 |
CrCl <50 mL/min | CG, 186 MDRD, CKD-EPI | Not mentioned | Not measured | Total prevalence (CKD-EPI with coefficient for black race): 2.5% CG: 3.4% (MDRD with coefficient for black race): 2.5% Prevalence by age <30 years: 29.8% 30–39 years: 57.1% ≥40 years: 13.1% Prevalence by gender Female: 66.7% |
Medium |
| Longo99 | 2012, Congo, Central | Consecutive patients with HIV from clinic | 300 | HIV-infected (ART treated=264) (ART-naïve=36) |
Age (years): 43±9 Male gender: 23% Hypertension: 13% BMI: 24±5 kg/m2 |
eGFR <60 mL/min/1.73 m2 or proteinuria defined as 1+ or greater | Proteinuria by dipstick and 24-hour proteinuria, eGFR by MDRD, CG | Kinetic Jaffe and IDMS | 20.50% | Total prevalence: 20.5% 3% of the patients had eGFR <60 mL/min/1.73 m2 by MDRD |
Low |
| Sarfo109 | 2013, Ghana, West | HIV clinic | 3137 | HIV-infected patients starting ART | Age (years): 38 (32–45) Male gender: 33% BMI: 20.3 (IQR: 17.6–22.7) kg/m2 |
eGFR <60 mL/min/1.73 m2, or proteinuria ≥+1 (confirmed by uPCR >45 mg/mmol) | Proteinuria by urinary strip, ACR, PCR, eGFR by CG, MDRD, CKD-EPI | Not mentioned | Total prevalence (CKD-EPI): 13.8% | Low | |
| Gupta161 | 2011, Cameroon, Central-West | Electronic medical records of patients from 18 sites throughout Western Kenya | 7383 | Patients with HIV without ART | Age (years): 35.5 (29.3–44.0) Male gender: 26.9% |
eGFR <60 mL/min/1.73 m2 | CG, MDRD | Not mentioned | Total prevalence (MDRD): 9.4% CG: 20.2% Prevalence by gender Female: 79.1% |
Medium | |
| Ekat146 | 2013, Congo, Central | Ambulatory treatment centre | 562 | Newly diagnosed patients with HIV | Age (years): 38.84 (IQR: 33.18–46.23) Male gender: 33.9% BMI: 20.31 (IQR: 17.97–22.89) kg/m2 |
eGFR <60 mL/min/1.73 m2 | 186 MDRD | Kinetic Jaffe | Not measured | Total prevalence: 8.5% | Low |
| Wools-Kaloustian80 | 2007, Kenya, East | Academic Model for the Prevention and Treatment of HIV/AIDS clinic |
373 | HIV-infected patients naïve to ART | Age (years): 35.0 (range, 19–60) Male gender: 32.1% SBP: 104.7 (range, 80–140) mm/Hg |
CrCl <60 mL/min/1.73 m2 | Proteinuria by urinary strip, CG, full and abbreviated MDRD | Kinetic assay | 6.2% (proteinuria ≥1+) | Total prevalence: 11.50% | Low |
| Emem81 | 2008, Nigeria, West | HIV/AIDS outpatient clinic | 400 | HIV-infected patients | Age (years): 34.6±9.4 Male gender: 48.5% Hypertension: 13.2% BMI categories <19.0 kg/m2: 59.2% 19–25 kg/m2: 37.5% >25 kg/m2: 3.3% |
Albuminuria +1 on at least two occasions (4 weeks apart) and/or serum creatinine >1.5 mg/dL | Proteinuria or albuminuria by urinary strip and 24-hour proteinuria, CG | Not mentioned | 38% proteinuria with dipstick 21.9% nephrotic range proteinuria |
Total prevalence : 38.8% Among patients, 8.8% had CrCl <15 mL/min. |
Medium |
| Wyatt82 | 2011, Rwanda, East | Community-based | 891 | 677 HIV-infected and 214 HIV-uninfected | Age (years): 34 (IQR: 30–39) HIV-positive: 43 (IQR: 34–50), HIV-negative Male gender: 0 Hypertension: HIV-positive: 4.8%/HIV-negative: 8.3% BMI (kg/m2): HIV-positive: 20.9 (IQR: 19.0–23.3)/HIV-negative: 20.5 (IQR: 18.5–23.3) |
eGFR <60 mL/min/1.73 m2 or proteinuria +1 or greater | Proteinuria by urinary strip, eGFR by MDRD, CKD-EPI, CG | Kinetic Jaffe | (9% among HIV-positive and 7.2% among non-infected) | Total prevalence among HIV-positive: 9% 2.7% had eGFR <60 mL/min/1.73 m2 CKD prevalence among HIV-negative: 7.2% 1.5% had eGFR <60 mL/min/1.73 m2 |
Medium |
| FolefackKaze83 | 2013, Cameroon, Central-West | HIV clinic of Yaoundé General Hospital | 104 | All newly diagnosed HIV-infected patients naïve to HAART | Age (years): 35±10.7 Male gender: 32% |
Presence of proteinuria +1 or more and eGFR <60 mL/min based on the average of eGFR by two equations | Proteinuria by urinary strip, eGFR by CG, 175 MDRD | Kinetic Jaffe | 36% | Total prevalence: 36% Among patients, 3% had eGFR<60 mL/min/1.73 m2. |
Low |
| Struik84 | 2011, Malawi, East | ART clinic in a central hospital in Malawi | 526 | Consecutive newly referred HIV-infected patients on ART | Age (years): 34.3±9.3 Male gender: 43.5% Hypertension: 11.2% DM: 0.8% |
Any proteinuria (≥+1), heavy proteinuria (≥+2), any proteinuria (≥+1) with renal dysfunction (eGFR <60 mL/min/1.73 m2), and heavy proteinuria (≥+2) with renal dysfunction (CrCl <60 mL/min) and the absence of any alternative cause for renal dysfunction or proteinuria | Proteinuria by urinary strip, eGFR by CG and MDRD | Not mentioned | 23.3% | Total prevalence: 23.3% Among patients with proteinuria, 5.3% had CrCl <60 mL/min. |
Low |
| Attolou118 | 1998, Benin, West | National Central Hospital | 92 | HIV-infected patients | Age (years): 22±4 Male gender: 68% |
Proteinuria >0.5 g/24 hours and SCr >14 mg/L | Serum creatinine measurement and 24-hour proteinuria | Not mentioned | Proteinuria >0.5 g/24 hours in 23.33% | Total prevalence: 27.16% | Low |
| Agaba170 | 2003, Nigeria, West | Infections unit of the Jos University Teaching Hospital | 126 | Consecutive 79 patients with AIDS and 57 controls | Not known | Not known | Not known | 25% (AIDS group) | Total prevalence among AIDS group: 51.80% CKD prevalence among control group: 12.2% |
Low | |
| Fana100 | 2011, Zimbabwe, South | Outpatient clinics | 159 | HIV-infected patients naïve to ART | CrCl <60 mL/min, proteinuria ≥+1 and/or PCR >20 mg/mg | Proteinuria by urinary strip and 24-hour proteinuria, eGFR by CG | Not mentioned | 45.90% | Total prevalence: 45.9% Among patients, 7.50% had CrCl <60 mL/min |
Low | |
| Han101 | 2006, South Africa, South | Medical centre | 615 | Patients with HIV not on ART | Age (years): 31 (range, 13–63) Male gender: 25% Proteinuria-negative: 117±14/70±9 Microalbuminuria: 121±15/81±10 Macroalbuminuria: 120±12/74±11 |
Microalbuminuria > urinary protein 30 and 300 mg/24 hours A cut-off serum creatinine level of 250 mmol/L was used to exclude those patients with advanced nephropathy. |
Proteinuria by urinary strip and 24-hour proteinuria, CG and MDRD | Not mentioned | 6% | Total prevalence (based on proteinuria): 6% | Low |
| Peters147 | 2008, Uganda, East | Home-based AIDS care | 508 | Patients with HIV starting HAART | Age (years): 39 (median) Male gender: 41% |
CrCl of 25–50 mL/min | CG, 175 MDRD | Kinetic Jaffe | Not measured | Total prevalence: 20% | Low |
| Jao110 | 2011, Cameroon, Central-West | Clinics | 389 | 199 HIV-positive and 190 HIV-negative pregnant women | Age (years): HIV-positive (27 (IQR: 24–31)) HIV-negative (27 (IQR: 22–31)) Male gender: 0 |
Proteinuria (PCR >200 mg/g) | Proteinuria by urinary strip and PCR | Not measured | HIV-positive: 39.2% HIV-negative: 20.9% |
Total prevalence among HIV-positive (based on proteinuria): 39.2% | Medium |
| Msango85 | 2011, Tanzania, East | Outpatient clinics | 355 | HIV-infected patients naïve to ART | Age (years): 36.1±7.9 Male gender: 35% BMI (kg/m2): 21.3±3.8 |
KDOQI | Proteinuria and albuminuria by urinary strip eGFR by CG, MDRD | Not mentioned | 36% proteinuria ≥+1 | Total prevalence: 85.6% | Low |
| Myer162 | 2013, South Africa, South | Primary healthcare clinic | 1861 | Consecutive 238 pregnant women, 1014 non-pregnant, 609 men; HIV-infected patients eligible for ART | Age (years): pregnant, 28 (IQR: 25–32), men, 37 (IQR: 32–45), women, 33 (IQR: 28–39) Male gender: 33% |
CrCl <60 mL/min | Absolute SCr and CG | Not mentioned | Not measured | Total prevalence: 5.8% | Low |
| Mulenga163 | 2008, Zambia, South | Clinic | 25 249 | HIV-infected, ART-naïve adults initiating treatment | Age (years): normal CrCl, 33.7±7.9, decreased CrCl, 38.5±9.9 Male gender: 39.7% |
CrCl <60 mL/min | Absolute SCr, eGFR by CG and MDRD | Not mentioned | Not measured | Total prevalence (MDRD): 3.2% | Medium |
| Adedeji158 | 2015, Nigeria, West | The University of Ilorin Teaching Hospital | 183 | Newly diagnosed HIV-infected ART-naïve patients | Age (years): 37.9+10.5 Male gender: 42.6% BMI (kg/m2): 20.88+3.56 |
eGFR <60 mL/min/1.73 m2 | Absolute SCr, eGFR by MDRD | Kinetic Jaffe and IDMS | Not measured | Total prevalence: 24% | Low |
| Anyabolu135 | 2016, Nigeria, West | Federal Medical Centre | 529 | 393 newly diagnosed drug-naïve patients with HIV, 136 age-matched and sex-matched HIV-seronegative controls | Age (years): 38.84±10.65 Male gender: 28% BMI categories <18.50.0 kg/m2: 7% 18.5–24.9 kg/m2: 35% 25–29.9 kg/m2: 32% ≥30 kg/m2: 23% |
24-hour urine protein ≥0.300 g and/or GFR <60 mL/min | Quantitative assessment of protienuira, SCr and eGFR | Not mentioned | Not mentioned | Total prevalence among HIV-positive patients: 22.9% Prevalence among HIV-negative: 8.1% |
Low |
| Ayokunle113 | 2015, Nigeria, West | Medical Out-patient Department of University of Ilorin Teaching Hospital | 335 | 227 newly diagnosed, ART-naïve patients with HIV/AIDS, 108 age-matched and sex-matched control | Age (years): 40.3±10.3 Male gender: 44% BMI (kg/m2): 20.5±4.8 among patients with HIV, 26.7±5.3 among control group SBP (mm Hg): 111.9±1 among patients with HIV, 126.1±12.0 among control group DBP (mm Hg): 72.9±9.5 among patients with HIV, 80.6±6.8 among control group |
Albuminuria ≥30 mg/g and/or eGFR <60 mL/mL/1.73 m2 | Proteinuria by dipstick, and ACR and eGFR by MDRD | Kinetic Jaffe | Not mentioned | Total prevalence among patients with HIV: 47.6% The prevalence among HIV-negative: 16.7% |
Low |
| Chadwick114 | 2015, Ghana, West | Komfo Anokye Teaching Hospital | 330 | Patients with HIV on ART | Age (years): 39 (IQR: 35–46) Male gender: 25% BMI (kg/m2): 22.9 (IQR: 20.5–26.6) |
Proteinuria or CrCl <60 mL/min | Proteinuria (dipsticks, PCR and ACR) and GFR by CG | Not mentioned | 37% by dipstick and 12% by PCR | Total prevalence (proteinuria): 37% CrCl <60 mL/min among 7% |
Low |
| Edwards166 | 2015, Kenya, East | Two primary care clinics | 2206 | 210 HIV-positive patients and 1996 HIV-negative | Age (years): HIV-positive: 43 (IQR: 39–50), HIV-negative: 49 (IQR: 40–56) Male gender: HIV-positive: 31%; HIV-negative: 28.7% Hypertension: HIV-positive: 44%; HIV-negative: 33.2% DM: HIV-positive: 5%; HIV-negative: 15.2% |
CrCl <60 mL/min | eGFR by CKD-EPI | Not mentioned | Not measured | Total prevalence: 12.1% HIV-positive: 17% HIV-negative: 11% |
Medium |
| Glaser14 | 2016, Malawi, East | Lighthouse Clinic | 363 | 116 HIV-positive ART-naïve patients and 247 HIV-negative patients | Age (years): 31 (IQR: 26–39) Male gender: 52% |
eGFR <60 mL/min | eGFR by CG, MDRD and CKD-EPI with and without correction factor | IDMS-calibrated creatinine and cystatin-C | Not measured | Total prevalence among HIV-positive (creatinine-based CKD-EPI): 1.9% | Medium |
| Glaser115 | 2016, Malawi, East | Lighthouse Clinic | 363 | 116 HIV-positive patients and 247 HIV-negative patients | Age (years): 34.1±10.9 Male gender: 52% BMI (kg/m2): 23.2±4.8 Hypertension: 13.5% |
KDOQI | Proteinuria by dipstick and ACR, eGFR by CG, MDRD and CKD-EPI | IDMS-calibrated creatinine and cystatin-C | 12.1% | Total prevalence: 13% Prevalence among HIV-positive: 22% Prevalence among HIV-negative: 9% |
Medium |
| Kamkuemah167 | 2015, South Africa, South | Gugulethu Community Health Centre |
1092 | HIV-infected patients initiated ART therapy | Age (years): 34 (IQR: 29–41) Male gender: 38% |
eGFR <60 mL/min | eGFR by CG | Not mentioned | Not measured | Total prevalence: 2% Prevalence by age <29 years: 17% 29–34 years: 28% 34–41 years: 5% >41 years: 50% Prevalence by gender Male: 28% Female: 72% |
Medium |
| Nsagha149 | 2015, Cameroon, Central-West | Government hospitals | 200 | Patients with HIV on HAART, DOTS or on the combined therapy (HAART/DOTS) | Age (years): 38.04±10.52 Male gender: 50.5% |
eGFR <60 mL/min per 1.73 m2 | eGFR by MDRD | Kinetic Jaffe | Not measured | Total prevalence: 8% | Low |
| Odongo94 | 2015, Uganda, East | Infectious Diseases Clinic of Gulu Regional Referral Hospital | 361 | Newly diagnosed patients with HIV not receiving ART | Age (years): 31.4±9.5 Male gender: 36.3% BMI (kg/m2)<18: 33% |
eGFR <60 mL/min/1.73 m2 | Proteinuria by dipstick and eGFR by MDRD | Not mentioned | Proteinuria ≥+1: 52% | Total prevalence: 14.4% Prevalence by gender Female: 16.5% Male: 10.4% |
Low |
| Okafor136 | 2016, Nigeria, West | University of Benin Teaching Hospital | 383 | HIV-infected naïve patients | Age (years): 36.03±9.08 Male gender: 41% |
eGFR <60 mL/min/1.73 m2 and/or evidence of kidney injury as detected when the PCR (mg/g) was ≥200 | Quantitative assessment of proteinuria by PCR and eGFR by MDRD | Kinetic Jaffe | Not mentioned | Total prevalence: 53.5% | Low |
| Seape156 | 2016, South Africa, South | Medical inpatients at the Chris Hani Baragwanath Hospital |
100 | HIV-infected naïve patients | Age (years): 37.0±9.6 Male gender: 60% BMI (kg/m2): 20.9±5.1 |
eGFR <60 mL/min/1.73 m2 | eGFR by CG, MDRD, CKD-EPI | IDMS | Not measured | Total prevalence: 16% | Low |
| Wensink137 | 2015, South Africa, South | Rural Medical Centre | 903 | HIV-infected adult patients | Age (years): 40 (IQR: 34–48) Male gender: 31% DM: 4% Hypertension: 23% |
Albuminuria or eGFR<60 mL/min/1.73 m2 | Albuminuria by ACR and eGFR by MDRD and CKD-EPI | Not mentioned | 21% | Total prevalence (albuminuria): 21% 2% had eGFR<60 mL/min/1.73 m2 |
Medium |
| Zachor157 | 2016, South Africa, South | Outpatient infectious clinic at an academic hospital | 650 | HIV-infected patients initiating ART | Age (years): 37.9±9.4 Male gender: 35.5% DM: 2.2% Hypertension: 7.8% |
eGFR <60 mL/min/1.73 m2 | eGFR by MDRD and CKD-EPI | IDMS | Not measured | Total prevalence: 2% | Medium |
| Mekuria150 | 2016, Ethiopia, East | Jimma University Specialised Hospital |
446 | 223 HAART-naïve and 223 HAART-experienced | Age (years): HAART-naïve: 38.25±10.8, HAART-positive: 35.14±9.2 Male gender: 37% BMI (kg/m2): HAART-naïve: 20.7±3.2, HAART-positive: 21.6±3.5 Hypertension: 3.36% DM: 21.4% |
eGFR <60 mL/min/1.73 m2 | eGFR by CG | Kinetic Jaffe | Not measured | Total prevalence: 18.2% | Medium |
ACR, albumin to creatinine ratio; ART, antiretroviral therapy; BMI, body mass index; CG, Cockroft-Gault; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease-Epidemiology; CrCl, creatinine clearance; DBP, diastolic blood pressure; DM, diabetes mellitus; DOTS, directly observed treatment short course; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; HAART, highly active antiretroviral therapy; IDMS, isotope dilution mass spectrometry; KDOQI, Kidney Disease Outcome Quality Initiative; MDRD, Modification of Diet in Renal Disease; SBP, systolic blood pressure; SCr, serum creatinine; uPCR, urinary protein to creatinine ratio.
