Abstract
Objectives
Population studies on hearing loss (HL) associated with rheumatoid arthritis (RA) are lacking. This study investigated the risk of developing HL in patients with RA using a nationwide population cohort.
Setting
The population-based insurance claims data in the Taiwan National Health Insurance Research Database.
Design
Retrospective cohort study followed up RA cohort and control cohort without RA frequency matched by sex, age and diagnosis year.
Study population
18 267 patients with RA newly diagnosed in 2000–2006 and 73 068 controls without RA.
Main outcomes
Incidences of HL by the end of 2011 and the RA cohort to non-RA cohort HRs after adjusting for sex, age and comorbidities.
Results
The HL incidence was higher in the RA cohort than in the non-RA cohort (3.08 vs 1.62 per 1000 person-years), with an adjusted HR (aHR) of 1.91 (95% CI 1.70 to 2.14) for the RA cohort relative to the non-RA cohort after controlling for age, sex and comorbidities. Men and the elderly are at a higher risk. Cardiovascular comorbidities were associated with a further increased HL risk for patients with RA. Medications were associated with reduced HL incidence; patients with RA who used non-steroidal anti-inflammatory drugs (NSAIDs) had an aHR of 0.12 (95% CI 0.07 to 0.20), compared with non-users.
Conclusions
This study demonstrates that patients with RA are at an increased risk of developing HL. Findings highlight the need of disease-modifying treatment and scheduled auditory examinations for HL prevention and early detection for patients with RA.
Keywords: rheumatoid arthritis, hearing loss, insurance data, retrospective cohort study
Strengths and limitations of this study.
The strength of this study is the use of a nationwide population-based cohort to identify hearing loss (HL) risk in an Asian population with rheumatoid arthritis (RA). Our findings can be generalised to the general population.
The inclusion of the Catastrophic Illness Patient Database confirmed the diagnoses of all RA cases with increased reliability of our data; the large sample size reduced the tendency for selection bias, enhanced statistical power and precision of risk appraisal.
Limitations in this study: information on several suspected risk factors for HL, such as smoking and chronic exposure to occupational and environmental noise, which could be associated with HL in the general population, was not available in the insurance database.
Information on laboratory test results and HL by severity and sound frequency (high, mid or low frequency) and on RA severity scale, such as disease activity, functional impairment and physical damage, was also unavailable.
Introduction
Rheumatoid arthritis (RA) is a disease predominantly characterised by chronic joint inflammation and is often accompanied by several peripheral inflammatory manifestations.1 RA may lead to the destruction of the cartilage and bone due to chronic synovitis and may consequently impair joint function.2 In addition, patients with RA may have extra-articular manifestations involving other organ systems,3 such as auditory system alteration, although with a different putative mechanism of damage.4–6 With respect to the auditory system, previous studies have shown conflicting findings, in both hearing loss (HL) and the RA disease activity and severity.7–10
There is a wide variation in the reported prevalence of HL in patients with RA. Sensorineural hearing loss (SNHL) is the most common hearing impairment in patients with RA, ranging from 25% to 72%,11 whereas conductive HL and mixed HL are less frequently reported.4 6 12 SNHL could be induced by a direct immune response of either T or B cells against inner ear proteins.13 Neurovascular inflammation and drugs used for RA treatment could also damage the cochlea.14 Thus, HL may be a manifestation of systemic vascular involvement in patients with RA and may have a significant effect on the health of patients with RA. However, the risk of developing HL in patients with RA has not been well examined using population data.
Hence, the purpose of this study was to investigate the risk of HL in patients with RA, using representative insurance claims data obtained from the Taiwan National Health Insurance (NHI). The HL risk associated with other comorbidities, such as coronary heart disease, hypertension, stroke, diabetes, hyperlipidaemia, hyperthyroidism, hypothyroidism, chronic renal disease and autoimmune diseases, was also evaluated.
Materials and methods
Data source
The Taiwan NHI system is a single-payer compulsory programme with a coverage of over 99% of 23.74 million people.15 We conducted this study using two data sets: the Registry for Catastrophic Illness Patient Database (CIPD) and the Longitudinal Health Insurance Database (LHID2000), obtained from the Taiwan National Health Research Institutes. Patients with major diseases, such as cancer, chronic mental illness, end-stage renal disease and several autoimmune diseases requiring long-term care, are eligible for the CIPD coverage for exemption from making copayment. The LHID2000 contains the claims data of 1 000 000 people randomly sampled from all populations being registered in 2000 for the insurance coverage. Reimbursement claims data for medical services from 1996 to 2011 in both data sets were used in this study. For privacy protection, all personal identifications were replaced with surrogate identifications suitable for public use and data linkage. The claims data contained information on the demographic status of the insured people, dates of treatment and treatments received, diagnostic codes, prescriptions and costs. Diagnoses of diseases were coded with the International Classification of Disease Diagnoses, Ninth Revision of Clinical Modification (ICD-9-CM). Several studies in Taiwan using the insurance claims data have demonstrated high accuracy and validity of ICD-9 diagnosis.16 17
Study population
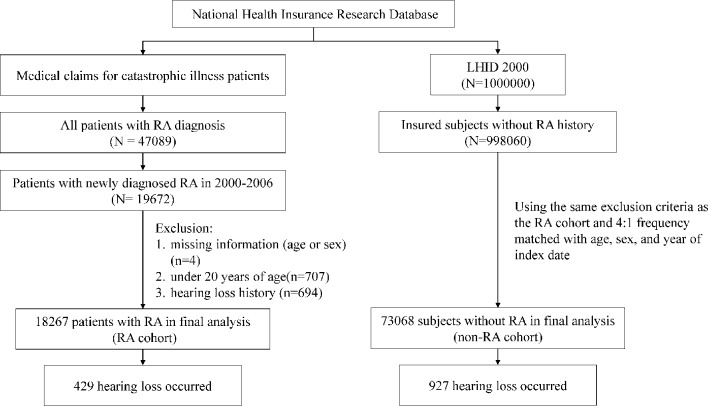
Figure 1 shows the flow chart for identifying and selecting study population using a population-based retrospective cohort study design. We identified an RA cohort from the registry for CIPD and a non-RA cohort from the LHID2000. Patients newly diagnosed with RA (ICD-9-CM 714.0) from 2000 to 2006 without HL were included in the RA cohort. The date with RA certificated as the catastrophic illness was considered as the index date for the approved patients. Patients who met four or more of the diagnostic criteria based on the 1987 American College of Rheumatology criteria were considered as having RA and those diagnosed by rheumatologists were included in the RA cohort.18 The application for catastrophic illness status was scrutinised by peer review.
