Abstract
Introduction
The number of children and young people living with life-limiting and life-threatening conditions is rising. Providing high-quality, responsive healthcare for them and for their families presents a significant challenge. Their conditions are often complex and highly unpredictable. Palliative care is advocated for people with life-limiting and life-threatening conditions, but these services for children are highly variable in terms of availability and scope. Little is known about the lived experiences and preferences of children and their families in terms of the palliative care that they do, or do not, receive. This study aims to produce an in-depth insight into the experiences and preferences of such children and families in order to develop recommendations for the future provision of services. The study will be carried out in the West Midlands, UK.
Methods and analysis
A qualitative study comprising longitudinal interviews over a 12-month period with children (aged 5–18 years) living with life-limiting or life-threatening conditions and their family members. Data analysis will start with thematic analysis, followed by narrative and cross-case analysis to examine changing experiences and preferences over time, at the family level and within the wider healthcare system. Patient and public involvement (PPI) has informed the design and conduct of the study. Findings will be used to develop recommendations for an integrated model of palliative care for children in partnership with the patient and public involvement (PPI) group.
Ethics and dissemination
Ethical approval was granted in September 2016 by the National Health Service Health Research Authority (IRAS ID: 196816, REC reference: 16/WM/0272). Findings will be of immediate relevance to healthcare providers, policy-makers, commissioners and voluntary sector organisations in the UK and internationally. Reports will be prepared for these audiences, as well as for children and their families, alongside academic outputs.
Keywords: organisation of health services, paediatric palliative care, palliative care, qualitative research
Strengths and limitations of this study.
An in-depth, contextual, longitudinal qualitative study with multiple child and family member stories captured over time.
New insights will be provided because all of the children and families included in the study could benefit from palliative care as it is currently defined, however, not all will have had conversations about this or have been referred to specialist palliative care services. Findings will focus on healthcare, but there is a wider applicability and relevance to social care and joint planning of services.
A diverse study population in terms of age, clinical condition, cultural background and family structure will allow detailed consideration of the role of healthcare services in effectively recognising and supporting children and families with their individual needs. However, all will speak English.
Neonates, preschool children and young people at transition (over the age of 18 years) are all excluded and warrant research in their own right.
There are multiple potential sources of bias which will be addressed throughout the study, including recruitment bias and the unconscious bias of the researcher.
Introduction
Children and young people with life-limiting conditions and life-threatening conditions represent a growing concern in healthcare.1 With advances in clinical practice, the number of children living with these conditions is rising.1–3 The nature of their conditions is complex and unpredictable; the risk of a sudden deterioration and death is an everyday reality for many. Family carers can experience enormous emotional, physical and financial pressures.4
Research suggests that families of children with life-limiting and life-threatening conditions wish for continuous and holistic healthcare, with the option that this is delivered in the home environment.4 However, most children who die have a pre-existing life-limiting condition,5 and most die in hospital,6 7 most frequently in an intensive care environment, where the mode of death is often withdrawal or limitation of life-sustaining treatments.8–10 The length of stay in the intensive care unit before death is increasing and the costs of hospital care at the end of life are significant.11 12 This situation presents complex clinical and ethical dilemmas at individual, organisational and societal levels.
Palliative care is an approach to care, which is advocated for all people who live with a life-limiting or life-threatening condition. Current definitions of palliative care are broad (box), which can cause difficulties and lack of clarity for those designing and commissioning specific services.
Box. Children’s palliative care definitions15 62 .
Palliative care for children with life-threatening conditions is defined by WHO as ‘a special, albeit closely related field to adult palliative care; the principles apply to other paediatric chronic disorders:
The active total care of the child’s body, mind and spirit, and support to the family.
It begins when illness is diagnosed, and continues regardless of whether or not a child receives treatment directed at the disease.
Health providers must evaluate and alleviate a child’s physical, psychological and social distress.
Effective palliative care requires a broad multidisciplinary approach that includes the family and makes use of available community resources; it can be successfully implemented even if resources are limited.