Table 4.
Studies on CKD among patients with diabetes
| Study ID | Year, country, region | Location | N | Study group | Population characteristics | Definition of CKD | Methods of outcome assessment | Creatinine assay | Proteinuria | CKD prevalence | Quality assessment |
| Janmohamed86 | 2013, Tanzania, East | Diabetes mellitus clinic of Bugando Medical Centre in Mwanza | 369 | Consecutive patients with diabetes | Age (years): 54 (IQR: 45–62) Male gender: 46.6% Hypertension: 57.5% BMI (kg/m2): 25.6 (IQR: 22.6–29.6) Duration of DM (years): 6 (3–11) 93.8% type 2 DM 6.2% type 1 DM |
eGFR ≤60 mL/min/1.73 m2 or evidence of kidney damage (microalbuminuria or overt proteinuria) | Microalbuminuria, proteinuria by urinary strips, eGFR by CG | Kinetic Jaffe | Overt proteinuria (34.1%), microalbuminuria (45.8%) | Total prevalence: 83.7% | Low |
| Wanjohi87 | 2002, Kenya, East | Outpatient diabetic clinic at Kenyatta National Hospital | 100 | Patients with type 2 diabetes | Age (years): 53.7±9.3 Male gender: 37% Hypertension: 50% BMI (kg/m2): 27.8±6.0 Duration of DM (months): 10.3±7.5 |
Albuminuria >20 mg/ L | Albuminuria by urinary strip, CG | Not mentioned | 26% had albuminuria | Total prevalence (based on albuminuria): 26% | Low |
| Bouzid119 | 2011, Tunis, North | Endocrinology centre at the National Institute of Nutrition | 689 | Patients with type 2 diabetes from computerised hospital database | Age (years): 60±11 Male gender: 39% Hypertension: 84.6% (renal insufficiency), 57.2% (no renal disease) Duration of DM (years): 11±8 BMI (kg/m2): 28.8±5.5 |
eGFR <60 mL/min | CG, 24-hour proteinuria | Not mentioned | 10.1% macroalbuminuria, 13% microalbuminuria | Total prevalence: 19.8% | Low |
| Choukem88 | 2012, Cameroon, Central-West | Two main referral centres | 420 | Consecutive patients with type 2 diabetes | Age (years): 56.7±9.9 Male gender: 49% Hypertension: 50% BMI (kg/m2): 28.5±5.2 Duration of DM (years): 4 (IQR: 1–9) |
Presence of positive proteinuria with or without low CrCl <90 mL/min/1.73 m2 | Proteinuria by urinary strip/eGFR by CG | Not mentioned | Total prevalence: 31% | Low | |
| Keeton120 | 2004, South Africa, South | Groote Schuur Hospital Outpatients Diabetic Clinic or the Somerset Hospital Outpatients |
59 | Patients with type 2 diabetes | Age (years): 62±9.4 Male gender: 36% BMI (kg/m2): (31±6) Duration of DM (years): 17 (range: 14–33) |
Double SCr level | Proteinuria by PCR and serum creatinine | Not mentioned | Total prevalence: 66.1% | Low | |
| Bouaziz121 | 2012, Tunisia, North | Basic Health Group of Sousse | 115 | 73 patients with type 2 diabetes and 42 healthy volunteers | Age (mean±SE in years): 59.3±1.1 Male gender: 35% SBP (mean±SE mm Hg): 136.3±3.1 DBP (mean±SE): 76.8±1.9 BMI (mean±SE in kg/m2): 30.5±0.7 Duration of DM (years): 10.6±1 |
Microalbuminuria (defined as <2.8 g/mmol for women and <2.3 for men) and eGFR ≤60 mL/min/1.73 m2 | Measurement of microalbuminuria, eGFR by MDRD | Not mentioned | Total prevalence: 11% | Low | |
| Katchunga122 | 2010, Congo, Central | Referral general hospital | 98 | Medical records of patients with type 2 diabetes | Age (years): 58±10.4 Male gender: 35.7% Hypertension: 59.2% BMI (kg/m2): 25.2±4.7 Duration of DM (years): 17.3±8.5 |
KDOQI | Microalbuminuria (>20 mg/L and <200 mg/L) eGFR by MDRD | Not mentioned | Total prevalence: 66% | Low | |
| Djrolo123 | 2001, Benin, West | National University Hospital Centre | 152 | Patients with type 1 and 2 diabetes | Age (years): 53.3 (range, 21–90) Male gender: 65.8% Duration of DM (years): <1–16 or more |
Presence of proteinuria | 24-hour proteinuria | Not measured | 28% | Total prevalence (based on proteinuria level): 28% | Low |
| Balogun102 | 2011, Nigeria, West | Tertiary hospital | 40 | Randomly selected patients with type 2 diabetes | Age (years): 59.4±11.25 Male gender: 37.5% Hypertension: 45% |
Not mentioned | Proteinuria by urinary strip and 24 hours, eGFR by CG | Jaffe method | 82.5% macroalbuminuria | Total prevalence: 90% | Low |
| Mafundikwa103 | 2007, Zimbabwe, South | Diabetic clinic | 75 | Consecutive insulin-dependent patients with diabetes | No available data | No available data | Proteinuria by urinary strips and 24-hour proteinuria | Overt proteinuria 21%, microalbuminuria 12%. |
Total prevalence: 33% | Low | |
| Lutale124 | 2007, Tanzania, East | Outpatient diabetic clinic | 204 | 91 patients with type 1 and 153 type 2 diabetes | 45% type 1 DM 55% type 2 DM Age (years): type 1, 21 (14–44.8), type 2, 53 (23.5–85) Male gender: 55% hypertension: 42% BMI (kg/m2): 19.3±3.8 (type 1), 27.8±4.8 (type 2) Duration of DM (years): 3(Range: 0–25) |
KDOQI | Quantitative assessment of albuminuria, CrCl by CG | Kinetic Jaffe | Type 1: microalbuminuria was 12.1% and macroalbuminuria 1.1%. Type 2: microalbuminuria 9.8% Macroalbuminuria 7.2% |
Total prevalence: 18.5% 4.6% of type 1 patients and 22% of type 2 had eGFR <60 mL/min/1.73 m2 |
Low |
| Gill125 | 2008, Ethiopia, East | Diabetic clinic at Mekelle Hospital | 105 | All patients with diabetes | Age (years): 41±16 Male gender: 70% Hypertension: 5% BMI (kg/m2): 20.6±5.4 Duration of DM (years): 7±6 |
Nephropathy was considered present if the urinary ACR was >25.0 mg/mmol and retinopathy was present. Microalbuminuria was diagnosed if the ACR was >2.5 and <25.0 mg/mmol in men and >3.5 and <25.0 mg/mmol in women. |
ACR, SCr | Not mentioned | 51% microalbuminuria | Total prevalence: 51% | Low |
| Makulo111 | 2010, Congo, Central | Community-based | 229 | 81 patients with diabetes and 148 with impaired fasting glucose | Age (years): 53.1±16.3 Male gender: 33% SBP (mm Hg): 128.0±5.7 DBP (mm Hg): 78.5±13.4 BMI (kg/m2): 22.6±5.2 |
eGFR of <60 mL/min/1.73 m2 | Urinary albumin by urinary strip and ACR, eGFR by 186 MDRD | Kinetic Jaffe | 29.6% | Total prevalence: 29.6% 10% of the patients had eGFR <60 mL/min/1.73 m2 |
Medium |
| Adebamowo151 | 2016, Nigeria, Ghana, Kenya (sub-Saharan) |
University medical centres and surrounding communities | 4815 | 2208 cases of type 2 DM and 2607 controls free from DM | Age (years): 48±15 Male gender: 41% Hypertension: 68.3% of type 2 DM and 35.3% of diabetic-free BMI (kg/m2): 26.9±5.4 (patients with diabetes), 25.5±5.7 (non-diabetics) |
eGFR of <60 mL/min/1.73 m2 | eGFR by MDRD and CKD-EPI | Kinetic Jaffe | Not measured | Total prevalence (MDRD): 9% 13.4% of type 2 DM and 4.8% of diabetic-free |
Medium |
| Feteh95 | 2016, Cameroon, Central-West | Outpatient section of the endocrine unit of the Douala General Hospital | 636 | Cases of type 2 DM | Age (years): 56.5±10.6 Male gender: 53.1% BMI (kg/m2): 29.3±14.7 Hypertension: 62.2% |
eGFR of <60 mL/min/1.73 m2 | Proteinuria by dipsticks and eGFR by 186 MDRD | Kinetic Jaffe | 68.4% among patients with anaemia, 57.6% non-anaemic | Total prevalence: 18.5% | Low |
| Fiseha152 | 2014, Ethiopia, East | Follow-up clinic at Butajira Hospital | 214 | Patients with diabetes | Age (years): 45±14.5 Male gender: 57.5% SBP (mm Hg): 121±17 DBP (mm Hg): 79±10 BMI (kg/m2): 25.26±4.35 |
eGFR of <60 mL/min/1.73 m2 | eGFR by CG and 186 MDRD | Kinetic Jaffe | Not measured | Total prevalence (MDRD): 18.2% Prevalence (CG): 23.8% |
Medium |
| Pillay96 | 2016, South Africa, South | All patients seen at Edendale Hospital Diabetic Clinic | 653 | Patients with diabetes with or without HIV (149 DM and HIV; 504 DM without HIV) | Among patients with diabetes with HIV: Age (years): 50–70 Male gender: 32% Among patients with diabetes without HIV Age (years): 51–60 |
eGFR of <60 mL/min/1.73 m2 | Proteinuria by dipstick and eGFR by 186 MDRD | Kinetic Jaffe | 18% | Total prevalence: 18.8% |
Medium |
| Eghan138 | 2007, Ghana, West | Outpatient diabetic clinic of the Department of Medicine at Komfo Anokye Teaching Hospital |
109 | Patients with diabetes | Age (years): 54.1±10.9 Male gender: 28% Hypertension: 39% BMI (kg/m2): 26.3±4.4 |
Microalbuminuria if urine albumin excretion was 30–300 mg/day | Albuminuria by urine albumin excretion and eGFR by CG | Not mentioned | 43.1% | Total prevalence (based on microalbuminuria): 43.1% Prevalence by gender: male: 31.9% |
Low |
ACR, albumin to creatinine ratio; BMI, body mass index; CG, Cockroft-Gault; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; CrCl, creatinine clearance; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IDMS, isotope dilution mass spectrometry; KDOQI, Kidney Disease Outcome Quality Initiative; MDRD, Modification of Diet in Renal Disease; SBP, systolic blood pressure; SCr, serum creatinine.
Table 5.