Figure 1.
Flow chart showing selection of study cohorts. LHID, Longitudinal Health Insurance Database; RA, rheumatoid arthritis.
For each patient with RA, four insured people without history of RA and HL were randomly selected from the LHID2000 for the non-RA cohort and were frequency matched by sex, age (each 5-year span) and index year. Individuals with missing information on age and/or sex or with history of HL (ICD-9-CM 388.2, 388.4, 389.00, 389.10, 389.12, 389.2 and 389.9) at baseline were excluded from the non-RA cohort.
Both cohorts were followed from the index date to the date with HL diagnosed, death, withdrawal from the NHI system or the end of 2011. In general, HL was diagnosed based on the audiometry test. To increase the validity of HL diagnosis, only patients with three or more diagnoses in outpatient claims or an inpatient record were included in the study. Patients who were suspected of having HL received comprehensive examinations and, subsequently, treatment was followed when the disorder was confirmed. In the insurance system, HL patients’ medical reimbursement and discharge notes are scrutinised by peer review. The insurance system also randomly reviewed insurance claims to prevent errors and violations. Therefore, diagnoses and codes of HL in the study were highly reliable.16
Statistical analysis
Distributions of sex and age (20–39, 40–59 and ≥60 years) and baseline comorbidities, including diabetes (ICD-9-CM 250), hyperlipidaemia (ICD-9-CM 272), hypertension (ICD-9-CM 401–405), hyperthyroidism (ICD-9-CM 242), ischaemic heart disease (IHD; ICD-9-CM 410–414), stroke (ICD-9-CM 430–438), chronic kidney disease (CKD; ICD-9-CM 580–589), hypothyroidism (ICD-9-CM 244) and autoimmune diseases (including psoriasis (ICD-9-CM 696), systemic lupus erythematosus (SLE; ICD-9-CM 710.0), systemic sclerosis (ICD-9-CM 710.1), Sjogren syndrome (ICD-9-CM 710.2), dermatomyositis (ICD-9-CM 710.3), polymyositis (ICD-9-CM 710.4) and vasculitis (ICD-9-CM 446.0, 446.2, 446.4, 446.5, 443.1, 446.7, 446.1 and 136.1), between the RA and non-RA cohorts were compared. A standardised mean difference of less than 0.1 was a negligible difference between two means or two prevalence rates.19 The incidence density of HL per 1000 person-years was calculated during the follow-up period by sex, age and comorbidity. The Kaplan-Meier method was employed to plot the cumulative incidence of HL for each cohort during the follow-up period, and the log-rank test was used to assess the differences between the two curves. Univariate and multivariate Cox proportional hazards regression analyses were used to measure the RA cohort to non-RA cohort crude HR (cHR) and adjusted HR (aHR) of HL, respectively, and their 95% CIs. Sex, age and comorbidities including diabetes, hyperlipidaemia, hypertension, hyperthyroidism, hypothyroidism, IHD, stroke, CKD and autoimmune diseases were included as covariates in the multivariate Cox regression analysis. To further assess the robustness of our results, we also evaluated the association between RA and HL risk in various subgroups by sex, age and each comorbidity. We further evaluated the treatment effectiveness of medications for patients with RA by calculating the incidence density and HRs of HL. The HL relating to medications for RA treatment was evaluated, including non-steroidal anti-inflammatory drugs (NSAIDs), prednisolone, disease-modifying antirheumatic drugs (DMARDs, including hydroxychloroquine, sulfasalazine, methotrexate and leflunomide), and tumour necrosis factor (TNF, including etanercept and adalimumab). Further analysis evaluated the HL risk by the type of HL: sensorineural, conductive or mixed.
All analyses were conducted using SAS statistical software (V.9.4 for Windows; SAS Institute), and all statistical tests were performed at the two-tailed significance level of 0.05.
Results
We identified 18 267 patients with RA newly diagnosed from 2000 to 2006 for the RA cohort and 73 068 persons without RA for the non-RA cohort as controls (table 1). There were more women than men (78.4 vs 21.6%) in both cohorts. Approximately 66.7% of the study populations were <60 years old. Prevalence rates of CKD and autoimmune diseases were more prevalent in patients with RA than in controls at the baseline.
Table 1.
Distribution of demographic factors and comorbidity compared between cohorts
| Variable | Non-RA cohort n=73 068 |
RA cohort n=18 267 |
Standardised mean difference | ||
| n | % | n | % | ||
| Sex | |||||
| Female | 57 288 | 78.4 | 14 322 | 78.4 | <0.001 |
| Male | 15 780 | 21.6 | 3945 | 21.6 | <0.001 |
| Age, years | |||||
| 20–39 | 12 224 | 16.7 | 3056 | 16.7 | <0.001 |
| 40–59 | 36 532 | 50.0 | 9133 | 50.0 | <0.001 |
| ≥60 | 24 312 | 33.3 | 6078 | 33.3 | <0.001 |
| Means (SD) | 53.3 | (14.2) | 53.6 | (13.9) | 0.021 |
| Comorbidity | |||||
| DM | 8102 | 11.1 | 2114 | 11.6 | 0.015 |
| Hyperlipidaemia | 14 078 | 19.3 | 3439 | 18.8 | 0.011 |
| Hypertension | 22 844 | 31.3 | 5964 | 32.7 | 0.030 |
| Hyperthyroidism | 1089 | 1.49 | 456 | 2.50 | 0.072 |
| IHD | 10 993 | 15.0 | 2941 | 16.1 | 0.029 |
| Stroke | 2128 | 2.91 | 483 | 2.64 | 0.016 |
| CKD | 4821 | 6.60 | 2061 | 11.3 | 0.165 |
| Hypothyroidism | 407 | 0.56 | 216 | 1.18 | 0.067 |
| Autoimmune diseases* | 433 | 0.59 | 534 | 2.92 | 0.178 |
*Autoimmune diseases including psoriasis, SLE, systemic sclerosis, Sjogren syndrome, dermatomyositis, polymyositis and vasculitis.