It can be provided in tertiary care facilities, in community health centres and even in children’s homes’.
The UK national charity for paediatric palliative care, Together for Short Lives, defines palliative care for children with life-limiting conditions as ‘an active and total approach to care, from the point of diagnosis or recognition, embracing physical, emotional, social and spiritual elements through to death and beyond. It focuses on enhancement of quality of life for the child and support for the family and includes the management of distressing symptoms, provision of short breaks and care through death and bereavement’.
For clarity, in this paper, children and young people will be referred to throughout as ‘children’.
Which children?
There has been a range of previous work concerned with the identification of clinical conditions where palliative care could be beneficial,13 14 and the following categorisation is provided by Together for Short Lives15:
Group 1: life-threatening conditions where access to palliative care services is necessary alongside attempts at curative treatment and/or if treatment fails, such as cancer.
Group 2: conditions such as Duchenne muscular dystrophy, where premature death is inevitable, but where there may be long periods where the child is well.
Group 3: progressive conditions without curative treatment options, such as Batten disease.
Group 4: irreversible but non-progressive conditions, with complex disabilities and healthcare needs which lead to increased likelihood of premature death, such as severe brain injury.
Organisational issues
Currently, there is a wide geographic variation in terms of paediatric palliative care services, and a poorly understood range of commissioning arrangements to support these services. Many services exist as a result of significant contributions from the voluntary sector (including children’s hospices), through the efforts of motivated individuals, and through non-recurring funding opportunities rather than the implementation of national policy.16 A significant development is the emergence of paediatric palliative medicine as a subspecialty of paediatrics.17 18
Effective palliative care services for children require strong partnerships between providers, and may require cross-boundary, collaborative commissioning between the statutory and voluntary sectors.19 In the UK, palliative care for children has specifically been included in national policy, a service specification for paediatric palliative care exists and NICE (National Institute for Health and Care Excellence) Guidelines for end-of-life care for infants, children and young people were published in 2016.20–24
Ontology, epistemology and theoretical perspective
Much of the evidence base that guides policy and practice in medicine is derived from experimental research grounded in a positivist paradigm, for example, randomised controlled trials, where a hypothesis can be generated and tested. The positivist approach does not lend itself to research which aims to investigate more complex interventions, such as palliative care, and an interpretive approach is more appropriate. The experience of healthcare services by children with a life-limiting or life-threatening condition and members of their family are shaped and influenced by many interlinked factors including their own personal experiences, values and cultural influences, the values of the healthcare team and the healthcare system, the specific context in which care is delivered and the relationships between those involved in providing and receiving care.25–27
The proposed research seeks to understand the mechanisms and influences that shape the experience of care in order to inform both the development and implementation of policy for palliative care services for young children with life-limiting conditions. The methodological approach identified as most appropriate for this research aim is realism, an approach which is increasingly used in healthcare and health sector management research.28–31 First described by Bhaskar in the 1970s,32 and subsequently by Pawson and Tilley 33realism seeks to understand how phenomena come about as a result of hidden mechanisms, enacted under certain circumstances.33 34 It acknowledges that there are a wide variety of dynamic contexts and mechanisms which can affect outcomes, including geographical and environmental factors, social and cultural issues and historical factors, and provides a generative approach allowing for the proposal of theories to guide the implementation of policy into practice.35
Rationale for research
Despite the range of recommendations for the provision and development of paediatric palliative care services, there remains a lack of research evidence to support the implementation of these guidelines.1 18 36–38 The proposed research seeks to address this gap using a realist approach to address research questions that correlate with the practical concerns associated with service delivery. The findings and theories that are generated will provide in-depth insights that will be of immediate relevance to clinicians, commissioners and policy makers, as well as to patients and their families.
Research questions
How do children with life-limiting and life-threatening conditions and their family members perceive healthcare services, and in particular ‘palliative care’?