Studies on CKD among patients with hypertension
| Study ID | Year, country, region | Location | N | Study group | Population characteristics | Definition of CKD | Methods of outcome assessment | Creatinine assay | Proteinuria | CKD prevalence | Quality assessment |
| Osafo126 | 2011, Ghana, West | Four polyclinics | 712 | Patients with hypertension | Age (years): 59 (range, 19–90) Male gender: 21.3% DM: 14.7% SBP (mm Hg): 150 (range, 100–280) DBP (mm Hg): 90 (range, 60–160) BMI (kg/m2): 29.7 (range, 12.2–67.4) BMI categories (kg/m2) <25: 22.3% 25–29.9: 26% >30: 45.7% |
KDOQI | Proteinuria by PCR (men >0.3, women >0.2 mg/mg) eGFR by MDRD | Kinetic Jaffe | 28.90% | Total prevalence: 46.90% Prevalence by stage Stages 1–2: 19.1% Stages 3–5: 27.8% Prevalence by gender Female: 46.6% Male: 48% |
Low |
| Ajayi164 | 2014, Nigeria, West | Tertiary health centre | 628 | Records of patients with hypertension and diabetes | Age (years): 49.71±13.22 Male gender: 49% DM: 8.6% SBP (mm Hg): 135.9±27.4 DBP (mm Hg): 87.0±16.3 BMI (kg/m2): 27.8±8.7 |
eGFR <60 mL/min/1.73 m2 | eGFR by MDRD | Not mentioned | Not measured | Total prevalence: 38.5% Prevalence by age <20 years: 0.1% 21–40 years: 31.5% 41–60 years: 34.7% 61–75 years: 40% >75 years: 62.9% Prevalence by gender Female: 57% Male: 18.9% |
Low |
| Lengani127 | 2000, Burkina Faso, West | Department of Cardiology or Internal Medicine | 342 | Patients with hypertension | Age (years): 50.6±13.8 Male gender: 58% |
Serum creatinine ≥650 µmol/L and or blood urea ≥35 mL/L plus long history with clinical manifestations | Measurement of SCr, 24-hour proteinuria | Not mentioned | Total prevalence: 50.8% | Low | |
| Nwankwo165 | 2006, Nigeria, West | University of Maiduguri Teaching Hospital | 185 | All hospitalised patients with hypertension | Age (years): 44.6±14.9 Male gender: 49% |
SCr >135 µmol/L | Measurement of SCr | Not mentioned | Not measured | Total prevalence: 45.50% | Low |
| Rayner128 | 2006, South Africa, South | 100 general practice centres | 1091 | Random patients with hypertension | Age (years): ≥35 years Male gender: 48.5% BMI: 23.6% of the patients had a normal BMI. 41.9% were overweight and 34.2% were frankly obese. |
Albuminuria defined as (mg/mmol) microalbuminuria 3–30, macroalbuminuria >30 | Quantitative assessment of albuminuria by ACR | Not measured | 21.3% microalbuminuria, 4.1% macroalbuminuria | Total prevalence (based on albuminuria): 25.4% | Medium |
| Plange-Rhule89 | 1999, Ghana, West | Komfo Anokye Teaching Hospital | 448 | Patients with hypertension | Age (years): 50.5±13.0 Male gender: 36% SBP (mm Hg): 165.0±27.8 DBP (mm Hg): 101.9±17.9 |
Plasma creatinine ≥140 mol/L | Proteinuria by urinary strips and serum creatinine | Not mentioned | 25.50% | Total prevalence: 30.2% | Low |
| Addo141 | 2009, Ghana, West | Seven central government ministries in Accra | 219 | Patients with hypertension | Age (years): 50.4±6.6 Male gender: 64% SBP (mm Hg): 156.0±21.5 DBP (mm Hg): 95±13 BMI (kg/m2): 27.5±5.4 |
Persistent proteinuria on urinalysis in the absence of urinary tract infection and/or impaired GFR <60 mL/min/ 1.73 m2 |
Proteinuria and eGFR by MDRD | Enzymatic assessment | 13.4% | Total prevalence: 13.4% 4.1% had eGFR <60 mL/min/1.73 m2 |
Medium |
| Aryee139 | 2016, Ghana, West | Komfo Anokye Teaching Hospital and the surrounding community |
242 | 180 non-diabetic patients with hypertension and 61 age-matched controls | Age (years): 22–87 Male gender:37% SBP (mm Hg): patients with hypertension (on antihypertensive therapy: 155.46±1.82, no antihypertensive therapy: 152±3.27), control (117.38±0.96) DBP (mm Hg): patients with hypertension (on antihypertensive therapy: 101.46±0.94, no antihypertensive therapy: 100.50±1.34), control (73.28±0.77) BMI (kg/m2): patients with hypertension (on antihypertensive therapy: 29.52±0.39, no antihypertensive therapy: 29.8±0.71), control (29.36±0.65) |
eGFR <60 mL/min/1.73 m2 | Urine albumin excretion, and eGFR by CG, 186 MDRD and CKD-EPI | Not mentioned | 30% | Total prevalence (CKD-EPI): 14.5% Prevalence by MDRD: 13.3% Prevalence by CG: 16.6% |
Low |
| Nabbaale140 | 2015, Uganda, East | Outpatient hypertension clinic | 256 | Newly diagnosed eligible black adult patients with hypertension | Age (years): 54.3±6.2 Male gender: 36.7% |
Microalbuminuria as a random urine albumin level between 30 and 299 mg/dL | Quantitative assessment of albumin in urine | Not measured | 39.5% | Total prevalence (based on microalbuminuria): 39.5% | Low |
BMI, body mass index; CG, Cockroft-Gault; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; CrCl, creatinine clearance; DBP, diastolic blood pressure; DM, diabetes mellitus; GFR, glomerular filtration rate; IDMS, isotope dilution mass spectrometry; KDOQI, Kidney Disease Outcome Quality Initiative; MDRD, Modification of Diet in Renal Disease; SBP, systolic blood pressure; eGFR, estimtaed glomerular filtration rate; ACR, albumin to creatinine ratio.
Table 6.
Studies on CKD among other populations
| Study ID | Year, country, region | Location | N | Study group | Population characteristics | Definition of CKD | Methods of outcome assessment | Creatinine assay | Proteinuria | CKD prevalence | Quality assessment |
| Ka19 | 2013, Senegal, West | Nephrology Department of the Aristide Le Dantec University Hospital Centre |
43 | Patients with lupus | Age (years): 32.9 Male gender: 7% Hypertension: 30% |
Proteinuria >0.5 g/24 hours with or without haematuria/renal insufficiency/abnormal renal biopsy | 24-hour proteinuria and eGFR by CG | Not mentioned | 51% | Total prevalence: 72% | Low |
| Abd ElHafeez29 | 2009, Egypt, North | Nephrology Department at the Main Alexandria University Hospital | 400 | Relatives of ESRD patients | Age (years): 35.2±11.6 Male gender: 50.8% Hypertension: 60% DM: 11.5% BMI (kg/m2): 28.5±5.89 |
KDOQI | Proteinuria by urinary strips, 186 MDRD | Kinetic Jaffe | 21.3% | Total prevalence: 57% Prevalence by stage Stage 1: 9% Stage 2: 44% Stage 3: 4% Stage 4: 0.3% |
Medium |
| Raji28 | 2015, Nigeria, West | Nephrology outpatient clinic at Lagos University Teaching Hospital | 469 | 230 first-degree relatives of patients with CKD and 230 age-matched and gender-matched controls with no personal or family history of CKD | Age (years): 33.49±12.0 BMI (kg/m2): first-degree relatives: 25.5±5.3, controls: 23.8±4.0 SBP (mm Hg): first-degree relatives: 116.5±22.5, controls: 112.1±18.1 DBP (mm Hg): first-degree relatives: 74.9±12.7, controls: 71.4±10.5 |
Reduced eGFR | Albuminuria by ACR and eGFR by MDRD | Not mentioned | 46% | Total prevalence: 4% | Low |
| Elsharif24 | 2013, Sudan, East | Primary healthcare | 252 | Patients attending the primary healthcare facilities | Age (years): 43.35±12.80 Male gender: 16% Hypertension: 10% DM: 5.95% BMI (kg/m2): 28.67±6.43 BMI categories (kg/m2) <18: 2.38% >25.13: 71.83 |
eGFR of <60 mL/min/1.73 m2 with or without proteinuria | Proteinuria by urinary strip and eGFR by MDRD | Not mentioned | 24.21% | Total prevalence: 10.32% | Low |
| Afolabi26 | 2009, Nigeria, West | Family practice clinic | 250 | Newly registered patients who attended the Family Practice Clinic | Age (years): 50.52+13.03 Male gender: 27.2% 32% elevated SBP, 30% elevated DBP DM: 6% Obesity: 32% |
Persistently abnormal ACR irrespective of GFR level or persistent eGFR <60 mL/min/1.73 m2 irrespective of the presence or absence of kidney damage after 3 months | Proteinuria by urinary strip, eGFR by MDRD | Standardised IDMS | 14.4% | Total prevalence: 14.4% 10.4% had persistent eGFR <60 mL/min/1.73 m2 |
Medium |
| Sumaili25 | 2009, Congo, Central | Primary and secondary healthcare | 527 | At-risk population randomly selected | Age (years): 53.9±15.5 Male gender: 43% Hypertension: 58.2% DM: 54.5% Obesity: 16% |
KDOQI | Proteinuria by urinary strip, 24-hour proteinuria, 175 MDRD | Kinetic Jaffe | 19% | Total prevalence: 36% Prevalence by stage Stage 1: 4.2% Stage 2: 6.1% Stage 3: 18.3% Stage 4: 1.9% Stage 5: 5.7% |
High |
| Anyabolu30 | 2016, Nigeria, West | Federal Medical Centre | 136 | Subjects from medical outpatient department of the hospital | Age (years): 38.58±11.79 Male gender: 27.9% BMI (kg/m2): 25.51±6.47 |
Proteinuria as 24-hour protein ≥0.300 g and impaired renal filtration function as CrCl <90mL/min | Proteinuria by quantitative assessment and SCr | Kinetic Jaffe | 14.1% had proteinuria | Total prevalence: 14.1% | Low |
| Dessein20 | 2015, South Africa, South | Charlotte Maxeke Johannesburg and Milpark Hospitals | 233 | African patients with rheumatoid arthritis | Age (years): 57.1±10.8 Male gender: 17.2% BMI (kg/m2): 27.4±6.0 Hypertension: 57.5% DM: 12.5% |
eGFR <60 mL/min/1.73 m2 | eGFR by CG, MDRD, CKD-EPI | Kinetic Jaffe and IDMS-calibrated | Not measured | Total prevalence: 39% | Low |
| Ephraim21 | 2015, Ghana, West | Tema General Hospital |
194 | Patients with sickle cell anaemia | Age (years): 23.25±12.04 Male gender: 43.3% SBP (mm Hg): 110.06±8.27 DBP (mm Hg): 67.16±8.23 BMI (kg/m2): 18.85±11.19 |
eGFR<60 mL/min/1.73 m2 or evidence of kidney damage as albuminuria or overt proteinuria | Proteinuria by dipstick and eGFR by CKD-EPI | IDMS | 13.4% | 39.2% | Low |
| van Rensburg27 | 2010, South Africa, South | Tertiary hospital | 1216 | New patients referred to the renal unit | Age (years): 39.6±15.9 Male gender: 51.1% Hypertension: 13.2% DM: 10.8% |
Elevated SCr (>130 μmol/L) and small kidneys on imaging without evidence of reversible causes | Proteinuria by quantitative assessment and SCr measurement | Not mentioned | 16.7% proteinuria >3.5 g/dL | Total prevalence: 37.9% | Low |
| Hamdouk104 | 2011, Sudan, East | Hairdressing saloons | 72 | Hairdressers | Age (years): 40±8 Male gender: 0% Hypertension: 19.4% |
SCr level ≥2 mg/dL | Proteinuria by urinary strip and 24-hour SCr measurement and renal biopsy | Not mentioned | 26.4% had albuminuria | Total prevalence: 26.4% 14% had SCr ≥2 mg/dL |
Low |
| EL-Safty129 | 2003, Egypt, North | Male workers attending the outpatient clinic of the Health Insurance Organisation | 81 | Male workers attending the outpatient clinic of the Health Insurance Organisation Workers (29 non-silicotics, 24 silicotics and 28 referent) |
Age (years): 39.83±7.27 Male gender: 100% Hypertension: 19.4% |
Elevated proteinuria | Assessment of proteinuria quantitatively | Not measured | 93% among non-silica-exposed 100% silica-exposed |
Total prevalence (among those with silica exposure): 100% | Low |
BMI, body mass index; CG, Cockroft-Gault; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; CrCl, creatinine clearance; DBP, diastolic blood pressure; DM, diabetes mellitus; GFR, glomerular filtration rate; IDMS, isotope dilution mass spectrometry; KDOQI, Kidney Disease Outcome Quality Initiative; MDRD, Modification of Diet in Renal Disease; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; ACR, albumin to creatine ratio.
bmjopen-2016-015069supp002.pdf (118.4KB, pdf)
The studies that were included covered all regions of Africa. The highest number of the studies came from the Western macro-area (n=54), followed by the Eastern macro-area (n=32) and Southern macro-area (n=25). Twenty studies were retrieved from Northern Africa, and eight studies from each of the Central macro-area and the Central-Western macro-area. Three studies were conducted in both the Eastern and Southern regions and two studies in the sub-Saharan region.
Assessment of kidney function impairment
Urinary markers for kidney disease were assessed in 78 (71%) among 110 studies conducted in the general population, high-risk groups, occupational or hospital-based studies. Proteinuria was assessed by a semiquantitative method (urinary strips) in 28 studies.21 24 26 29 73–96 Twenty studies used dipstick with confirmation by quantitative methods, nine of which used dipsticks to identify proteinuria/albuminuria with confirmation by 24-hour proteinuria,25 97–104 whereas 11 studies used dipstick with confirmation by the protein-to-creatinine ratio or albumin-to-creatinine ratio.105–115 Quantitative methods for the assessment of proteinuria/albuminuria (24-hour proteinuria or albuminuria, Protein to Creatine Ratio (PCR), immunoassay or Albumin to Creatinine Ratio (ACR) were applied in 29 studies.19 27 28 30 116–140 In one study, the method of proteinuria assessment was not mentioned.141
Serum creatinine was measured in 95 studies (86%). The Jaffe assay was used in 30 studies,29 30 76 80 82 83 86 90 95 97 102 105 111 113 124 126 130 131 136 142–152 whereas the isotope dilution mass spectrometry (IDMS)-calibrated method was used in 15 studies.12 14 21 26 115 117 132–134 141 153–157 In nine studies, both the Jaffe assay and the calibrated serum creatinine were used.13 20 25 91 98 99 106 112 158 The remaining 41 studies provided no information on the method of creatinine measurement.19 24 27 28 78 79 81 84 85 87–89 93 94 96 100 101 104 109 114 116 118–122 125 127 135 137–139 159–167 With respect to the formula used for estimating GFR, the MDRD equation was used in 30 studies24–26 28 29 94–97 105 106 111 113 116 117 121 122 126 130 133 134 136 141 146 149 153 154 158 159 164 and the CG equation was used in 18.19 76 81 86–88 93 100 102 114 119 124 138 143 145 150 162 167 The other 14 studies used both the CG and the MDRD equations,78–80 83–85 98 99 101 144 147 152 161 163 whereas 15 studies estimated GFR by the CG, MDRD and the CKD-EPI methods.12–14 20 82 90 91 109 112 115 139 142 155 156 160 Six studies used MDRD and CKD-EPI131 132 137 148 151 157 and two studies used CKD-EPI.21 166 In other two studies the formula was not mentioned.30 135
Definition of CKD
Thirty-one studies defined the presence of CKD as an estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2,12 14 20 80 93–96 111 117 119 139 146 148–159 161–164 166 167 with chronicity confirmed by repeated testing in four other studies.142–145 Moreover, 28 studies reported CKD prevalence based on eGFR below 60 mL/min/1.73 m2 and/or the presence of proteinuria or albuminuria.21 24 26 76 78 82–84 86 91 99 100 105 106 109 112–114 121 130–137 141 Proteinuria/albuminuria was used alone to identify CKD in 14 studies.73–75 77 87 92 107 108 110 123 128 129 138 140 KDOQI staging168 of CKD was used in 13 studies.13 25 29 79 85 90 97 98 115 116 122 124 126 The serum creatinine level (either doubling, or an increase above a certain threshold) was considered to be a marker of the presence of CKD in four studies.89 104 120 165 In 16 studies, the definition of CKD was either not mentioned or was defined in various ways, including personal history, creatinine clearance (CrCl) ≤50 mL/min, clinical manifestations, the presence of albuminuria, elevated serum creatinine and the average of two measurements of eGFR <90 mL/min/1.73 m2.19 27 28 30 81 88 101–103 118 125 127 147 160 169 170
Paper quality
Paper quality was high in 16 studies.13 25 75 90 91 97 98 105 106 112 116 132–134 148 155 Thirty-five studies were of medium quality.12 14 26 29 73 74 77–79 81 82 96 110 111 115 117 128 130 131 137 141 143–145 150–152 154 157 159–161 163 166 167 The rest of the studies were of low quality.