CKD, chronic kidney disease; DM, diabetes mellitus; IHD, ischaemic heart disease; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
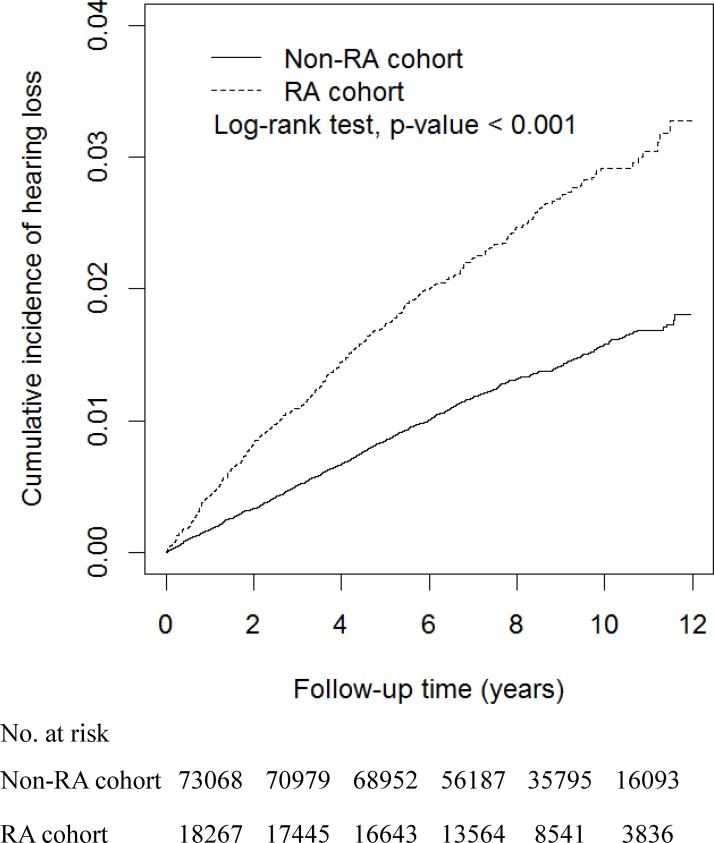
The Kaplan-Meier method estimated cumulative incidence of HL was 1.5% greater in the RA cohort than in the non-RA cohort (3.3 vs 1.8%; P value <0.001 in the log-rank test; figure 2). The incidence density of HL was approximately twofold greater in the RA cohort than in the non-RA cohort (3.08 vs 1.62 per 1000 person-years), with an aHR of 1.91 (95% CI 1.70 to 2.14; table 2). Men were at a greater risk of HL than women, and the risk increased with age. Compared with 20–39 years old, the aHRs of HL were 2.89 (95% CI 2.21 to 3.79) and 5.27 (95% CI 3.99 to 6.95) for those aged 40–59 years and those aged ≥60 years, respectively. The HL risk for individuals with comorbidities was also elevated. Patients with hypertension and IHD were significantly associated with higher risk of HL compared with their counterparts without the disorder, with aHRs of 1.21 (95% CI 1.07 to 1.38) and of 1.36 (95% CI 1.19 to 1.56), respectively.
Figure 2.
Kaplan-Meier method estimated cumulative incidence curves of hearing loss in the two cohorts. RA, rheumatoid arthritis.
Table 2.
Cox model measured HRs and 95% CIs of hearing loss associated with RA and covariates
| Variable | Event (n) | Person-years | Incidence density† | HR (95% CI) |
|
| Univariate | Multivariate‡ | ||||
| RA | |||||
| No | 927 | 572 031 | 1.62 | Ref | Ref |
| Yes | 429 | 139 085 | 3.08 | 1.90 (1.70 to 2.13)*** | 1.91 (1.70 to 2.14) *** |
| Sex | |||||
| Female | 977 | 565 205 | 1.73 | Ref | Ref |
| Male | 379 | 145 912 | 2.60 | 1.49 (1.33 to 1.68)*** | 1.40 (1.24 to 1.58)*** |
| Age, years | |||||
| 20 – 39 | 59 | 123 836 | 0.48 | Ref | Ref |
| 40 – 59 | 563 | 368 175 | 1.53 | 3.21 (2.45 to 4.19)*** | 2.89 (2.21 to 3.79)*** |
| ≥ 60 | 734 | 219 105 | 3.35 | 6.98 (5.35 to 9.10)*** | 5.27 (3.99 to 6.95)*** |
| Comorbidity | |||||
| DM | |||||
| No | 1127 | 638 984 | 1.76 | Ref | Ref |
| Yes | 229 | 72 133 | 3.17 | 1.78 (1.55 to 2.06)*** | 1.14 (0.98 to 1.33) |
| Hyperlipidaemia | |||||
| No | 974 | 578 643 | 1.68 | Ref | Ref |
| Yes | 382 | 132 474 | 2.88 | 1.70 (1.51 to 1.92)*** | 1.10 (0.97 to 1.26) |
| Hypertension | |||||
| No | 714 | 499 747 | 1.43 | Ref | Ref |
| Yes | 642 | 211 370 | 3.04 | 2.11 (1.90 to 2.35)*** | 1.21 (1.07 to 1.38)* |
| Hyperthyroidism | |||||
| No | 1325 | 699 624 | 1.89 | Ref | Ref |
| Yes | 31 | 11 492 | 2.70 | 1.41 (0.99 to 2.02) | 1.33 (0.92 to 1.92) |
| IHD | |||||
| No | 987 | 610 475 | 1.62 | Ref | Ref |
| Yes | 369 | 100 642 | 3.67 | 2.25 (2.00 to 2.54)*** | 1.36 (1.19 to 1.56)*** |
| Stroke | |||||
| No | 1310 | 695 656 | 1.88 | Ref | Ref |
| Yes | 46 | 15 461 | 2.98 | 1.55 (1.15 to 2.07)** | 0.85 (0.63 to 1.14) |
| CKD | |||||
| No | 1204 | 662 599 | 1.82 | Ref | Ref |
| Yes | 152 | 48 517 | 3.13 | 1.71 (1.45 to 2.03)*** | 1.06 (0.89 to 1.26) |
| Hypothyroidism | |||||
| No | 1343 | 706 448 | 1.90 | Ref | Ref |
| Yes | 13 | 4668 | 2.78 | 1.46 (0.85 to 2.52) | 1.15 (0.65 to 2.01) |
| Autoimmune diseases§ | |||||
| No | 1334 | 704 142 | 1.89 | Ref | Ref |
| Yes | 22 | 6975 | 3.15 | 1.65 (1.08 to 2.51)* | 1.34 (0.88 to 2.05) |
*P<0.05, **P<0.01, ***P<0.001.