What are the experiences and preferences of children living with a life-limiting or life-threatening condition and/or their families, in relation to the delivery of healthcare services?
What are the facilitators and barriers to the delivery of palliative care for children, and how might these be overcome?
What should an integrated model of palliative care for children look like?
Methods and analysis
In order to conduct an exploration of the experiences of healthcare from the perspective of children with life-limiting and life-threatening conditions and their families, we will adopt qualitative research methods and a narrative-based approach, suitable for complex, emotionally charged subject areas.39 40 Active listening, reflection, a flexible approach and insight into the narratives being co-constructed between participant and researcher will be necessary throughout.
This is the protocol for an in-depth longitudinal qualitative study using semistructured interviews with school-aged children (5–18 years) and one or two of their household family members.
Benefits of longitudinal studies include being able to describe the changing needs of the children and their families, and their experience of services, over time,41 and enabling rapport to build between researcher and participant.
Neonates, preschool children and young people aged over the age of 18 years are excluded from this study. Specific issues around healthcare services arise when considering neonatal care and young people who are making the transition from paediatric to adult services, both of which warrant research in their own right. Research methods would need to be tailored to interview preschool children; this is also an area for potential future research.
The research plan has been informed by review of relevant literature, patient and public involvement (PPI) work and advice from local experts via the West Midlands Paediatric Palliative Care Network.
Sampling and recruitment
Recruitment to a study of this nature depends on many factors, including the clinical condition of the child, conflicting demands on the family’s time and the motivation and understanding of their clinical teams. Recruitment began following ethical approval in October 2016 and will continue until January 2018.
The approach to participants is through:
direct invitation via their clinical team
leaflets and posters displayed in public areas in the hospital (such as notice boards on wards and in outpatients).
The research will be introduced to clinical teams in both the hospital and the community through formal presentations at departmental meetings and to individual clinicians at their request, as well as to the paediatric palliative care network. The researcher, SM, will undertake a period of shadowing with clinical teams, on hospital wards, in outpatient clinics and in the community.
Potential participants will be provided with a participant information sheet, with details of the researcher, the project, how to get involved and a contact telephone number and email address.
Inclusion and exclusion criteria are outlined in table 1. Our aim initially is to purposively sample children so that each of the four Together for Short Lives categories are represented. However, since children live with such individual and highly complex conditions, we anticipate that achieving this may be difficult. The study population will therefore be children with life-limiting or life-threatening conditions, aged from 5 to 18 years, and their family members, some of whom have experience of a palliative care service, and some who do not.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | 1. Children aged 5–18 years (school age) with a life-limiting or life-threatening condition who are under the care of the community children’s nursing team and/or the children’s hospital and who either:
|
| Exclusion criteria | 2. Their family members, who live in the same household.63
|
The study has been carefully designed to ensure that all of the children have the opportunity to participate and that wherever possible the views of the child are included, by tailoring each individual interview to their needs and capabilities (including the consent and agreement process). This may include having a learning disability or communication difficulties associated with their condition.
Ethical approval has been granted for the recruitment of 12–14 families to take part in a series of interviews (longitudinal interviews). The aim is to continue to conduct interviews until data saturation is achieved,42 however, given the uniqueness and individuality of the stories of children and families, it is possible that new themes will continue to emerge such that data saturation is impossible. We will aim for saturation of the main themes that emerge from the data, and identify emergent themes, which may form the basis of future research.
Interview plan
Interviews will be carried out by SM, a researcher who is also a general practitioner (GP) with advanced communication skills training and previous experience in qualitative interviewing. According to the preference of participants, interviews will be conducted with individuals, or with the child and parent together, in their preferred location. One or two family members will be interviewed in each family, either individually or together, depending on their preference and what is most convenient for them.
Interviews will be open and conversational, using a blended approach of interview techniques, with passive interviewing allowing the participant space and time to tell their story (narrative), and more active techniques, including appreciative inquiry, which asks ‘What works well?’ and ‘Why does it work well?’,4 43 employed. A topic guide (table 2) will guide the interview; this will continue to develop iteratively throughout the research, with adaptations made during each interview and in response to each individual participant.