Prevalence of CKD
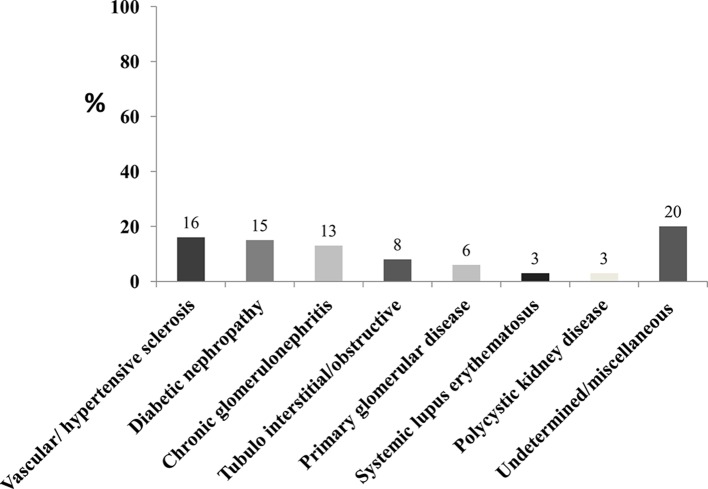
The included medium-quality/high-quality studies in the general population in Africa provided estimates of CKD prevalence by disparate criteria (table 2). The prevalence of CKD ranged from 2% to 41% (pooled prevalence: 10.1%; 95% CI 9.8% to 10.5%). The prevalence was reported to range from 2% to 41% (pooled estimate: 16.5%) in the West/Central-West, followed by the Central region where the prevalence ranged from 12% to 17% (pooled estimate: 16%), in the Southern where the CKD prevalence range was 6%–29% (pooled estimate: 12.2%), in Eastern where the prevalence ranged from 7% to 15% (pooled estimate: 11.0%), and in the North where the prevalence ranged from 3% to 13% (pooled estimate: 4%) (figure 2). In sub-Saharan Africa, the prevalence ranged from 2% to 14% (pooled prevalence: 14.02%; 95% CI 13.5% to 14.5%). In studies defining CKD as eGFR <60 mL/min, the prevalence of CKD ranged from 7% to 29% (pooled estimate: 13.2%), while in those who adopted the combined criterion GFR <60 mL/min/1.73 m2 and/or the presence of proteinuria or albuminuria, the prevalence ranged from 3% to 22% (pooled estimate: 5.6%). When defined according to KDOQI, the prevalence ranged from 2% to 28% (pooled estimate: 10.8%). Finally, in studies reporting on proteinuria/albuminuria only, the prevalence ranged from 3% to 41% (pooled estimate: 18.9%). The CKD prevalence for each age or gender group was not reported in the majority of the studies. In online supplementary figure 1 we show graphically the relationship between gender and age and CKD prevalence in the medium-high-quality studies of this systematic review.
Figure 2.
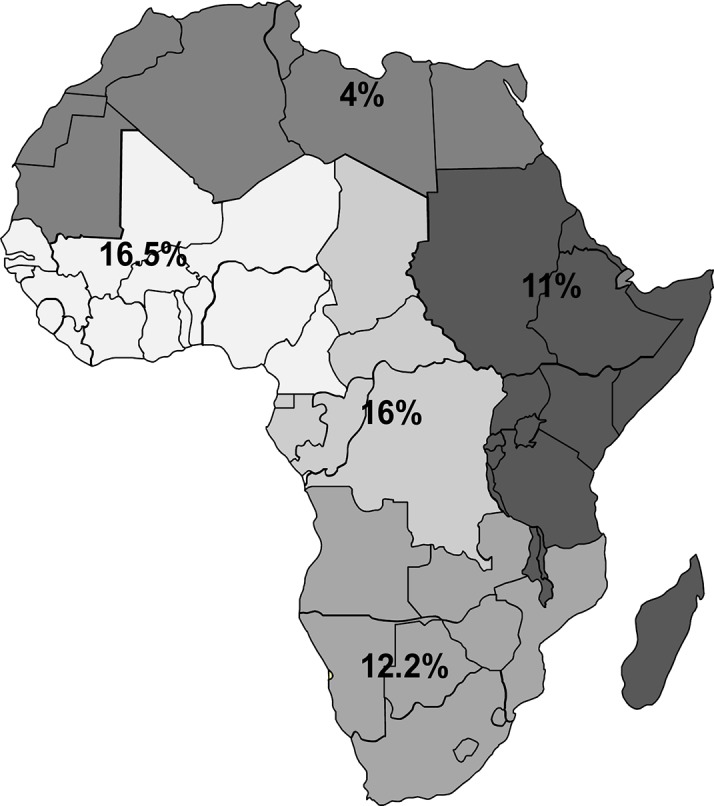
Prevalence of chronic kidney disease among the entire general population. Estimates from this figure should be presented with caution as it is bound to be imprecise and inaccurate due to its tentative way of estimation.
bmjopen-2016-015069supp003.jpg (298.8KB, jpg)
Among patients with HIV (table 3), the prevalence of CKD in the 18 medium-quality studies ranged from 1% to 46% (pooled prevalence: 5.6%; 95% CI 5.4% to 5.8%). The prevalence of CKD in the West/Central West macro-areas, which ranged from 9% to 39% (pooled estimate: 11.6%), and the East macro-areas, where the prevalence ranged from 1% to 46% (pooled estimate: 11.2%), had seemingly similar figures, which were higher than in the South (3.5%) macro-areas. Based on the treatment status, the prevalence of renal dysfunction ranged from 1% to 47% (pooled prevalence: 9.9%; 95 % CI 9.4% to 10.4%) among patients with HIV not receiving treatment, while it ranged from 7% to 33% (pooled prevalence: 5.2%; 95 % CI 5.0% to 5.4%) among patients with HIV on antiretroviral therapy. The prevalence was reported to be 5.7% (range: 3.1%–7.2%) among the three studies done in both the East and South macro-areas and 2.5% from the study done in the sub-Saharan area. According to the definition, the prevalence of CKD ranged from 1% to 18% (pooled estimate: 4.7%) in studies that defined CKD as eGFR <60 mL/min. In studies that defined CKD as eGFR <60 mL/min/1.73 m2 and/or the presence of proteinuria or albuminuria, the CKD prevalence ranged from 9% to 21% (pooled estimate: 5.6%). There are other four studies that defined CKD based on either the presence of proteinuria, KDOQI, CrCl <50 mL/min, or albuminuria and serum creatinine. In these four studies, the prevalence of CKD ranged from 3% to 46% (pooled estimate: 12.6%). The CKD prevalence for each age or gender group was not reported in the majority of the studies. In online supplementary figure 1 we show graphically the relationship between gender and age and CKD prevalence among patients with HIV in the medium-high-quality studies.
Among patients with diabetes (table 4, all studies are of low quality except for four with medium quality), the prevalence of CKD ranged from 11% to 90% (pooled prevalence: 24.7%; 95% CI 23.6% to 25.7%). The highest prevalence was in the Eastern, which ranged from 18% to 84% (pooled estimate: 46.9%), followed by the Central, where the CKD prevalence ranged from 30% to 66% (pooled estimate: 40.8%). In the West/Central-West, CKD prevalence ranged from 18% to 90% (pooled estimate: 27.7%), while in the South the CKD prevalence ranged from 18% to 66% (pooled estimate: 23.0%), and in the North CKD prevalence ranged from 11% to 20% (pooled estimate: 18.9%). One study done in sub-Saharan reported that the prevalence was 13%. Among patients with diabetes, CKD prevalence ranged from 11% to 83% (pooled estimate: 51.8%) when CKD was defined as eGFR <60 mL/min/1.73 m2 and/or the presence of proteinuria or albuminuria. When CKD was defined based on proteinuria/albuminuria, CKD prevalence ranged from 26% to 51% (pooled estimate: 36.3%). In patients with diabetes who had CKD based on eGFR <60 mL/min/1.73 m2, the prevalence ranged from 13% to 30% (pooled estimate: 16.6%). When KDOQI was used to define CKD, the prevalence of CKD ranged from 19% to 66% (pooled estimate: 34.2%). The CKD prevalence for each age or gender group was not reported in the majority of the studies. In online supplementary figure 1 we show graphically the relationship between gender and age and CKD prevalence among patients with diabetes in the included studies.
The prevalence of CKD among patients with hypertension (table 5, 9 studies; all of low quality except for two with medium quality) ranged from 13% to 51% (pooled prevalence: 34.5%; 95% CI 34.04% to 36%). The highest prevalence was reported from one study in the East macro-area (39.5%), followed by the West/Central-West, where the prevalence ranged from 13% to 51% (pooled estimate: 37.7%). In South Africa, the CKD prevalence reported from one study was 25.4%. No data were found for other African macro-areas. In studies that defined CKD as eGFR <60 mL/min/1.73 m2, the prevalence of CKD ranged from 38.5% to 40% (pooled estimate: 38.9%). When serum creatinine was used to define CKD, the prevalence ranged from 30% to 51% (pooled estimate: 40.3%). When CKD was defined according to albuminuria/proteinuria, the prevalence of CKD ranged from 15% to 25% (pooled estimate: 23.6%). In one study, CKD was defined according to KDOQI criteria and it was prevalent among 47% of patients with hypertension. The CKD prevalence for each age or gender group was not reported in the majority of the studies. In online supplementary figure 1 we show graphically the relationship between gender and age and CKD prevalence among patients with diabetes in the included studies.
Among other patient populations (studies reported in table 6), almost three-quarters of patients with lupus had CKD (prevalence=72.0%) based on low-quality study.19 Hospital-based surveys revealed that (the calculation was based on the total prevalence reported from all studies including three of high-medium quality and four of low quality in the same table) more than one-third of patients attending either primary care centres or tertiary hospitals had CKD (range: 11%–57%, pooled prevalence: 36%, 95% CI 34.4% to 37.7%). In hospital-based studies, when CKD was defined as eGFR <60 mL/min/1.73 m2 and/or the presence of proteinuria or albuminuria, the prevalence ranged from 10% to 14% (pooled estimate: 12.4%), while the prevalence ranged from 49% to 57% (pooled estimate: 45.1%) when CKD was defined according KDOQI. CKD was prevalent among almost 39% of patients with rheumatoid arthritis20 or sickle cell.21 The study (low quality) conducted among hairdressers exposed to paraphenylenediamine104 reported that 26.4% of these subjects had renal impairment. Of note, 100% of silica-exposed workers experienced proteinuria (reported from low-quality study).129
Causes of CKD
Forty-two studies were conducted specifically to clarify the underlying cause of CKD31–72 (online supplementary table 2). The diagnosis was biopsy-proven in 17 studies.33 39 41 43–45 48 54 55 58 60 63 67–70 72 Vascular/hypertensive sclerosis was the main cause of CKD (16%), followed by diabetic nephropathy (15%), chronic glomerulonephritis (13%), tubulointerstitial/obstructive (8%), primary glomerular diseases (6%), systemic lupus erythematosus (3%) and polycystic kidney disease (3%). The causes of CKD were undetermined/miscellaneous causes in one-fifth of the patients (20%) (figure 3).
Figure 3.
Main causes of chronic kidney disease.
Discussion
This systematic review focuses on the burden of CKD on the entire African continent. We assessed 152 papers published between 1 January 1995 and 7 April 2017 reporting the epidemiology of CKD in the general population and in specific chronic conditions in Africa. The CKD prevalence reported in our review should be interpreted with caution. Our estimates may be affected by the analytical heterogeneity used to measure creatinine and albuminuria. Serum creatinine concentrations are affected by intraindividual variability with over 20% changes within a 2-week period171 and most Jaffe assays overestimate serum creatinine.172 The resulting bias could vary according to the creatinine concentration, specific assay, manufacturer and calibration material used. Although the IDMS calibration standardisation has reduced the bias and improved the inter-laboratory comparability,173 the number of studies reported using IDMS was low in Africa. Moreover, CKD prevalence may additionally be influenced by albuminuria assays, which are affected by inter-laboratory differences.174 The different equations used to estimate GFR could be a source of bias. The systematic underestimation of measured GFR at higher estimated GFR by the MDRD equation is well known, and may reflect higher creatinine generation in healthy individuals compared with individuals with CKD in whom the MDRD equation was derived. This bias is reduced substantially, but not completely, by the CKD-EPI equation, which was derived from studies including people without CKD.175 In addition, differences in sample size, demographics and clinical characteristics are all significant limitations in this systematic review for making accurate estimates of the prevalence of CKD in African countries. Age and gender are well-known determinants of the risk of CKD development, progression and complication. While the prevalence of CKD tends to be higher in women, the disease is more severe in men, who also have a higher risk of all-cause and cardiovascular disease (CVD) mortality across different levels of renal function. However, the risk relationships of reduced eGFR and higher albuminuria with mortality were steeper in women than in men. Moreover, the risk of progression to ESRD at a given eGFR rate and urinary albumin-to-creatinine ratio seemed equivalent in men and women.176 177 The lack of information on the prevalence of CKD by age and gender in studies included in this systematic review—only 11% of the included studies reported CKD prevalence by either age or gender groups—limits the value and the reliability of pooled estimates of CKD prevalence in Africa and in its macro-areas. To circumvent this limitation, we showed the prevalence of CKD in the various studies in relationship to the proportion of men and age in the same studies. However the number of studies is too small for reliably capturing the effect of age and gender on CKD prevalence in Africa. Furthermore, only five studies79 142–145 assessed the KDOQI chronicity criterion, which is a fundamental element of the current definition of CKD by this organisation. A single elevated serum creatinine, reduced eGFR or an abnormal urinalysis should initially be viewed as a screening test, and the diagnosis of CKD should be confirmed with repeated tests, additional work-up and clinical judgement.178 Thus, estimates in this review should be seen as a pragmatic attempt to evaluate the dimension of CKD as a public health issue on the African continent.