†Per 1000 person-years.
‡Multivariate Cox proportional hazards regression model, including RA, sex, age (categorical), DM, hyperlipidaemia, hypertension, hyperthyroidism, IHD, stroke, CKD, hypothyroidism and autoimmune diseases.
§Autoimmune diseases including psoriasis, SLE, systemic sclerosis, Sjogren syndrome, dermatomyositis, polymyositis and vasculitis.
CKD, chronic kidney disease; DM, diabetes mellitus; IHD, ischaemic heart disease; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Table 3 shows that incidence rates of HL stratified by sex, age and comorbidity were consistently greater in the RA cohort than in the non-RA cohort. Comorbidity was associated with further increased HL risk for patients with RA. Patients with RA with comorbid IHD had the highest incident HL, 5.60 per 1000 person-years.
Table 3.
Incidence density and RA cohort to non-RA cohort HRs of hearing loss by sex, age and comorbidity in the two cohorts
| Variables | Non-RA cohort | RA cohort | RA cohort to non-RA cohort | |||||
| HR (95% CI) | ||||||||
| Event (n) | Person-years | Incidence density† | Event (n) | Person-years | Incidence density† | Crude | Adjusted‡ | |
| Sex | ||||||||
| Women | 663 | 454 249 | 1.46 | 314 | 110 956 | 2.83 | 1.94 (1.69 to 2.22)*** | 1.95 (1.70 to 2.23)*** |
| Men | 264 | 117 782 | 2.24 | 115 | 28 130 | 4.09 | 1.82 (1.46 to 2.27)*** | 1.85 (1.48 to 2.30)*** |
| Age, years | ||||||||
| 20–39 | 40 | 98 817 | 0.40 | 19 | 25 020 | 0.76 | 1.89 (1.09 to 3.26)* | 1.80 (1.02 to 3.16)* |
| 40–59 | 355 | 295 193 | 1.20 | 208 | 72 982 | 2.85 | 2.37 (2.00 to 2.81)*** | 2.32 (1.95 to 2.76)*** |
| ≥60 | 532 | 178 021 | 2.99 | 202 | 41 084 | 4.92 | 1.63 (1.39 to 1.92)*** | 1.62 (1.37 to 1.90)*** |
| Comorbidity | ||||||||
| DM | ||||||||
| No | 765 | 514 262 | 1.49 | 362 | 124 722 | 2.90 | 1.95 (1.72 to 2.21)*** | 1.94 (1.71 to 2.20)*** |
| Yes | 162 | 57 770 | 2.80 | 67 | 14 363 | 4.66 | 1.66 (1.25 to 2.21)*** | 1.74 (1.30 to 2.32)*** |
| Hyperlipidaemia | ||||||||
| No | 654 | 464 753 | 1.41 | 320 | 113 890 | 2.81 | 2.00 (1.75 to 2.28)*** | 1.96 (1.72 to 2.25)*** |
| Yes | 273 | 107 279 | 2.54 | 109 | 25 195 | 4.33 | 1.69 (1.36 to 2.11)*** | 1.74 (1.39 to 2.18)*** |
| Hypertension | ||||||||
| No | 481 | 402 716 | 1.19 | 233 | 97 031 | 2.40 | 2.01 (1.72 to 2.35)*** | 1.94 (1.66 to 2.28)*** |
| Yes | 446 | 169 316 | 2.63 | 196 | 42 054 | 4.66 | 1.76 (1.49 to 2.08)*** | 1.82 (1.54 to 2.16)*** |
| Hyperthyroidism | ||||||||
| No | 909 | 563 891 | 1.61 | 416 | 135 733 | 3.06 | 1.90 (1.69 to 2.13)*** | 1.92 (1.71 to 2.16)*** |
| Yes | 18 | 8140 | 2.21 | 13 | 3352 | 3.88 | 1.76 (0.86 to 3.58) | 1.72 (0.84 to 3.55) |
| IHD | ||||||||
| No | 671 | 491 566 | 1.37 | 316 | 118 909 | 2.66 | 1.95 (1.70 to 2.23)*** | 1.96 (1.71 to 2.24)*** |
| Yes | 256 | 80 466 | 3.18 | 113 | 20 176 | 5.60 | 1.75 (1.40 to 2.18)*** | 1.75 (1.40 to 2.19)*** |
| Stroke | ||||||||
| No | 896 | 559 458 | 1.60 | 414 | 136 197 | 3.04 | 1.90 (1.69 to 2.13)*** | 1.90 (1.69 to 2.14)*** |
| Yes | 31 | 12 573 | 2.47 | 15 | 2888 | 5.19 | 2.10 (1.13 to 3.89)* | 2.18 (1.16 to 4.12)* |
| CKD | ||||||||
| No | 835 | 538 065 | 1.55 | 369 | 124 534 | 2.96 | 1.91 (1.69 to 2.16)*** | 1.94 (1.71 to 2.19)*** |
| Yes | 92 | 33 967 | 2.71 | 60 | 14 551 | 4.12 | 1.53 (1.11 to 2.12)* | 1.73 (1.25 to 2.42)** |
| Hypothyroidism | ||||||||
| No | 917 | 569 055 | 1.61 | 426 | 137 393 | 3.10 | 1.92 (1.71 to 2.16)*** | 1.94 (1.73 to 2.18)*** |
| Yes | 10 | 2977 | 3.36 | 3 | 1692 | 1.77 | 0.53 (0.15 to 1.93) | 0.69 (0.19 to 2.57) |
| Autoimmune diseases§ | ||||||||
| No | 916 | 568 956 | 1.61 | 418 | 135 186 | 3.09 | 1.92 (1.71 to 2.15)*** | 1.94 (1.73 to 2.18)*** |
| Yes | 11 | 3076 | 3.58 | 11 | 3899 | 2.82 | 0.79 (0.34 to 1.82) | 0.89 (0.38 to 2.07) |
*P<0.05, **P<0.01, ***P<0.001.