Table 2.
Topic guide
| For all families | For those aware of ‘palliative care’ |
|
Introduction
Please tell me your story, in any way that you can/want to
Do you talk to other children/young people/families about your healthcare/services?
|
Palliative care and you (if appropriate)
Do you receive services from the hospice? Can you tell me how you came to receive palliative care/know the palliative care nursing team/the hospice?
|
Questions in bold are leading questions. Bulleted questions are prompts.
For interviews with children, a range of techniques will be used including depersonalising questions, developing a narrative in the third person, and using props and toys to encourage storytelling. Arts-based activities will be used, where appropriate, as a mutual point of focus for the researcher and participant, or as a focus of the interview, as in the draw–write–tell technique.44 PPI advice has been sought on the format of interviews for children (table 3).
Table 3.
Feedback from PPI groups on interview plans
| January 2016 | “Those who are passionate about improving palliative care will take part regardless of how sensitive this may be” |
| July 2016 | “Remember young people who are seriously ill are more mature, they have to grow up” “Keep it simple as often a child will openly speak anyway” “‘Do you talk about it to your friends?’ is a good question, a good way to talk to most ages” “Children are more eloquent, mature and more capable than you think” |
| October 2016 | “Use pictures and images, more emojis” |
| February 2017 | “Doesn’t make me uncomfortable as I think it is very important and relevant” |
PPI, patient and public involvement.
Each interview will be audiorecorded, with field notes made to include any additional comments from the child or family made once the audiorecording has stopped,45 reflections on the interview and observation of the family situation, environment, behaviour and any other interactions that may take place (for example, with other members of the family and clinical staff in hospital or on the phone).
Participants will be asked whether they would like to participate in interviews that will take place over a period of up to 12 months. These are intended to allow the identification of common themes over time and for theories generated through analysis of earlier interviews to be tested out during later interviews.41 The time intervals between interviews will be individually agreed, depending on the child and family circumstances. The method of communication with each family will also be individually agreed (phone or email). Up to three interviews with each participant are aimed for.
We anticipate practical challenges with conducting longitudinal interviews relating to fluctuations and changes in the clinical condition of each child. Depending on their condition, some children will respond well to treatments and get better. Others may suffer unexpected complications of their condition or treatment, and some may suffer deteriorations which bring about the possibility of dying. To manage the research in this context, we will check the family understanding of the situation before every interview. On occasions, interviews may need to be postponed and rearranged at late notice due to a change in circumstances.
For children who are unable to participate in interviews due to their condition, family members will be interviewed. Children and families are under no obligation to take part in follow-up interviews if they do not wish to. In these cases, and with their consent, data from previous interviews will still be included in the study.
Data analysis
Interviews will be transcribed verbatim, and NVivo used for data handling. Analysis of interview transcripts and field notes will commence alongside data collection, with an initial broad thematic analysis. All data will be coded, and codes grouped into broad overarching themes.
This initial analysis will be followed by an in-depth, narrative analysis, using structure form analysis to examine not just what is being said, but how it is being said, and to propose what works, for whom, in what circumstance at a micro (immediate clinical team), meso (local organisation) and macro (wider healthcare system) level perspective.33 46 47
The collection of longitudinal data allows for innovative approaches to be taken in data analysis.41 Matrices will be developed to identify key times for families and identification of cross-cutting themes at these times, for example, the time of diagnosis, an admission to intensive care or referral to a palliative care team.
Peer review and respondent validation will take place throughout the data analysis as follows48 49 :
Peer review: SM will code all of the data. A selection of transcripts will be reviewed and independently coded by other members of the research team in order to decrease lone researcher bias.48 The coding frameworks will be discussed and compared, allowing further development of categories and themes.
Respondent validation: by returning to participants to conduct longitudinal interviews, there is an opportunity to check, validate or refute emergent themes from the initial data analysis.