CKD is now considered to be an important component of the epidemic of non-communicable diseases in economically developed and low–income/middle-income countries alike. In a seminal meta-analysis published in 2014, Stanifer et al9 for the first time drew attention to the public health relevance of CKD in the sub-Saharan Africa, a vast area comprising 85% (947.4 million) of the whole African population.9 In the present systematic review, the lowest prevalence of CKD (4%) was reported in the Northern Africa macro-area, including Egypt, Libya, Tunisia, Algeria, Morocco, the Western Sahara and Mauritania, and the highest (16.5%) was observed in West/Central West Africa, which includes Benin, Burkina Faso, the island nation of Cape Verde, Gambia, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Liberia, Mali, Mauritania, Niger, Nigeria, Cameroon, the island of Saint Helena, Senegal, Sierra Leone, São Tomé and Príncipe and Togo. The average prevalence in the entire African continent was 10.1%. The global CKD prevalence was reported to be 13.4%.179 In sub-Saharan Africa in Stanifer et al’s meta-analysis, the prevalence of CKD was 13.2%,9 which is close to that reported in the same area in our review (14.02%). Among the general population of economically developed countries, CKD has 13.6% prevalence in the USA.180 In Europe, the reported prevalence is lower and more homogeneous, being 8.9% in the Netherlands, 6.8% in Italy, 5.2% in Portugal, 4.7% in Spain and 3.3% in Norway.181 CKD prevalence in some Asian countries was higher than the estimates in the USA and in Europe, being 17.5% in Thailand,182 15% in India,183 13% in Japan,184 11.9% in Taiwan185 and 9.9% in China.186 Overall, the estimated prevalence of CKD at the general population level in African countries appears to be comparable and possibly even higher than that reported in other continents. This may be at least in part due to the low-quality data for the prevalence of CKD in Africa related to poor sampling techniques, unreliable kidney function measurements and the different definitions used.
In our review, the prevalence of CKD in surveys based on hospitals or primary care centres (36%) is close to that in Swiss primary care centres (36%).187
Poverty-related factors such as infectious diseases secondary to poor sanitation, inadequate supply of safe water, environmental pollutants and high concentrations of disease-transmitting vectors continue to play an important role in the development of CKD in low-income countries. Although rates of diabetic nephropathy are rising, chronic glomerulonephritis and interstitial nephritis are among the principal causes of CKD in many countries.188
In Africa, infectious diseases such as HIV, bilharziasis, malaria, hepatitis B and C represent an almost unique cluster of risk factors responsible for CKD.189 HIV/AIDS is pandemic in Africa, with a prevalence ranging from 0.5% in Senegal190 to 27.4% in Swaziland.191 The global success in bringing effective antiretroviral treatment (highly active antiretroviral therapy (HAART)) to HIV-infected patients in Africa has determined the emergence of chronic medical illnesses such as HIV-related CKD.192 Up to 50% of kidney diseases in HIV-infected persons result from a wide array of non-HIV-associated nephropathy pathologies, ranging from glomerulonephritis to diabetic nephropathy.193 We found that 5.6% of patients with HIV complained of renal dysfunction. This figure is lower than that reported in economically developed countries such as France, USA, China, Spain and Brazil.194–198 CKD was higher among patients with HIV not receiving HAART compared with those on HAART. Variation in the proportion of patients with HIV affected by CKD depends on the heterogeneity in the definition used to determine renal dysfunction, the proportion of the study population on HAART, diverse ethnicities, the associated comorbidities and the nutritional status of the study population. Patients with HIV are more prone to nutritional deficiencies due to malabsorption, impaired oral intake and the wasting syndrome. Increased availability of HAART has led to some improvement of the nutritional status of patients. However, for certain individuals, undernutrition and weight loss persist despite therapy. Malnutrition exacerbates side effects, alters drug pharmacokinetics and impinges on adherence, thereby limiting the beneficial effects of the therapy.199 Furthermore, differences in HIV clades or strains in African patients200 and genetic factor201 may influence the replication capacities within the isolated renal reservoir and thus lead to a diversity in clinical presentations.80
Regarding systemic autoimmune diseases such as lupus, a study conducted among patients with lupus from Senegal showed that almost three-quarters (71.0%) of the patients with this disease had evidence of renal involvement.19 This isolated figure is higher than that reported in other countries.202–204 More than one-third (39%) of patients with rheumatoid arthritis had CKD,20 which is higher than that reported from Taiwan.205
Even though there are no sufficient data to precisely reconstruct historical trends, the profile of CKD causes has changed during the last decades. Interstitial nephritis and glomerulonephritis were the main causes of CKD in North Africa,206 and CKD was principally caused by chronic glomerulonephritis and hypertension in East and Tropical Africa.207 208 Today, the spectrum of causes of CKD in Africa is dominated by diabetes mellitus and hypertension.209 We found that the prevalence of vascular/hypertensive and diabetic nephropathies as a cause of CKD (16% and 15%, respectively) exceeded that caused by chronic glomerulonephritis (13%).
Our review has both strengths and limitations. The major strengths include a thorough systematic search of electronic databases and the inclusion of all comprehensive studies with a transparent assessment of CKD prevalence by two independent reviewers. The fact that our literature search was limited to PubMed and Ovid Medline but did not include the African Index Medicus, like it was done by Stanifer et al in the meta-analysis of CKD in sub-Saharan Africa9, is a limitation of our study. Because there was a huge discrepancy in the definitions used to identify CKD, the methods of creatinine measurement, urine protein assessment and in the quality of the reporting, we decided to adopt an inclusive strategy. Our primary interest was to identify all studies conducted among different population groups in Africa providing information on CKD and to reconstruct a tentative scenario of the epidemiological dimension concerning disease in the entire African continent. Methodological limitations notwithstanding this review compiled estimates suggesting that the CKD burden in Africa is at least as concerning as that in economically developed countries. The lack of a consistent definition of CKD makes it difficult to compare the burden of CKD across studies in various countries. Moreover, the failure to demonstrate chronicity when defining CKD is a common limitation of studies investigating CKD prevalence in Africa. It was reported that a single test in time has an extremely poor positive predictive value for confirmation of CKD compared with repeated testing 3 months later. Failure to repeat testing may lead to a significant overestimation of CKD prevalence and underestimation of the burden of CVD in CKD.210 In addition, observational studies are subject to bias and residual confounding, which are difficult to account for and there are limitations due to the heterogeneity that arises from differences in age and sex distributions. This poor data quality reported in different studies is considered as a cumbersome problem limiting the accuracy in assessing the burden of CKD in Africa.
In conclusion, CKD in Africa appears to be at least as common as in other continents, and as such it constitutes a true public health priority with major cost burden to healthcare systems worldwide. Targeted screening of high-risk groups (including those patients with with hypertension, diabetes mellitus and HIV, and persons with occupational exposures) should likely be instituted as the first step in kidney disease prevention whenever and wherever affordable and feasible. Education to increase awareness of CKD among healthcare workers and patients, and the promotion of healthy lifestyles, should be engrained in preventive programmes. The treatment of hypertension and diabetes mellitus is of obvious relevance. Nurses and other health workers should be trained to manage these conditions at the local level if we are to curb the incidence of CKD and to avert the added burden of CKD complications to diabetes, hypertension and infectious diseases, the deadly trio of risk factors underlying the CKD epidemic in Africa.
Supplementary Material
Acknowledgments
We would like to thank the following professors and physicians for their help in providing the articles we evaluated in our review:
Professor Olutayo Alebiosu, Professor Ahmed Donia, Professor Rashad Barsoum, Professor Carel IJsselmuiden, Professor Laurent Forcard, Professor Anatole Laleye, Professor Nestor Pakasa, Professor Imaobong Etuk, Professor Ifeoma Ulasi, Professor Abubakr Abefe Sanusi, Professor Gbenga Ayodele, Professor Raida S Yahya, Professor Mohammed Benghanem Gharbi, Professor Fatma Ben Moussa, Dr Ikechi Okpechi, Dr Alaya Akram, Dr Adebowale Ademola, Dr Oluyombo Rotimi, Dr KS Nayak, Dr Guy Neild, Dr Rasheed Gbadegesin, Dr Sidy Mohamed Seck, Dr Amr El-Husseini Mohamed, Dr Fasika M Tedla, Professor Adewale Akinsola, Professor Olanrewaju Adedoyin, Dr Halle Marie Patrice, Dr Emmanuel Agaba, Professor Miriam Adhikari, Dr BT Bello and Dr Zidane Djelloul.
Footnotes
Contributors: SA, DB and CZ: conceptualised and designed the study. SAE, GD and ED: participated in revising the articles included in the review and retrieved the necessary information. DB and GT: supervised the data capture and analysis. SAE, DB and GT: analysed and interpreted the data. SAE, DB and CZ: drafted and critically revised the manuscript. All of the authors read and approved the final manuscript.
Funding: SA was granted a European Renal Association-European Dialysis and Transplantation Association (ERA-EDTA) fellowship at CNR-IFC/IBIM, Clinical Epidemiology and Physiopathology of Renal Disease and Hypertension of Reggio Calabria, Italy, and this work was completed during her training. This article was written in the framework of the Advisory Program of the ERA-EDTA YNP (Young Nephrologists’ Platform), which is an official body of the ERA-EDTA (European Renal Association-European Dialysis and Transplant Association). SA was an advisee of ERA-EDTA YNP Adviser-Advisee Program (Adviser: DB).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data are published in the manuscript.
References
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007;72:247–59. 10.1038/sj.ki.5002343 [DOI] [PubMed] [Google Scholar]
- 2.Zoccali C, Kramer A, Jager KJ. Epidemiology of CKD in Europe: an uncertain scenario. Nephrol Dial Transplant 2010;25:1731–3. 10.1093/ndt/gfq250 [DOI] [PubMed] [Google Scholar]
- 3.Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bello AK, Peters J, Rigby J, et al. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol 2008;3:1316–23. 10.2215/CJN.00680208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Nahas AM, Bello AK. Chronic kidney disease: the global challenge. Lancet 2005;365:331–40. 10.1016/S0140-6736(05)17789-7 [DOI] [PubMed] [Google Scholar]
- 6.UN World Population Prospects: The 2015 Revision, Key Findings and Advance Tables: United Nations. 2015. http://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf (accessed 8 Nov 2015).
- 7.Ad-G A, Unwin N, Agyemang C, et al. Commentary Tackling Africa’s chronic disease burden: from the local to the global. 2010. [DOI] [PMC free article] [PubMed]
- 8.World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. 2013.
- 9.Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e174–81. 10.1016/S2214-109X(14)70002-6 [DOI] [PubMed] [Google Scholar]
- 10.Anothaisintawee T, Rattanasiri S, Ingsathit A, et al. Prevalence of chronic kidney disease: a systematic review and meta-analysis. Clin Nephrol 2009;71:244–54. 10.5414/CNP71244 [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analysis of studies that evaluate health care interventions: explanation and elaboration. Italian Journal of Public Health 2012;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsha TE, Yako YY, Rensburg MA, et al. Chronic kidney diseases in mixed ancestry south African populations: prevalence, determinants and concordance between kidney function estimators. BMC Nephrol 2013;14:75 10.1186/1471-2369-14-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastwood JB, Kerry SM, Plange-Rhule J, et al. Assessment of GFR by four methods in adults in Ashanti, Ghana: the need for an eGFR equation for lean African populations.[Erratum appears in Nephrol Dial Transplant. 2011 Dec;26(12):4153 Note: Emmett, Lynsey [added]; Miller, Michelle A [added]]. Nephrol Dial Transplant 2010;25:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaser N, Deckert A, Phiri S, et al. Comparison of Various Equations for Estimating GFR in Malawi: How to Determine Renal Function in Resource Limited Settings? PLoS One 2015;10:e0130453 10.1371/journal.pone.0130453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323:42–6. 10.1136/bmj.323.7303.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol 2010;63:1061–70. 10.1016/j.jclinepi.2010.04.014 [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. A Coefficient of Agreement for Nominal Scales. Educ Psychol Meas 1960;20:37–46. 10.1177/001316446002000104 [DOI] [Google Scholar]
- 19.Ka EF, Cisse MM, Lemrabott AT, et al. [Lupus nephropathy in black patients with systemic lupus erythematosus in Senegal: 43 cases]. Med Sante Trop 2013;23:328–31. 10.1684/mst.2013.0200 [DOI] [PubMed] [Google Scholar]
- 20.Dessein PH, Hsu HC, Tsang L, et al. Kidney function, endothelial activation and atherosclerosis in black and white Africans with rheumatoid arthritis. PLoS One 2015;10:e0121693 10.1371/journal.pone.0121693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ephraim RK, Osakunor DN, Cudjoe O, et al. Chronic kidney disease is common in sickle cell disease: a cross-sectional study in the Tema Metropolis, Ghana. BMC Nephrol 2015;16:75 10.1186/s12882-015-0072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghahramani N. Silica nephropathy. Int J Occup Environ Med 2010;1:108-115. [PubMed] [Google Scholar]