†Per 1000 person-years.
‡Model mutually adjusted for sex, age (continuous), DM, hyperlipidaemia, hypertension, hyperthyroidism, IHD, stroke, CKD, hypothyroidism and autoimmune diseases.
§Autoimmune diseases including psoriasis, SLE, systemic sclerosis, Sjogren syndrome, dermatomyositis, polymyositis and vasculitis.
CKD, chronic kidney disease; DM, diabetes mellitus; IHD, ischaemic heart disease; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Table 4 shows that medications were associated with reduced incident HL for patients with RA. Near 99% of patients with RA used NSAIDs, and users had an HL incidence of 2.98 per 1000 person-years, with an aHR of 0.12 (95% CI 0.07 to 0.20) compared with non-users who had an incidence of 30.1 per 1000 person-years for HL. Patients with RA on medications of adalimumab (n=950) had the lowest HL incidence of 0.64 per 1000 person-years with an aHR of 0.23 (95% CI 0.10 to 0.55), compared with non-users who had an incidence of 3.23 per 1000 person-years.
Table 4.
Incidence density and HRs of hearing loss associated with medication in patients with RA
| Medicine use | N | Event (n) | Person-years | Incidence density† | HR (95% CI) | |
| Crude‡ | Adjusted‡ | |||||
| NSAIDs | ||||||
| No | 169 | 16 | 532 | 30.1 | Ref | Ref |
| Yes | 18 098 | 413 | 138 553 | 2.98 | 0.11 (0.07 to 0.18)*** | 0.12 (0.07 to 0.20)*** |
| Prednisolone | ||||||
| No | 1673 | 60 | 11 529 | 5.20 | Ref | Ref |
| Yes | 16 594 | 369 | 127 556 | 2.89 | 0.56 (0.43 to 0.74)*** | 0.53 (0.40 to 0.70)*** |
| DMARDs | ||||||
| Hydroxychloroquine | ||||||
| No | 12 200 | 309 | 91 284 | 3.39 | Ref | Ref |
| Yes | 6067 | 120 | 47 801 | 2.51 | 0.75 (0.60 to 0.92)** | 0.77 (0.62 to 0.95)* |
| Sulfasalazine | ||||||
| No | 5141 | 148 | 36 176 | 4.09 | Ref | Ref |
| Yes | 13 126 | 281 | 102 909 | 2.73 | 0.68 (0.56 to 0.83)*** | 0.74 (0.61 to 0.91)** |
| Methotrexate | ||||||
| No | 9261 | 268 | 67 188 | 3.99 | Ref | Ref |
| Yes | 9006 | 161 | 71 897 | 2.24 | 0.57 (0.47 to 0.69)*** | 0.65 (0.53 to 0.79)*** |
| Leflunomide | ||||||
| No | 15 393 | 405 | 116 118 | 3.49 | Ref | Ref |
| Yes | 2874 | 24 | 22 967 | 1.04 | 0.30 (0.20 to 0.45)*** | 0.33 (0.22 to 0.50)*** |
| TNF | ||||||
| Etanercept | ||||||
| No | 16 259 | 408 | 122 506 | 3.33 | Ref | Ref |
| Yes | 2008 | 21 | 16 579 | 1.27 | 0.39 (0.25 to 0.60)*** | 0.44 (0.28 to 0.68)*** |
| Adalimumab | ||||||
| No | 17 317 | 424 | 131 303 | 3.23 | Ref | Ref |
| Yes | 950 | 5 | 7783 | 0.64 | 0.20 (0.08 to 0.48)*** | 0.23 (0.10 to 0.55)** |
*P<0.05, **P<0.01, ***P<0.001.
†Per 1000 person-years.
‡Model adjusted for sex, age, DM, hyperlipidaemia, hypertension, hyperthyroidism, IHD, stroke, CKD, hypothyroidism and autoimmune diseases.
CKD, chronic kidney disease; DM, diabetes mellitus; DMARD, disease-modifying antirheumatic drug; IHD, ischaemic heart disease; NSAID, non-steroidal anti-inflammatory drug; RA, rheumatoid arthritis; TNF, tumour necrosis factor.
Further evaluation on the subtype HL showed that patients with RA had only few cases of conductive HL, but were at increased risk of sensorineural HL and mixed HL with aHRs of 2.35 (95% CI 1.91 to 2.89) and 1.77 (95% CI 1.54 to 2.03), respectively (table 5).
Table 5.