Healthcare professional perspectives
There are 12 paediatric palliative care networks in the UK, which include professionals from a range of organisations within paediatric palliative care. Several have patient and family representatives. Arrangements will be made to present study findings to four of the UK networks at existing meetings. The presentation will be followed by a structured focus group which will aim to first to test out and validate with palliative care professionals the themes from the research findings, and second aims to collect views of professionals. These multiple perspectives will inform and guide the formulation of recommendations for healthcare services in the future.50
An expression of interest email will be circulated to network chairs via Together for Short Lives, and arrangements made to attend meetings from networks who respond. Audiorecorded focus group discussions will be carried out at those meetings by SM.
Patient and public involvement
PPI has been integral to the design and conduct of the study. Members of existing groups at a children’s hospital and children’s hospice have provided advice on the study proposal and design. Smaller groups have been recruited for specific activities, including conference presentations. Group members range in age from 12 to 22 years. PPI activities are outlined in table 4, and will continue throughout the project, with the aim of coproducing the recommendations for the model of care. This will involve structured group sessions during which anonymised findings of the data analysis will be presented to the group for feedback and comment. A patient experience framework will be used to structure the discussion and to build recommendations.51
Table 4.
PPI activities
| Completed PPI activities |
|
| Work in progress |
|
| Future plans |
|
PPI, patient and public involvement.
Strengths and limitations
The strength of this study lies in the in-depth, contextual qualitative nature of the data, with multiple child and family member stories captured over time. Our anticipated study population is diverse in terms of age, clinical condition, cultural background and family structure, allowing detailed consideration of the role of healthcare services in effectively recognising and supporting children and families with their individual needs. All of the children and families included in the study could benefit from palliative care as it is currently defined,15 however, not all will have had conversations about this with their clinicians, or been referred to specialist palliative care services. Given the nature of their clinical conditions, including for some the inability to communicate verbally or deterioration in their health, recruitment and retention within the study is likely to become a challenge and will require a reflexive, flexible approach.
Potential limitations in the study include our exclusion of neonates, preschool children and young people at transition (over the age of 18 years). These groups all warrant research in their own right. Given the time and resource constraints of the study, all interviews will be carried out in English. Further research into the experiences of children and families who cannot communicate in English is necessary. There will be ongoing consideration of sources of bias. Recruitment bias is being addressed by aiming for a diverse sample and providing access to project information independent of the clinical teams. Data saturation will be sought during data analysis, with an ongoing process of reflection and peer review to address any possible unconscious bias of the researcher (SM).
Ethics and dissemination
Research with children raises ethical and legal considerations around recruitment, consent and data collection.52 53 In addition, research regarding palliative and end-of-life care can be emotionally demanding and distressing for those involved. There are also particular ethical issues to consider given the longitudinal nature of the study.54
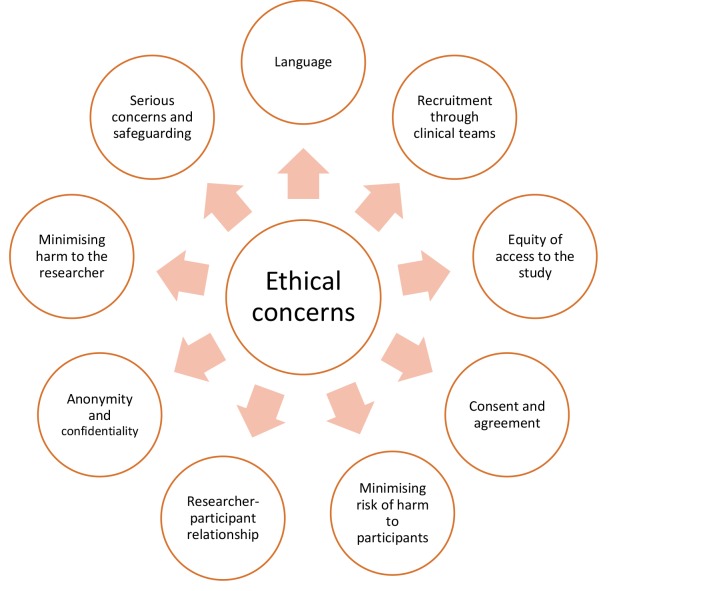
We are recruiting children and families who are potentially vulnerable and may be experiencing considerable distress. The justification for our approach is that children and their families in this situation are rarely asked about their experiences, but talking to them and understanding their experiences is essential in order to be able to design and develop services that respond to their actual needs. Here, we summarise our approach to the ethical issues the study raises (figure 1).