- 23.Sampathkumar K, Yesudas S. Hair dye poisoning and the developing world. J Emerg Trauma Shock 2009;2:129 10.4103/0974-2700.50749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsharif ME, Abdullha SM, Abdalla SM, et al. The magnitude of chronic kidney diseases among primary health care attendees in Gezira state, Sudan. Saudi J Kidney Dis Transpl 2013;24:807–9. 10.4103/1319-2442.113900 [DOI] [PubMed] [Google Scholar]
- 25.Sumaili EK, Cohen EP, Zinga CV, et al. High prevalence of undiagnosed chronic kidney disease among at-risk population in Kinshasa, the Democratic Republic of Congo. BMC Nephrol 2009;10:18 10.1186/1471-2369-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afolabi MO, Abioye-Kuteyi EA, Arogundade FA, et al. Prevalence of chronic kidney disease in a Nigerian family practice population. S Afr Fam Pract 2009;51:132–7. 10.1080/20786204.2009.10873828 [DOI] [Google Scholar]
- 27.van Rensburg BW, van Staden AM, Rossouw GJ, et al. The profile of adult nephrology patients admitted to the Renal Unit of the Universitas Tertiary Hospital in Bloemfontein, South Africa from 1997 to 2006. Nephrol Dial Transplant 2010;25:820–4. 10.1093/ndt/gfp535 [DOI] [PubMed] [Google Scholar]
- 28.Raji Y, Mabayoje O, Bello T. Familial clustering of risk factors for cardiovascular disease among first-degree relatives of patients with chronic kidney disease in a sub-Saharan African population. Cardiovasc J Afr 2015;26(2 Suppl 1):S11–14. 10.5830/CVJA-2015-041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The unrecognized prevalence of chronic kidney disease among family members of end stage renal disease patinets [IEA-EEF abstract 264]. Eur J Epidemiol 2009. [Google Scholar]
- 30.Anyabolu EN, Chukwuonye II, Anyabolu AE, et al. A look at risk factors of proteinuria in subjects without impaired renal filtration function in a general population in Owerri, Nigeria. Pan Afr Med J 2016;23:257 10.11604/pamj.2016.23.257.8189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Khayat SS, Hallal K, Gharbi MB, et al. Fate of patients during the first year of dialysis. Saudi J Kidney Dis Transpl 2013;24:605–9. 10.4103/1319-2442.111085 [DOI] [PubMed] [Google Scholar]
- 32.Seck SM, Diallo IM, Diagne SI. Epidemiological patterns of chronic kidney disease in black African elders: a retrospective study in West Africa. Saudi J Kidney Dis Transpl 2013;24:1068–72. 10.4103/1319-2442.118104 [DOI] [PubMed] [Google Scholar]
- 33.Seck SM, Elhadj FK, Fall S, et al. [Adherence to therapy in sub-Saharan non-dialysed patients with chronic kidney diseases]. Nephrol Ther 2008;4:325–9. 10.1016/j.nephro.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 34.Bourquia A. Société marocaine des maladies rénales. [Autosomal dominant polycystic kidney disease (ADPKD). in Morocco. Multicenter study about 308 families]. Nephrologie 2002;23:93–6. [PubMed] [Google Scholar]
- 35.Ouattara B, Kra O, Yao H, et al. [Characteristics of chronic renal failure in black adult patients hospitalized in the Internal Medicine department of Treichville University Hospital]. Nephrol Ther 2011;7:531–4. 10.1016/j.nephro.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 36.Lengani A, Coulibaly G, Laville M, et al. [Epidemiology of severe chronic renal insufficiency in Burkina Faso]. Sante 1997;7:379–83. [PubMed] [Google Scholar]
- 37.Afifi AM, Mady GE, Ahmad AA, et al. Pattern of renal diseases among elderly Egyptians patients with acute or chronic renal diseases in Ain Shams University and Nasser Institute Hospitals, Cairo, Egypt. J Egypt Soc Parasitol 2005;35:911–24. [PubMed] [Google Scholar]
- 38.Diouf B, Ka EF, Niang A, et al. [Etiologies of chronic renal insufficiency in a adult internal medicine service in Dakar]. Dakar Med 2000;45:62–5. [PubMed] [Google Scholar]
- 39.Niang A, Dial C, Ka EF, et al. [Nephrotic syndrom with focal and segmental glomerulosclerosis in Dakar: epidemiological and clinicopathological characteristics (about 134 cases)]. Dakar Med 2008;53:45–51. [PubMed] [Google Scholar]
- 40.Sabi KA, Gnionsahe DA, Amedegnato D. [Chronic kidney failure in Togo: clinical, laboratory, and etiological aspects]. Med Trop 2011;71:74–6. [PubMed] [Google Scholar]
- 41.Ulasi II, Ijoma CK. The enormity of chronic kidney disease in Nigeria: the situation in a teaching hospital in South-East Nigeria. J Trop Med 2010;2010:1–6. 10.1155/2010/501957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abderrahim E, Zouaghi K, Hedri H, et al. Renal replacement therapy for diabetic end-stage renal disease. Experience of a Tunisian hospital centre. Diabetes Metab 2001;27:584–90. [PubMed] [Google Scholar]
- 43.Abdou N, Boucar D, El Hadj Fary KA, et al. Histopathological profiles of nephropathies in senegal. Saudi J Kidney Dis Transpl 2003;14:212–4. [PubMed] [Google Scholar]
- 44.Afifi A, El Setouhy M, El Sharkawy M, et al. Diabetic nephropathy as a cause of end-stage renal disease in Egypt: a six-year study. East Mediterr Health J 2004;10:620–6. [PubMed] [Google Scholar]
- 45.Afifi A, Karim MA. Renal replacement therapy in Egypt: first annual report of the Egyptian Society of Nephrology, 1996. East Mediterr Health J 1999;5:1023–9. [PubMed] [Google Scholar]
- 46.Agaba EI, Wigwe CM, Agaba PA, et al. Performance of the Cockcroft-Gault and MDRD equations in adult Nigerians with chronic kidney disease. Int Urol Nephrol 2009;41:635–42. 10.1007/s11255-008-9515-8 [DOI] [PubMed] [Google Scholar]
- 47.Alashek WA, McIntyre CW, Taal MW. Epidemiology and aetiology of dialysis-treated end-stage kidney disease in Libya. BMC Nephrol 2012;13:33 10.1186/1471-2369-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alasia DD, Emem-Chioma P, Wokoma FS. A single-center 7-year experience with end-stage renal disease care in Nigeria-a surrogate for the poor state of ESRD care in Nigeria and other sub-saharan african countries: advocacy for a global fund for ESRD care program in sub-saharan african countries. Int J Nephrol 2012;2012:1–7. 10.1155/2012/639653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alebiosu CO, Ayodele OO, Abbas A, et al. Chronic renal failure at the Olabisi Onabanjo University Teaching Hospital, Sagamu, Nigeria. Afr Health Sci 2006;6:132–8. 10.5555/afhs.2006.6.3.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amira CO, Braimoh RW, Bello BT. Pattern of intradialytic complications at the Lagos University Teaching Hospital. Afr J Med Med Sci 2012;41:411–6. [PubMed] [Google Scholar]
- 51.Arogundade FA, Sanusi AA, Hassan MO, et al. The pattern clinical characteristics and outcome of ESRD in Ile-Ife, Nigeria: is there a change in trend? Afr health sci 2011;11:594–601. [PMC free article] [PubMed] [Google Scholar]
- 52.Counil E, Cherni N, Kharrat M, et al. Trends of incident dialysis patients in Tunisia between 1992 and 2001. Am J Kidney Dis 2008;51:463–70. 10.1053/j.ajkd.2007.10.032 [DOI] [PubMed] [Google Scholar]
- 53.Chijioke A, Makusidi AM, Kolo PM. Electrocardiographic abnormalities among dialysis naïve chronic kidney disease patients in Ilorin Nigeria. Ann Afr Med 2012;11:21–6. 10.4103/1596-3519.91011 [DOI] [PubMed] [Google Scholar]
- 54.Madala ND, Thusi GP, Assounga AG, et al. Characteristics of South African patients presenting with kidney disease in rural KwaZulu-Natal: a cross sectional study. BMC Nephrol 2014;15:61 10.1186/1471-2369-15-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okpechi IG, Ayodele OE, Rayner BL, et al. Kidney disease in elderly South Africans. Clin Nephrol 2013;79:269–76. 10.5414/CN107746 [DOI] [PubMed] [Google Scholar]
- 56.Laleye A, Awede B, Agboton B, et al. Autosomal dominant polycystic kidney disease in University Clinic of Nephrology and Haemodialysis of Cotonou: clinical and genetical findings. Genet Couns 2012;23:435–45. [PubMed] [Google Scholar]
- 57.Okunola Y, Ayodele O, Akinwusi P, et al. Haemodialysis practice in a resource-limited setting in the tropics. Ghana Med J 2013;47:4–9. [PMC free article] [PubMed] [Google Scholar]
- 58.Bello BT, Raji YR, Sanusi I, et al. Challenges of providing maintenance hemodialysis in a resource poor country: Experience from a single teaching hospital in Lagos, Southwest Nigeria. Hemodial Int 2013;17:427–33. 10.1111/hdi.12024 [DOI] [PubMed] [Google Scholar]
- 59.El Minshawy O. End-stage renal disease in the El-Minia Governorate, upper Egypt: an epidemiological study. Saudi J Kidney Dis Transpl 2011;22:1048–54. [PubMed] [Google Scholar]
- 60.Okpechi IG, Rayner BL, Swanepoel CR. Nephrotic syndrome in adult black South Africans: HIV-associated nephropathy as the main culprit. J Natl Med Assoc 2010;102:1193–7. 10.1016/S0027-9684(15)30774-4 [DOI] [PubMed] [Google Scholar]
- 61.Madala ND, Nkwanyana N, Dubula T, et al. Predictive performance of eGFR equations in South Africans of African and Indian ancestry compared with ⁹⁹mTc-DTPA imaging. Int Urol Nephrol 2012;44:847–55. 10.1007/s11255-011-9928-7 [DOI] [PubMed] [Google Scholar]
- 62.El Farouki MR, Bahadi A, Hamzi MA, et al. [Profile of chronic renal failure in diabetes at initiation of hemodialysis in the nephrology and dialysis service of the military hospital in Rabat, Morocco]. Pan Afr Med J 2013;15:124 10.11604/pamj.2013.15.124.2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okpechi I, Swanepoel C, Duffield M, et al. Patterns of renal disease in Cape Town South Africa: a 10-year review of a single-centre renal biopsy database. Nephrol Dial Transplant 2011;26:1853–61. 10.1093/ndt/gfq655 [DOI] [PubMed] [Google Scholar]
- 64.Niang A, Cisse MM, Mahmoud SM, et al. Pilot experience in senegal with peritoneal dialysis for end-stage renal disease. Perit Dial Int 2014;34:539–43. 10.3747/pdi.2011.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buargub MA. 5-year mortality in hemodialysis patients: a single center study in Tripoli. Saudi J Kidney Dis Transpl 2008;19:268–73. [PubMed] [Google Scholar]
- 66.Chijioke A, Aderibigbe A, Olarenwaju TO, et al. Prevalence and pattern of cystic kidney diseases in Ilorin, Nigeria. Saudi J Kidney Dis Transpl 2010;21:1172–8. [PubMed] [Google Scholar]
- 67.Elsharif ME, Elsharif EG. Causes of end-stage renal disease in Sudan: a single-center experience. Saudi J Kidney Dis Transpl 2011;22:373–6. [PubMed] [Google Scholar]
- 68.Elkhatib M, Elnahed MS, Fadda S, et al. The change in the spectrum of glomerulonephritis in Egypt over the past decade. Saudi J Kidney Dis Transpl 2012;23:1065–7. 10.4103/1319-2442.100955 [DOI] [PubMed] [Google Scholar]
- 69.Ibrahim S, Fayed A, Fadda S, et al. A five-year analysis of the incidence of glomerulonephritis at Cairo University Hospital-Egypt. Saudi J Kidney Dis Transpl 2012;23:866–70. 10.4103/1319-2442.98191 [DOI] [PubMed] [Google Scholar]
- 70.Ayach G, El-Filali H, Saidi S, et al. Histopathological study of pure primary nephrotic syndrome in adolescents and young Moroccan adults. Arab J Nephrol Transplant 2011;4:137–40. 10.4314/ajnt.v4i3.71025 [DOI] [PubMed] [Google Scholar]
- 71.Ramilitiana B, Ranivoharisoa EM, Dodo M, et al. [A retrospective study on the incidence of chronic renal failure in the Department of Internal Medicine and Nephrology at University Hospital of Antananarivo (the capital city of Madagascar)]. Pan Afr Med J 2016;23:141 10.11604/pamj.2016.23.141.8874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zajjari Y, Benyahia M, Ibrahim DM, et al. La néphropathie non diabétique chez les patients diabétiques de type 2 à l’hôpital militaire Mohammed V de Rabat (Maroc). EMHJ 2012;18. [PubMed] [Google Scholar]
- 73.Fatiu A, Abubakr S, Muzamil H, et al. Undiagnosed hypertension and proteinuria in a market population in Ile-Ife, Nigeria. Arab J Nephrol Transplant 2011;4:141–6. 10.4314/ajnt.v4i3.71026 [DOI] [PubMed] [Google Scholar]
- 74.Traore M, Traore HA, Kardorff R, et al. The public health significance of urinary schistosomiasis as a cause of morbidity in two districts in Mali. Am J Trop Med Hyg 1998;59:407–13. 10.4269/ajtmh.1998.59.407 [DOI] [PubMed] [Google Scholar]
- 75.Sumaili EK, Nseka NM, Lepira FB, et al. Screening for proteinuria and chronic kidney disease risk factors in Kinshasa: a World Kidney Day 2007 study. Nephron Clin Pract 2008;110:c220–8. 10.1159/000167869 [DOI] [PubMed] [Google Scholar]
- 76.Egbi OG, Okafor UH, Miebodei KE, et al. Prevalence and correlates of chronic kidney disease among civil servants in Bayelsa state, Nigeria. Niger J Clin Pract 2014;17:602–7. 10.4103/1119-3077.141426 [DOI] [PubMed] [Google Scholar]
- 77.Ayodele OE, Okunola OO, Afolabi MO, et al. Prevalence of hypertension, diabetes and chronic kidney disease in participants of the 2009 World Kidney Day screening exercise in Southwest Nigeria. HKJN 2011;13:55–63. 10.1016/j.hkjn.2011.09.004 [DOI] [Google Scholar]
- 78.Abu-Aisha H, Elhassan A, Khamis A, et al. Chronic kidney disease in police forces households in Khartoum, Sudan: pilot report. Arab J Nephrol Transplant 2009;2:21–6. [Google Scholar]
- 79.Cailhol J, Nkurunziza B, Izzedine H, et al. Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: a cross-sectional study. BMC Nephrol 2011;12:40 10.1186/1471-2369-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wools-Kaloustian K, Gupta SK, Muloma E, et al. Renal disease in an antiretroviral-naive HIV-infected outpatient population in Western Kenya. Nephrol Dial Transplant 2007;22:2208–12. 10.1093/ndt/gfm223 [DOI] [PubMed] [Google Scholar]
- 81.Emem CP, Arogundade F, Sanusi A, et al. Renal disease in HIV-seropositive patients in Nigeria: an assessment of prevalence, clinical features and risk factors. Nephrol Dial Transplant 2008;23:741–6. 10.1093/ndt/gfm836 [DOI] [PubMed] [Google Scholar]
- 82.Wyatt CM, Shi Q, Novak JE, et al. Prevalence of kidney disease in HIV-infected and uninfected Rwandan women. PLoS One 2011;6:e18352 10.1371/journal.pone.0018352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.FolefackKaze F, Kengne AP, Pefura Yone EW, et al. Renal function, urinalysis abnormalities and correlates among HIV-infected Cameroonians naive to antiretroviral therapy. Saudi J Kidney Dis Transpl 2013;24:1291–7. 10.4103/1319-2442.121280 [DOI] [PubMed] [Google Scholar]
- 84.Struik GM, den Exter RA, Munthali C, et al. The prevalence of renal impairment among adults with early HIV disease in Blantyre, Malawi. Int J STD AIDS 2011;22:457–62. 10.1258/ijsa.2011.010521 [DOI] [PubMed] [Google Scholar]