Incidence density and HRs for subtypes of HL according to RA status
| Types of HL | RA | Compared with non-RA group | ||||
| No | Yes | HR (95% CI) | ||||
| Event (n) | Incidence density* | Event (n) | Incidence density* | Crude | Adjusted† | |
| Sensorineural | 249 | 0.44 | 144 | 1.04 | 2.38 (1.94 to 2.92)*** | 2.35 (1.91 to 2.89)*** |
| Conductive | 10 | 0.02 | 1 | 0.01 | 0.41 (0.05 to 3.21) | 0.41 (0.05 to 3.23) |
| Mixed | 668 | 1.17 | 284 | 2.04 | 1.75 (1.52 to 2.01)*** | 1.77 (1.54 to 2.03)*** |
ICD-9-CM: sensorineural, 389.10 and 389.12; conductive, 389.00; mixed, 388.2, 388.4, 389.2 and 389.9.
*Per 1000 person-years.
†Model adjusted for sex, age (continuous), DM, hyperlipidaemia, hypertension, hyperthyroidism, IHD, stroke, CKD, hypothyroidism and autoimmune diseases.
***P<0.001.
CKD, chronic kidney disease; DM, diabetes mellitus; HL, hearing loss; ICD-9-CM, International Classification of Disease Diagnoses, Ninth Revision of Clinical Modification; IHD, ischaemic heart disease; RA, rheumatoid arthritis.
Discussion
This retrospective cohort study showed that patients with RA were nearly twofold more likely to develop HL than those without RA. The risk of HL associated with RA increased with age. In the RA cohort, those ≥60 years old had an HL incidence of 4.92 per 1000 person-years, which was 4.16 per 1000 person-year greater than that of patients aged 20–39 years. The corresponding difference was 2.49 per 1000 person-years between the two age groups in the non-RA cohort, reflecting the natural HL by ageing in the non-RA cohort. Similar to reports in other studies, HL is age dependent in patients and control subjects.6 14 20 This finding is also consistent with previous studies for patients with sudden SNHL comorbid with SLE and psoriasis.21 22 The excess HL risk could be 50% in patients with psoriasis.
We also found that, in the RA cohort, men had an incidence of 4.09 per 1000 person-years for HL, which was 1.26 per 1000 person-years greater than women had. The corresponding difference was 0.78 per 1000 person-years in the non-RA cohort, indicating that the relationship between RA and HL risk may be slightly greater for men. In the entire study population, the overall aHR was 1.40 for men compared with women (table 2). There is a remarkable imbalance between the number of males and females with autoimmune diseases, with females representing the majority of cases. Although reasons for this over-representation of women are unclear, genetic (X-linked) factors and hormonal aspects are likely involved. Halligan et al23 investigated patients with RA and also demonstrated that the prevalence of abnormal hearing is significantly greater in males (86% or 12/14) than in females (33% or 5/15) (P=0.008). However, no significant gender difference in HL among those without RA was found (P=0.715).
Evidences have shown that patients with RA are prevalent with comorbidities, such as IHD,24–26 stroke,27 hypertension,28 29 diabetes,30–32 dyslipidaemia,27 33 CKD34 35 and thyroid disorders.36–38 In this study, the study populations in both cohorts were young. The baseline prevalence rates of most comorbidities between the two cohorts were not significantly different, except that CKD and autoimmune diseases were more prevalent in patients with RA than in controls without RA at the baseline (table 1). However, it is interesting to note that most of the comorbidities are associated with further increased incidence of HL, greater for the RA cohort than for the non-RA cohort, except hypothyroidism, and autoimmune diseases (table 3). Autoimmune disease is a possible pathology associated with SNHL because of the destruction of the cochlear hair cells.39 Our study failed to prove this relationship.
The development of RA and the breakdown of atherosclerotic plaques possibly share common factors contributing to inflammatory cells and proinflammatory cytokines.25 Proinflammatory cytokines may contribute to the oxidative damage in the inner ear.26 For example, both TNF-α and interleukin (IL)-6 are involved in the pathogenesis of both RA and atherosclerosis.40 However, Takatsu et al showed that the proinflammatory cytokines (IL-6) and matrix matalloproteinase-3 may contribute to harm in inner ear cells by an oxidative process.6 Both RA and HL may have a shared mechanism associating with cardiovascular diseases which account for the higher risk of HL in patients with RA. IHD alone may associate with HL. An earlier study found that patients with IHD are prevalent with HL for up to over 30%.41 Moreover, in this study, we found that patients with RA with IHD had the highest HL incidence among patients with cardiovascular disorders. Hence, RA and cardiovascular disorders may have a shared contribution to the HL risk.
Furthermore, several studies have reported elevated plasma renin and ACE activities in patients with RA.42 43 Poor blood pressure control could induce changes in the renin–angiotensin system. Higher oxidative stress in patients with RA could also impair the vasodilatory mechanism of the endothelium,43 which could be associated with the higher HL risk in patients with RA. Hence, hypertension is likely another risk factor contributing to HL. The findings in our study further demonstrate the association between autoimmune disease and HL risk.
After adjustment for sex, age and comorbidity, we found reduced HL risk for patients with RA on medication of NSAID, prednisolone, DMARDs and TNF. Conversely, Halligan et al23 described an association between HL and hydroxychloroquine, and Dikici et al44 observed a dose relation between HL using methotrexate. On the other hand, some studies found no relationship between HL and RA treatment using NSAID, corticosteroid and DMARDs.6 9 20 45 The inconsistent results may be due to the relatively small study sample sizes, whereas our study is a nationwide population-based cohort with large sample size. It is likely that the reduced inflammation in patients with RA on medications of NSAID, corticosteroid, DMARDs and TNF could be associated with reducing the HL risk.
The strength of this study is the use of a nationwide population-based cohort to evaluate HL risk in an Asian population with RA. Our findings can be generalised to the general population. The large sample size allowed the identification of risk factors associated with the development of HL in Taiwan with a minimal tendency for selection bias and enhanced the statistical power and precision of risk appraisal. In addition, the inclusion of the CIPD confirmed the diagnoses of all RA cases in the NHIRD database, which increased the reliability of our data.