Figure 1.
Ethical issues in longitudinal qualitative research for children and families in palliative care.
Language
Published literature suggests that the term ‘palliative care’ is poorly understood and perceived negatively,55–59 a view confirmed by our PPI group. The scope of our study is therefore to investigate the experiences of children with ‘life-limiting’, ‘life-threatening’ and ‘conditions which may or may not get better’, whether or not they have heard of palliative care or receive care from specialist services. ‘Palliative care’ will be avoided in participant information sheets and interviews, unless individuals are already familiar with palliative care services or bring it up themselves.
Recruitment
There is an ethical challenge in terms of potential coercion to the study by clinicians who know the family well. Clinicians will therefore only provide study information but will not actively recruit families; the initial expression of interest is from the family to the researcher. The researcher (SM) will then discuss the study in person or by phone with the child and their family member(s) and answer any questions before arranging a time for interview. Participants will be made aware that they can decline to take part or to withdraw at any stage without having to give a reason. Interviews are only carried out at a time that is mutually agreed and minimises any potential inconvenience or intrusion.
Equity of access to the study
Recruitment through clinical teams is widely used in palliative care research but may be limited by ‘gatekeeping’.60 There may also be families who wish to participate who do not find out about the study through their clinical team. In order to address this, we have designed posters for display on hospital wards and in outpatients, and at the local children’s hospice, and a paragraph for organisational newsletters. These provide the direct contact details for the researcher (email, text or phone).
The study setting is Birmingham, UK, a city where the population is highly diverse in terms of family situation and multiculturalism. Over 50% of families with a child known to palliative care services in Birmingham and Solihull are from black or minority ethnic backgrounds.61 Many of these families speak English as a first or second language, so within the time and resource constraints of this study, interviews will be carried out with those who can provide informed consent and take part in an interview in English.
Consent
Consent for the study raises ethical and legal issues with children who are under the age of 16 years and/or do not have the capacity to consent. We will aim for written and/or verbal consent and agreement from every individual for every interview.
For children under the age of 16, written consent will be obtained from the parent and then verbal or written agreement obtained from the child.
In keeping with the Mental Capacity Act, there is an assumption of capacity in young people aged 16 years and over, so they will be asked for consent first, followed by agreement from their parent(s). Parental agreement is not a legal requirement, but conducting an interview with a young person about a potentially difficult subject without the knowledge or agreement of their parents is an ethical concern. If there is a concern that the young person lacks capacity or is considered particularly vulnerable, for example, with a learning disability, parents will be asked to provide verbal and written consent in addition to the young person’s agreement.
Parental consent is required for any interview to be carried out in the family home.53
For a child on a full care order, social worker consent would replace that of parental consent. Where possible parental consent/agreement will also be sought.
Interviews
Subject areas discussed during interviews may cause distress to participants, and recruitment may occur soon after sensitive conversations. We have designed the study to ensure that the risks and burden associated with taking part in the study are minimal.
Qualitative interviews will be informal and reflexive to the needs of the participant. In the event that a participant experiences any difficulties during the interview, such as tiredness or distress, the interview will be halted, and if necessary brought to an end. Adequate time will be given for debrief, and the researcher will provide information about local resources for support if necessary. Interviews will be carried out at a time and in a location that is convenient to the participants. If this is in hospital, the researcher (SM) will liaise closely with clinical teams so that the research does not interfere with routine clinical care and ward work.