- 85.Msango L, Downs JA, Kalluvya SE, et al. Renal dysfunction among HIV-infected patients starting antiretroviral therapy. AIDS 2011;25:1421–5. 10.1097/QAD.0b013e328348a4b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Janmohamed MN, Kalluvya SE, Mueller A, et al. Prevalence of chronic kidney disease in diabetic adult out-patients in Tanzania. BMC Nephrol 2013;14:183 10.1186/1471-2369-14-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wanjohi FW, Otieno FC, Ogola EN, et al. Nephropathy in patients with recently diagnosed type 2 diabetes mellitus in black Africans. East Afr Med J 2002;79:399–404. 10.4314/eamj.v79i8.8824 [DOI] [PubMed] [Google Scholar]
- 88.Choukem SP, Dzudie A, Dehayem M, et al. Comparison of different blood pressure indices for the prediction of prevalent diabetic nephropathy in a sub-Saharan African population with type 2 diabetes. Pan Afr Med J 2012;11:67. [PMC free article] [PubMed] [Google Scholar]
- 89.Plange-Rhule J, Phillips R, Acheampong JW, et al. Hypertension and renal failure in Kumasi, Ghana. J Hum Hypertens 1999;13:37–40. 10.1038/sj.jhh.1000726 [DOI] [PubMed] [Google Scholar]
- 90.Kalyesubula R, Nankabirwa JI, Ssinabulya I, et al. Kidney disease in Uganda: a community based study. BMC Nephrol 2017;18:116 10.1186/s12882-017-0521-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaze FF, Halle MP, Mopa HT, et al. Prevalence and risk factors of chronic kidney disease in urban adult Cameroonians according to three common estimators of the glomerular filtration rate: a cross-sectional study. BMC Nephrol 2015;16:96 10.1186/s12882-015-0102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lunyera J, Stanifer JW, Ingabire P, et al. Prevalence and correlates of proteinuria in Kampala, Uganda: a cross-sectional pilot study. BMC Res Notes 2016;9:97 10.1186/s13104-016-1897-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wachukwu CM, Emem-Chioma PC, Wokoma FS, et al. Prevalence of risk factors for chronic kidney disease among adults in a university community in southern Nigeria. Pan Afr Med J 2015;21:120. 10.11604/pamj.2015.21.120.7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Odongo P, Wanyama R, Obol JH, et al. Impaired renal function and associated risk factors in newly diagnosed HIV-infected adults in Gulu Hospital, Northern Uganda. BMC Nephrol 2015;16:43 10.1186/s12882-015-0035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feteh VF, Choukem SP, Kengne AP, et al. Anemia in type 2 diabetic patients and correlation with kidney function in a tertiary care sub-Saharan African hospital: a cross-sectional study. BMC Nephrol 2016;17:29 10.1186/s12882-016-0247-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pillay S, Aldous C, Mahomed F. A deadly combination - HIV and diabetes mellitus: Where are we now? S Afr Med J 2016;106:378 10.7196/SAMJ.2016.v106i4.9950 [DOI] [PubMed] [Google Scholar]
- 97.Seck SM, Doupa D, Guéye L, et al. Chronic kidney disease epidemiology in northern Senegal: a cross-sectional study. Iran J Kidney Dis 2014;8:286–91. [PubMed] [Google Scholar]
- 98.Sumaili EK, Krzesinski JM, Zinga CV, et al. Prevalence of chronic kidney disease in Kinshasa: results of a pilot study from the Democratic Republic of Congo. Nephrol Dial Transplant 2009;24:117–22. 10.1093/ndt/gfn469 [DOI] [PubMed] [Google Scholar]
- 99.Longo AL, Lepira FB, Sumaili EK, et al. Prevalence of low estimated glomerular filtration rate, proteinuria, and associated risk factors among HIV-infected black patients using Cockroft-Gault and modification of diet in renal disease study equations. J Acquir Immune Defic Syndr 2012;59:59–64. 10.1097/QAI.0b013e31823587b0 [DOI] [PubMed] [Google Scholar]
- 100.Fana GT, Ndhlovu CE. Renal dysfunction among anti-retroviral therapy naïve HIV infected patients in Zimbabwe. Cent Afr J Med 2011;57:1–5. [PubMed] [Google Scholar]
- 101.Han TM, Naicker S, Ramdial PK, et al. A cross-sectional study of HIV-seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int 2006;69:2243–50. 10.1038/sj.ki.5000339 [DOI] [PubMed] [Google Scholar]
- 102.Balogun WO, Abbiyesuku FM. Excess renal insufficiency among type 2 diabetic patients with dip-stick positive proteinuria in a tertiary hospital. Afr J Med Med Sci 2011;40:399–403. [PubMed] [Google Scholar]
- 103.Mafundikwa A, Ndhlovu CE, Gomo Z. The prevalence of diabetic nephropathy in adult patients with insulin dependent diabetes mellitus attending Parirenyatwa Diabetic Clinic, Harare. Cent Afr J Med 2007;53:1–6. [DOI] [PubMed] [Google Scholar]
- 104.Hamdouk M, Abdelraheem M, Taha A, et al. The association between prolonged occupational exposure to paraphenylenediamine (hair-dye) and renal impairment. Arab J Nephrol Transplant 2011;4:21–5. 10.4314/ajnt.v4i1.63151 [DOI] [PubMed] [Google Scholar]
- 105.Oluyombo R, Ayodele OE, Akinwusi PO, et al. A community study of the prevalence, risk factors and pattern of chronic kidney disease in Osun State, South West Nigeria. West Afr J Med 2013;32:85–92. [PubMed] [Google Scholar]
- 106.Prevalence of Chronic Kidney Disease and Associated Risk Factors: First Results from a Population Based Screening Program in Morocco(MAREMAR) [ASN abstract 353]. J Am Soc Nephrol 2012. [Google Scholar]
- 107.Masimango MI, Sumaili EK, Jadoul M, et al. Prevalence of microalbuminuria and diagnostic value of dipstick proteinuria in outpatients from HIV clinics in Bukavu, the Democratic Republic of Congo. BMC Nephrol 2014;15:146 10.1186/1471-2369-15-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fabian J, Naicker S, Venter WD, et al. Urinary screening abnormalities in antiretroviral-naive HIV-infected outpatients and implications for management–a single-center study in South Africa. Ethn Dis 2009;19(1 Suppl 1):S1–80. [PubMed] [Google Scholar]
- 109.Sarfo FS, Keegan R, Appiah L, et al. High prevalence of renal dysfunction and association with risk of death amongst HIV-infected Ghanaians. J Infect 2013;67:43–50. 10.1016/j.jinf.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 110.Jao J, Palmer D, Leus I, et al. Prevalence and predictors of proteinuria in HIV-infected and uninfected pregnant women in Cameroon. Nephrol Dial Transplant 2011;26:3051–3. 10.1093/ndt/gfr310 [DOI] [PubMed] [Google Scholar]
- 111.Makulo R, Nseka MN, Jadoul M, et al. Albuminurie pathologique lors du dépistage du diabète en milieu semi-rural (cité de Kisantu en RD Congo). Nephrol Ther 2010;6:513–9. 10.1016/j.nephro.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 112.Kaze FF, Kengne AP, Magatsing CT, et al. Prevalence and Determinants of Chronic Kidney Disease Among Hypertensive Cameroonians According to Three Common Estimators of the Glomerular Filtration Rate. J Clin Hypertens 2016;18:408–14. 10.1111/jch.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ayokunle DS, Olusegun OT, Ademola A, et al. Prevalence of chronic kidney disease in newly diagnosed patients with Human immunodeficiency virus in Ilorin, Nigeria. J Bras Nefrol 2015;37:177–84. 10.5935/0101-2800.20150029 [DOI] [PubMed] [Google Scholar]
- 114.Chadwick DR, Sarfo FS, Kirk ES, et al. Tenofovir is associated with increased tubular proteinuria and asymptomatic renal tubular dysfunction in Ghana. BMC Nephrol 2015;16:195 10.1186/s12882-015-0192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glaser N, Phiri S, Bruckner T, et al. The prevalence of renal impairment in individuals seeking HIV testing in Urban Malawi. BMC Nephrol 2016;17:186 10.1186/s12882-016-0403-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pruijm MT, Madeleine G, Riesen WF, et al. Prevalence of microalbuminuria in the general population of Seychelles and strong association with diabetes and hypertension independent of renal markers. J Hypertens 2008;26:871–7. 10.1097/HJH.0b013e3282f624d9 [DOI] [PubMed] [Google Scholar]
- 117.Gouda Z, Mashaal G, Bello AK, et al. Egypt information, prevention, and treatment of chronic kidney disease (EGIPT-CKD) programme: prevalence and risk factors for microalbuminuria among the relatives of patients with CKD in Egypt. Saudi J Kidney Dis Transpl 2011;22:1055. [PubMed] [Google Scholar]
- 118.Attolou V, Bigot A, Ayivi B, et al. [Renal complications associated with human acquired immunodeficiency virus infection in a population of hospital patients at the Hospital and University National Center in Cotonou]. Sante 1998;8:283–6. [PubMed] [Google Scholar]
- 119.Bouzid C, Smida H, Kacem A, et al. [Renal failure in Tunisian patients with type 2 diabetes: frequency and related factors]. Tunis Med 2011;89:10–15. [PubMed] [Google Scholar]
- 120.Keeton GR, Smit R, Bryer A. Renal outcome of type 2 diabetes in South Africa–a 12-year follow-up study. S Afr Med J 2004;94:771–5. [PubMed] [Google Scholar]
- 121.Bouaziz A, Zidi I, Zidi N, et al. Nephropathy following type 2 diabetes mellitus in Tunisian population. West Indian Med J 2012;61:881–9. 10.7727/wimj.2012.053 [DOI] [PubMed] [Google Scholar]
- 122.Katchunga P, Hermans MP, Manwa B, et al. [Hypertension, insulin resistance and chronic kidney disease in type 2 diabetes patients from South Kivu, DR Congo]. Nephrol Ther 2010;6:520–5. 10.1016/j.nephro.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 123.Djrolo F, Attolou VG, Avode DG, et al. [Diabetic nephropathy: an epidemiological study based on proteinuria in a population of black African diabetics in Cotonou, Benin]. Sante 2001;11:105–9. [PubMed] [Google Scholar]
- 124.Lutale JJ, Thordarson H, Abbas ZG, et al. Microalbuminuria among type 1 and type 2 diabetic patients of African origin in Dar Es Salaam, Tanzania. BMC Nephrol 2007;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gill G, Gebrekidan A, English P, et al. Diabetic complications and glycaemic control in remote North Africa. QJM 2008;101:793–8. 10.1093/qjmed/hcn096 [DOI] [PubMed] [Google Scholar]
- 126.Osafo C, Mate-Kole M, Affram K, et al. Prevalence of chronic kidney disease in hypertensive patients in Ghana. Ren Fail 2011;33:388–92. 10.3109/0886022X.2011.565140 [DOI] [PubMed] [Google Scholar]
- 127.Lengani A, Samadoulougou A, Cissé M. [Characteristics of renal disease in hypertensive morbidities in adults in Burkina Faso]. Arch Mal Coeur Vaiss 2000;93:1053–7. [PubMed] [Google Scholar]
- 128.Rayner B, Becker P. The prevalence of microalbuminuria and ECG left ventricular hypertrophy in hypertensive patients in private practices in South Africa. Cardiovasc J S Afr 2006;17:245–9. [PubMed] [Google Scholar]
- 129.EL-Safty IA, Gadallah M, Shouman AE, et al. Subclinical nephrotoxicity caused by smoking and occupational silica exposure among Egyptian industrial workers. Arch Med Res 2003;34:415–21. 10.1016/S0188-4409(03)00077-8 [DOI] [PubMed] [Google Scholar]
- 130.Laurence EC, Volmink J, Esterhuizen TM, et al. Risk of cardiovascular disease among teachers in Cape Town: Findings of the South African PaCT pilot study. S Afr Med J 2016;106:996–1001. 10.7196/SAMJ.2016.v106i10.10869 [DOI] [PubMed] [Google Scholar]
- 131.Mogueo A, Echouffo-Tcheugui JB, Matsha TE, et al. Validation of two prediction models of undiagnosed chronic kidney disease in mixed-ancestry South Africans. BMC Nephrol 2015;16:94 10.1186/s12882-015-0093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stanifer JW, Egger JR, Turner EL, et al. Neighborhood clustering of non-communicable diseases: results from a community-based study in Northern Tanzania. BMC Public Health 2016;16:226 10.1186/s12889-016-2912-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stanifer JW, Maro V, Egger J, et al. The epidemiology of chronic kidney disease in Northern Tanzania: a population-based survey. PLoS One 2015;10:e0124506 10.1371/journal.pone.0124506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stanifer JW, Turner EL, Egger JR, et al. Knowledge, Attitudes, and Practices Associated with Chronic Kidney Disease in Northern Tanzania: A Community-Based Study. PLoS One 2016;11:e0156336 10.1371/journal.pone.0156336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Anyabolu EN, Chukwuonye II, Arodiwe E, et al. Prevalence and predictors of chronic kidney disease in newly diagnosed human immunodeficiency virus patients in Owerri, Nigeria. Indian J Nephrol 2016;26:10–15. 10.4103/0971-4065.156115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Okafor UH, Unuigbe EI, Chukwuonye E. Prevalence and clinical and laboratory characteristics of kidney disease in anti-retroviral-naive human immunodeficiency virus-infected patients in South-South Nigeria. Saudi J Kidney Dis Transpl 2016;27:129–34. 10.4103/1319-2442.174155 [DOI] [PubMed] [Google Scholar]
- 137.Wensink GE, Schoffelen AF, Tempelman HA, et al. Albuminuria Is Associated with Traditional Cardiovascular Risk Factors and Viral Load in HIV-Infected Patients in Rural South Africa. PLoS One 2015;10:e0136529 10.1371/journal.pone.0136529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eghan BA, Frempong MT, Adjei-Poku M. Prevalence and predictors of microalbuminuria in patients with diabetes mellitus: a cross-sectional observational study in Kumasi, Ghana. Ethn Dis 2007;17:726–30. [PubMed] [Google Scholar]
- 139.Aryee C, Owiredu WK, Osei-Yeboah J, et al. An Analysis of Anthropometric Indicators and Modifiable Lifestyle Parameters Associated with Hypertensive Nephropathy. Int J Hypertens 2016;2016:1–14. 10.1155/2016/6598921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nabbaale J, Kibirige D, Ssekasanvu E, et al. Microalbuminuria and left ventricular hypertrophy among newly diagnosed black African hypertensive patients: a cross sectional study from a tertiary hospital in Uganda. BMC Res Notes 2015;8:198 10.1186/s13104-015-1156-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Addo J, Smeeth L, Leon DA. Hypertensive target organ damage in Ghanaian civil servants with hypertension. PLoS One 2009;4:e6672 10.1371/journal.pone.0006672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Owiredu WK, Quaye L, Amidu N, et al. Renal insufficiency in Ghanaian HIV infected patients: need for dose adjustment. Afr Health Sci 2013;13:101–11. 10.4314/ahs.v13i1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stöhr W, Reid A, Walker AS, et al. Glomerular dysfunction and associated risk factors over 4-5 years following antiretroviral therapy initiation in Africa. Antivir Ther 2011;16:1011–20. 10.3851/IMP1832 [DOI] [PubMed] [Google Scholar]
- 144.Stöhr W, Walker AS, Munderi P, et al. Estimating glomerular filtration rate in HIV-infected adults in Africa: comparison of Cockcroft-Gault and Modification of Diet in Renal Disease formulae. Antivir Ther 2008;13:761–70. [DOI] [PubMed] [Google Scholar]