However, several limitations to the interpretation of our findings should be considered. Information on several suspected risk factors for HL was unavailable, such as smoking and chronic exposure to occupational and environmental noise, which could be associated with HL for both cohorts. Moreover, information was also unavailable on laboratory test results, HL severity and RA severity scale and activity, functional impairment and physical damage of the disease. Hearing impairments by specific sound frequency were not measured to differentiate high, mid or low frequency HL.
In conclusion, this study demonstrated that patients with RA are at an elevated risk of developing HL. Our findings also suggest the need for prompt treatment and early detection of RA for HL prevention. Appropriate and timely medical interventions may improve the prognosis of HL for patients diagnosed with RA.
Supplementary Material
Footnotes
Contributors: The paper was conceived by C-MH, H-JC, P-HH, GJT and J-LL. C-MH, H-JC and F-CS wrote the first draft, with further contributions from all authors. Statistical analyses were undertaken by C-MH, H-JC and F-CS. C-MH and F-CS revised the article. All authors contributed to data interpretation and reviewed and approved the final version of the manuscript.
Funding: This study was supported by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW106-TDU-B-212-113004); China Medical University Hospital; Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM10601010036); Taiwan Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Competing interests: None declared.
Ethics approval: Research Ethics Committee of China Medical University Hospital (CMUH104-REC2-115).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094–108. 10.1016/S0140-6736(10)60826-4 [DOI] [PubMed] [Google Scholar]
- 2.Harris ED. Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med 1990;322:1277–89. 10.1056/NEJM199005033221805 [DOI] [PubMed] [Google Scholar]
- 3.Ozcan M, Karakuş MF, Gündüz OH, et al. Hearing loss and middle ear involvement in rheumatoid arthritis. Rheumatol Int 2002;22:16–19. [DOI] [PubMed] [Google Scholar]
- 4.Murdin L, Patel S, Walmsley J, et al. Hearing difficulties are common in patients with rheumatoid arthritis. Clin Rheumatol 2008;27:637–40. 10.1007/s10067-007-0802-z [DOI] [PubMed] [Google Scholar]
- 5.Emamifar A, Bjoerndal K, Hansen IM. Is hearing impairment associated with rheumatoid arthritis? a review. Open Rheumatol J 2016;10:26–32. 10.2174/1874312901610010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takatsu M, Higaki M, Kinoshita H, et al. Ear involvement in patients with rheumatoid arthritis. Otol Neurotol 2005;26:755–61. 10.1097/01.mao.0000178138.19848.bd [DOI] [PubMed] [Google Scholar]
- 7.Yildirim A, Surucu G, Dogan S, et al. Relationship between disease activity and hearing impairment in patients with rheumatoid arthritis compared with controls. Clin Rheumatol 2016;35:309–14. 10.1007/s10067-015-3129-1 [DOI] [PubMed] [Google Scholar]
- 8.Magaro M, Zoli A, Altomonte Z, et al. Sensorineural hearing loss in rheumatoid arthritis. Clin Exp Rheum 1990;8:487–90. [PubMed] [Google Scholar]
- 9.Lobo FS, Dossi MO, Batista L, et al. Hearing impairment in patients with rheumatoid arthritis: association with anti-citrullinated protein antibodies. Clin Rheumatol 2016;35:2327–32. 10.1007/s10067-016-3278-x [DOI] [PubMed] [Google Scholar]
- 10.Salvinelli F, Cancilleri F, Casale M, et al. Hearing thresholds in patients affected by rheumatoid arthritis. Clin Otolaryngol Allied Sci 2004;29:75–9. 10.1111/j.1365-2273.2004.00783.x [DOI] [PubMed] [Google Scholar]
- 11.Pascual-Ramos V, Contreras-Yáñez I, Rivera-Hoyos P, et al. Cumulative disease activity predicts incidental hearing impairment in patients with Rheumatoid Arthritis (RA). Clin Rheumatol 2014;33:315–21. 10.1007/s10067-014-2485-6 [DOI] [PubMed] [Google Scholar]
- 12.Bayazit YA, Yilmaz M, Gunduz B, et al. Distortion product otoacoustic emission findings in Behçet’s disease and rheumatoid arthritis. ORL J Otorhinolaryngol Relat Spec 2007;69:233–8. 10.1159/000101544 [DOI] [PubMed] [Google Scholar]
- 13.Mijovic T, Zeitouni A, Colmegna I. Autoimmune sensorineural hearing loss: the otology-rheumatology interface. Rheumatology 2013;52:780–9. 10.1093/rheumatology/ket009 [DOI] [PubMed] [Google Scholar]
- 14.Oztürk A, Yalçin S, Kaygusuz I, et al. High-frequency hearing loss and middle ear involvement in rheumatoid arthritis. Am J Otolaryngol 2004;25:411–7. 10.1016/j.amjoto.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 15.Cheng S-H, Chen C-C, Tsai S-L. The impacts of drg-based payments on health care provider behaviors under a universal coverage system: a population-based study. Health Policy 2012;107:202–8. 10.1016/j.healthpol.2012.03.021 [DOI] [PubMed] [Google Scholar]
- 16.Cheng CL, Kao YH, Lin SJ, et al. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:236–42. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
- 17.Yu YB, Gau JP, Liu CY, et al. A nation-wide analysis of venous thromboembolism in 497,180 cancer patients with the development and validation of a risk-stratification scoring system. Thromb Haemost 2012;108:225–35. 10.1160/TH12-01-0010 [DOI] [PubMed] [Google Scholar]
- 18.Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 19.Mamdani M, Sykora K, Li P, et al. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 2005;330:960–2. 10.1136/bmj.330.7497.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual-Ramos V, Contreras-Yanez I, Enriquez L, et al. Ramirez-Anguiano J hearing impairment in a tertiarycare- level population of mexican rheumatoid arthritis patients. JCl i n Rheumatol 2012;18:393–8. [DOI] [PubMed] [Google Scholar]
- 21.Lin C, Lin SW, Weng SF, et al. Risk of sudden sensorineural hearing loss in patients with systemic lupus erythematosus: a population-based cohort study. Audiol Neurootol 2013;18:95–100. 10.1159/000345512 [DOI] [PubMed] [Google Scholar]
- 22.Yen YC, Lin YS, Weng SF, et al. Risk of sudden sensorineural hearing loss in patients with psoriasis: a retrospective cohort study. Am J Clin Dermatol 2015;16:213–20. 10.1007/s40257-015-0117-9 [DOI] [PubMed] [Google Scholar]
- 23.Halligan CS, Bauch CD, Brey RH, et al. Hearing loss in rheumatoid arthritis. Laryngoscope 2006;116:2044–9. 10.1097/01.mlg.0000241365.54017.32 [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Hamada K, Nakayama T, et al. Risk for cardiovascular disease in Japanese patients with rheumatoid arthritis: a large-scale epidemiological study using a healthcare database. Springerplus 2016;5 10.1186/s40064-016-2800-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost 2009;7(Suppl 1):328–31. 10.1111/j.1538-7836.2009.03416.x [DOI] [PubMed] [Google Scholar]
- 26.Evans P, Halliwell B. Free radicals and hearing. cause, consequence, and criteria. Ann N Y Acad Sci 1999;884:19–40. 10.1111/j.1749-6632.1999.tb08633.x [DOI] [PubMed] [Google Scholar]
- 27.Semb AG, Kvien TK, Aastveit AH, et al. Lipids, myocardial infarction and ischaemic stroke in patients with rheumatoid arthritis in the Apolipoprotein-related Mortality RISk (AMORIS) Study. Ann Rheum Dis 2010;69:1996–2001. 10.1136/ard.2009.126128 [DOI] [PubMed] [Google Scholar]
- 28.Boyer JF, Gourraud PA, Cantagrel A, et al. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint Bone Spine 2011;78:179–83. 10.1016/j.jbspin.2010.07.016 [DOI] [PubMed] [Google Scholar]
- 29.Protogerou AD, Panagiotakos DB, Zampeli E, et al. Arterial hypertension assessed "out-of-office" in a contemporary cohort of rheumatoid arthritis patients free of cardiovascular disease is characterized by high prevalence, low awareness, poor control and increased vascular damage-associated "white coat" phenomenon. Arthritis Res Ther 2013;15:R142 10.1186/ar4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung CP, Oeser A, Solus JF, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum 2008;58:2105–12. 10.1002/art.23600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giles JT, Danielides S, Szklo M, et al. Insulin resistance in rheumatoid arthritis: disease-related indicators and associations with the presence and progression of subclinical atherosclerosis. Arthritis Rheumatol 2015;67:626–36. 10.1002/art.38986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dessein PH, Joffe BI, Stanwix AE. Inflammation, insulin resistance, and aberrant lipid metabolism as cardiovascular risk factors in rheumatoid arthritis. J Rheumatol 2003;30:1403–5. [PubMed] [Google Scholar]
- 33.Kavanaugh A. Dyslipoproteinaemia in a subset of patients with rheumatoid arthritis. Ann Rheum Dis 1994;53:551–2. 10.1136/ard.53.8.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kochi M, Kohagura K, Shiohira Y, et al. Inflammation as a risk of developing chronic kidney disease in rheumatoid arthritis. PLoS One 2016;11:e0160225 10.1371/journal.pone.0160225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu HY, Huang HL, Li CH, et al. Increased risk of chronic kidney disease in rheumatoid arthritis associated with cardiovascular complications - a national population-based cohort study. PLoS One 2015;10:e0136508 10.1371/journal.pone.0136508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan XF, Gu JQ, Shan ZY. Increased risk of thyroid autoimmunity in rheumatoid arthritis: a systematic review and meta-analysis. Endocrine 2015;50:79–86. 10.1007/s12020-015-0533-x [DOI] [PubMed] [Google Scholar]
- 37.Joshi P, Agarwal A, Vyas S, et al. Prevalence of hypothyroidism in rheumatoid arthritis and its correlation with disease activity. Trop Doct 2017;47:6–10. 10.1177/0049475515627235 [DOI] [PubMed] [Google Scholar]
- 38.Bourji K, Gatto M, Cozzi F, et al. Rheumatic and autoimmune thyroid disorders: a causal or casual relationship? Autoimmun Rev 2015;14:57–63. 10.1016/j.autrev.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 39.Goodall AF, Siddiq MA. Current understanding of the pathogenesis of autoimmune inner ear disease: a review. Clin Otolaryngol 2015;40:412–9. 10.1111/coa.12432 [DOI] [PubMed] [Google Scholar]
- 40.Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med 2008;121:S21–S31. 10.1016/j.amjmed.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 41.Susmano A, Rosenbush SW. Hearing loss and ischemic heart disease. Am J Otol 1988;9:403–8. [PubMed] [Google Scholar]
- 42.Samoriadova OS, Zharova EA, Masenko VP, et al. [The renin-angiotensin-aldosterone system and arterial hypertension in patients with rheumatoid arthritis]. Klin Med 1991;69:69–71. [PubMed] [Google Scholar]
- 43.Sakuta T, Morita Y, Satoh M, et al. Involvement of the renin-angiotensin system in the development of vascular damage in a rat model of arthritis: effect of angiotensin receptor blockers. Arthritis Rheum 2010;62:1319–28. 10.1002/art.27384 [DOI] [PubMed] [Google Scholar]
- 44.Dikici O, Muluk NB, Tosun AK, et al. Subjective audiological tests and transient evoked otoacoustic emissions in patients with rheumatoid arthritis: analysis of the factors affecting hearing levels. Eur Arch Otorhinolaryngol 2009;266:1719–26. 10.1007/s00405-009-0975-y [DOI] [PubMed] [Google Scholar]
- 45.Kastanioudakis I, Skevas A, Danielidis V, et al. Inner ear involvement in rheumatoid arthritis: a prospective clinical study. J Laryngol Otol 1995;109:713–8. 10.1017/S0022215100131135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.