- 145.Reid A, Stöhr W, Walker AS, et al. Severe renal dysfunction and risk factors associated with renal impairment in HIV-infected adults in Africa initiating antiretroviral therapy. Clin Infect Dis 2008;46:1271–81. 10.1086/533468 [DOI] [PubMed] [Google Scholar]
- 146.Ekat MH, Courpotin C, Diafouka M, et al. [Prevalence and factors associated with renal disease among patients with newly diagnoses of HIV in Brazzaville, Republic of Congo]. Med Sante Trop 2013;23:176–80. 10.1684/mst.2013.0170 [DOI] [PubMed] [Google Scholar]
- 147.Peters PJ, Moore DM, Mermin J, et al. Antiretroviral therapy improves renal function among HIV-infected Ugandans. Kidney Int 2008;74:925–9. 10.1038/ki.2008.305 [DOI] [PubMed] [Google Scholar]
- 148.Peck R, Baisley K, Kavishe B, et al. Decreased renal function and associated factors in cities, towns and rural areas of Tanzania: a community-based population survey. Trop Med Int Health 2016;21:393–404. 10.1111/tmi.12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nsagha DS, Pokam BT, Assob JC, et al. HAART, DOTS and renal disease of patients co-infected with HIV/AIDS and TB in the South West Region of Cameroon. BMC Public Health 2015;15:1040 10.1186/s12889-015-2331-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mekuria Y, Yilma D, Mekonnen Z, et al. Renal Function Impairment and Associated Factors among HAART Naïve and Experienced Adult HIV Positive Individuals in Southwest Ethiopia: A Comparative Cross Sectional Study. PLoS One 2016;11:e0161180 10.1371/journal.pone.0161180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Adebamowo SN, Adeyemo AA, Tekola-Ayele F, et al. Impact of Type 2 Diabetes on Impaired Kidney Function in Sub-Saharan African Populations. Front Endocrinol 2016;7:50 10.3389/fendo.2016.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fiseha T, Kassim M, Yemane T. Chronic kidney disease and underdiagnosis of renal insufficiency among diabetic patients attending a hospital in Southern Ethiopia. BMC Nephrol 2014;15:198 10.1186/1471-2369-15-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Odenigbo C, Oguejiofor O, Onwubuya E, et al. The prevalence of chronic kidney disease in apparently healthy retired subjects in asaba, Nigeria. Ann Med Health Sci Res 2014;4(Suppl 2):S128–32. 10.4103/2141-9248.138031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lucas GM, Clarke W, Kagaayi J, et al. Decreased Kidney Function in a Community-based Cohort of HIV-Infected and HIV-Negative Individuals in Rakai, Uganda. J Acquir Immune Defic Syndr 2010;55:491–4. 10.1097/QAI.0b013e3181e8d5a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Booysen HL, Woodiwiss AJ, Raymond A, et al. Chronic kidney disease epidemiology collaboration-derived glomerular filtration rate performs better at detecting preclinical end-organ changes than alternative equations in black Africans. J Hypertens 2016;34:1178–85. 10.1097/HJH.0000000000000924 [DOI] [PubMed] [Google Scholar]
- 156.Seape T, Gounden V, van Deventer HE, et al. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing Highly Active Antiretroviral Therapy. Ann Clin Biochem 2016;53:58–66. 10.1177/0004563215579695 [DOI] [PubMed] [Google Scholar]
- 157.Zachor H, Machekano R, Estrella MM, et al. Incidence of stage 3 chronic kidney disease and progression on tenofovir-based regimens. AIDS 2016;30:1221–8. 10.1097/QAD.0000000000001041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Adedeji TA, Adedeji NO, Adebisi SA, et al. Prevalence and Pattern of Chronic Kidney Disease in Antiretroviral-Naïve Patients with HIV/AIDS. J Int Assoc Provid AIDS Care 2015;14:434–40. 10.1177/2325957415587570 [DOI] [PubMed] [Google Scholar]
- 159.Matsha TE, Soita DJ, Hassan SM, et al. Deterioration, improvement of kidney function over time and determinants in the Cape Town Bellville South cohort. Nephrology 2014;19:638–47. 10.1111/nep.12313 [DOI] [PubMed] [Google Scholar]
- 160.Jao J, Lo W, Toro PL, et al. Factors associated with decreased kidney function in HIV-infected adults enrolled in the MTCT-Plus Initiative in sub-Saharan Africa. J Acquir Immune Defic Syndr 2011;57:40–5. 10.1097/QAI.0b013e31821008eb [DOI] [PubMed] [Google Scholar]
- 161.Gupta SK, Ong’or WO, Shen C, et al. Reduced renal function is associated with progression to AIDS but not with overall mortality in HIV-infected Kenyan adults not initially requiring combination antiretroviral therapy. J Int AIDS Soc 2011;14:31 10.1186/1758-2652-14-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Myer L, Kamkuemah M, Kaplan R, et al. Low prevalence of renal dysfunction in HIV-infected pregnant women: implications for guidelines for the prevention of mother-to-child transmission of HIV. Trop Med Int Health 2013;18:1400–5. 10.1111/tmi.12194 [DOI] [PubMed] [Google Scholar]
- 163.Mulenga LB, Kruse G, Lakhi S, et al. Baseline renal insufficiency and risk of death among HIV-infected adults on antiretroviral therapy in Lusaka, Zambia. AIDS 2008;22:1821–7. 10.1097/QAD.0b013e328307a051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ajayi S, Mamven M, Ojji D. eGFR and chronic kidney disease stages among newly diagnosed asymptomatic hypertensives and diabetics seen in a tertiary health center in Nigeria. Ethn Dis 2014;24:220–5. [PubMed] [Google Scholar]
- 165.Nwankwo EA, Nwankwo B, Mubi B. Prevalence of impaired kidney function in hospitalized hypertensive patients in Maiduguri, Nigeria. Intern J Intern Med 2006;6. [Google Scholar]
- 166.Edwards JK, Bygrave H, Van den Bergh R, et al. HIV with non-communicable diseases in primary care in Kibera, Nairobi, Kenya: characteristics and outcomes 2010-2013. Trans R Soc Trop Med Hyg 2015;109:440–6. 10.1093/trstmh/trv038 [DOI] [PubMed] [Google Scholar]
- 167.Kamkuemah M, Kaplan R, Bekker LG, et al. Renal impairment in HIV-infected patients initiating tenofovir-containing antiretroviral therapy regimens in a Primary Healthcare Setting in South Africa. Trop Med Int Health 2015;20:518–26. 10.1111/tmi.12446 [DOI] [PubMed] [Google Scholar]
- 168.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47. 10.7326/0003-4819-139-2-200307150-00013 [DOI] [PubMed] [Google Scholar]
- 169.Abdelsatir S, Al-Sofi A, Elamin S, et al. The potential role of nursing students in the implementation of community-based hypertension screening programs in Sudan. Arab J Nephrol Transplant 2013;6:51–4. [PubMed] [Google Scholar]
- 170.Agaba EI, Agaba PA, Sirisena ND, et al. Renal disease in the acquired immunodeficiency syndrome in north central Nigeria. Niger J Med 2003;12:120–5. [PubMed] [Google Scholar]
- 171.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 2002;39:920–9. 10.1053/ajkd.2002.32765 [DOI] [PubMed] [Google Scholar]
- 172.Liu WS, Chung YT, Yang CY, et al. Serum creatinine determined by Jaffe, enzymatic method, and isotope dilution-liquid chromatography-mass spectrometry in patients under hemodialysis. J Clin Lab Anal 2012;26:206–14. 10.1002/jcla.21495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Drion I, Cobbaert C, Groenier KH, et al. Clinical evaluation of analytical variations in serum creatinine measurements: why laboratories should abandon Jaffe techniques. BMC Nephrol 2012;13:133 10.1186/1471-2369-13-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bachmann LM, Nilsson G, Bruns DE, et al. State of the art for measurement of urine albumin: comparison of routine measurement procedures to isotope dilution tandem mass spectrometry. Clin Chem 2014;60:471–80. 10.1373/clinchem.2013.210302 [DOI] [PubMed] [Google Scholar]
- 175.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010;55:622–7. 10.1053/j.ajkd.2010.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cobo G, Hecking M, Port FK, et al. Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin Sci 2016;130:1147–63. 10.1042/CS20160047 [DOI] [PubMed] [Google Scholar]
- 177.Nitsch D, Grams M, Sang Y, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ 2013;346:f324 10.1136/bmj.f324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Poggio ED, Rule AD. A critical evaluation of chronic kidney disease–should isolated reduced estimated glomerular filtration rate be considered a ‘disease’? Nephrol Dial Transplant 2009;24:698–700. 10.1093/ndt/gfn704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hill NR, Fatoba ST, Oke JL, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0158765 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2015;66(1 Suppl 1):S1–305. 10.1053/j.ajkd.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brück K, Stel VS, Gambaro G, et al. CKD Prevalence Varies across the European General Population. J Am Soc Nephrol 2016;27:2135–47. 10.1681/ASN.2015050542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ingsathit A, Thakkinstian A, Chaiprasert A, et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant 2010;25:1567–75. 10.1093/ndt/gfp669 [DOI] [PubMed] [Google Scholar]
- 183.Singh AK, Farag YM, Mittal BV, et al. Epidemiology and risk factors of chronic kidney disease in India - results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol 2013;14:114 10.1186/1471-2369-14-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Imai E, Horio M, Watanabe T, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 2009;13:621–30. 10.1007/s10157-009-0199-x [DOI] [PubMed] [Google Scholar]
- 185.Hwang SJ, Tsai JC, Chen HC. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology 2010;15(Suppl 2):3–9. 10.1111/j.1440-1797.2010.01304.x [DOI] [PubMed] [Google Scholar]
- 186.Lin B, Shao L, Luo Q, et al. Prevalence of chronic kidney disease and its association with metabolic diseases: a cross-sectional survey in Zhejiang province, Eastern China. BMC Nephrol 2014;15:36 10.1186/1471-2369-15-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tomonaga Y, Risch L, Szucs TD, et al. The prevalence of chronic kidney disease in a primary care setting: a Swiss cross-sectional study. PLoS One 2013;8:e67848 10.1371/journal.pone.0067848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 189.Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med 2006;354:997–9. 10.1056/NEJMp058318 [DOI] [PubMed] [Google Scholar]
- 190.UNAIDS. HIV and AIDS estimates. UNAIDS, 2015. http://www.unaids.org/en/regionscountries/countries/senegal (accessed 15 Jul 2015). [Google Scholar]
- 191.UNAIDS. HIV and AIDS estimates. UNAIDS, 2015. http://www.unaids.org/en/regionscountries/countries/swaziland (accessed 1 Aug 2015). [Google Scholar]
- 192.Matic S, Lazarus JV, Donoghoe MC. HIV/AIDS in Europe: moving from death sentence to chronic disease management. World Health Organization, 2006. [Google Scholar]
- 193.Estrella M, Fine DM, Gallant JE, et al. HIV type 1 RNA level as a clinical indicator of renal pathology in HIV-infected patients. Clin Infect Dis 2006;43:377–80. 10.1086/505497 [DOI] [PubMed] [Google Scholar]
- 194.Déti EK, Thiébaut R, Bonnet F, et al. Prevalence and factors associated with renal impairment in HIV-infected patients, ANRS C03 Aquitaine Cohort, France. HIV Med 2010;11:308–17. 10.1111/j.1468-1293.2009.00780.x [DOI] [PubMed] [Google Scholar]
- 195.Fernando SK, Finkelstein FO, Moore BA, et al. Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci 2008;335:89–94. 10.1097/MAJ.0b013e31812e6b34 [DOI] [PubMed] [Google Scholar]
- 196.Cao Y, Gong M, Han Y, et al. Prevalence and risk factors for chronic kidney disease among HIV-infected antiretroviral therapy-naïve patients in mainland China: a multicenter cross-sectional study. Nephrology 2013;18:307–12. 10.1111/nep.12031 [DOI] [PubMed] [Google Scholar]
- 197.Rustarazo SB, Fuente SR, de Miguel SC, et al. Prevalence and spectrum of chronic kidney disease in HIV-positive patients: GRP031 Table 1. Eur J Hosp Pharm 2012;19:96.3–7. 10.1136/ejhpharm-2012-000074.31 [DOI] [Google Scholar]
- 198.Menezes AM, Torelly J, Real L, et al. Prevalence and risk factors associated to chronic kidney disease in HIV-infected patients on HAART and undetectable viral load in Brazil. PLoS One 2011;6:e26042 10.1371/journal.pone.0026042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sicotte M, Langlois ÉV, Aho J, et al. Association between nutritional status and the immune response in HIV + patients under HAART: protocol for a systematic review. Syst Rev 2014;3:9 10.1186/2046-4053-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Taylor BS, Sobieszczyk ME, McCutchan FE, et al. The challenge of HIV-1 subtype diversity. N Engl J Med 2008;358:1590–602. 10.1056/NEJMra0706737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wools-Kaloustian KK, Gupta SK. Will there be an epidemic of HIV-related chronic kidney disease in sub-Saharan Africa? Too soon to tell. Kidney Int 2008;74:845–7. 10.1038/ki.2008.326 [DOI] [PubMed] [Google Scholar]
- 202.Pokroy-Shapira E, Gelernter I, Molad Y. Evolution of chronic kidney disease in patients with systemic lupus erythematosus over a long-period follow-up: a single-center inception cohort study. Clin Rheumatol 2014;33:649–57. 10.1007/s10067-014-2527-0 [DOI] [PubMed] [Google Scholar]
- 203.Mak A, Mok CC, Chu WP, et al. Renal damage in systemic lupus erythematosus: a comparative analysis of different age groups. Lupus 2007;16:28–34. 10.1177/0961203306074469 [DOI] [PubMed] [Google Scholar]
- 204.Rabbani MA, Tahir MH, Siddiqui BK, et al. Renal involvement in systemic lupus erythematosus in Pakistan. J Pak Med Assoc 2005;55:328–32. [PubMed] [Google Scholar]
- 205.Chiu HY, Huang HL, Li CH, et al. Increased Risk of Chronic Kidney Disease in Rheumatoid Arthritis Associated with Cardiovascular Complications - A National Population-Based Cohort Study. PLoS One 2015;10:e0136508 10.1371/journal.pone.0136508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Barsoum RS. End-stage renal disease in North Africa. Kidney Int Suppl 2003;83:S111–4. 10.1046/j.1523-1755.63.s83.23.x [DOI] [PubMed] [Google Scholar]
- 207.Naicker S. End-stage renal disease in Sub-Saharan Africa. Kidney Int Suppl 2013;3:161–3. 10.1038/kisup.2013.4 [DOI] [PubMed] [Google Scholar]
- 208.Naicker S. Challenges for nephrology practice in Sub-Saharan Africa. Nephrol Dial Transplant 2010;25:649–50. [DOI] [PubMed] [Google Scholar]
- 209.Noubiap JJ, Naidoo J, Kengne AP. Diabetic nephropathy in Africa: A systematic review. World J Diabetes 2015;6:759–73. 10.4239/wjd.v6.i5.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brook MO, Bottomley MJ, Mevada C, et al. Repeat testing is essential when estimating chronic kidney disease prevalence and associated cardiovascular risk. QJM 2012;105:247–55. 10.1093/qjmed/hcr171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015069supp001.pdf (80.9KB, pdf)
bmjopen-2016-015069supp002.pdf (118.4KB, pdf)
bmjopen-2016-015069supp003.jpg (298.8KB, jpg)