Longitudinal interviews
Family views and understanding of what might happen next as a result of the condition of the child will be discussed sensitively, and any follow-up interviews scheduled around possible further treatments. If it seems likely that there will be a deterioration in the condition of the child, this is explored carefully and an agreement made with the individual family about whether they want to continue to participate in the study.
Anonymity and confidentiality
All interview data will be anonymised with personal identifiers removed. Any qualitative interview data that could identify child, families or any professionals involved in their care because of the individuality and context of the narrative is included in the data analysis, but will be excluded from reporting.
Field notes and anonymised interview transcripts will be stored securely on a password protected university hard drive.
Minimising harm to the researcher
There is a need for clearly defined boundaries for a researcher–participant relationship in a longitudinal study of this type. It will be made clear to participants at the time of consent that it is not the role of the researcher to provide personal support or clinical advice. With the risk of emotional distress for the researcher, plans to ensure adequate support through regular academic supervision and access to a counsellor are in place.
Serious concerns and safeguarding
If information contained in a participant’s response indicates a serious clinical or safeguarding concern or an issue which may jeopardise the safety of the participant or another person, this will be escalated appropriately in line with the protocols of the community or hospital trusts. This may on very rare occasions necessitate a breach of participant confidentiality in order to maintain their safety. Participants will be informed of any disclosure and to whom it is made.
Dissemination plan
The research is embedded in plans for impact. Table 5 outlines our planned outputs. We will work on traditional academic and clinical outputs, including manuscripts with the results of the study for publication in a peer-reviewed journal. Simultaneously work will be carried out with the PPI group to plan innovative, accessible outputs for patients, the public and commissioners which will include infographics and film based reports outlining our recommendations.
Table 5.
Planned outputs from the research
| Academic/clinical audiences | Patient, public and policy maker audiences |
|
|
Supplementary Material
Footnotes
Contributors: SM drafted the protocol with regular academic supervision from JD, A-MS and JC. The study was conceptualised by SM, JD, A-MS and JC, informed and guided by patient and public involvement. The protocol incorporates peer review feedback received during the application process for the NIHR Doctoral Research Fellowship, and the MPhil to PhD upgrade panel at the University of Warwick. JD, A-MS and JC have all reviewed the protocol for intellectual content. All authors have reviewed and agreed this version.
Funding: This work is supported by a National Institute of Health Research Doctoral Research Fellowship (Dr Sarah Mitchell DRF-2014-07-065).
Disclaimer: This article presents independent research funded in part by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethical approval was granted by the UK Health Research Authority on 14 September 2016 (IRAS ID: 196816, REC reference: 16/WM/0272, sponsor: University of Warwick).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fraser LK, Miller M, Hain R, et al. . Rising national prevalence of life-limiting conditions in children in England. Pediatrics 2012;129:e923–29. 10.1542/peds.2011-2846 [DOI] [PubMed] [Google Scholar]
- 2.Fraser LK, Parslow RC, McKinney PA, et al. . Life-limiting and life-threatening conditions in children and young people in the United Kingdom. 2012;129:1–7. [Google Scholar]
- 3. Office for National Statistics. Mortality statistics: deaths registered, 2007. [Google Scholar]
- 4.Coad J, Kaur J, Ashley N, et al. . Exploring the perceived met and unmet need of life-limited children, young people and families. J Pediatr Nurs 2015;30:45–53. 10.1016/j.pedn.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 5.Sidebotham P, Fraser J, Fleming P, et al. . Patterns of child death in England and Wales. Lancet 2014;384:904–14. 10.1016/S0140-6736(13)61090-9 [DOI] [PubMed] [Google Scholar]
- 6. Confidential Enquiry into Maternal and Child Health. Why children die: a pilot study. London: CEMACH, 2008. [Google Scholar]
- 7. Department of Health (2007). Palliative care statistics for children and young adults. London: Department of Health, 2007. [Google Scholar]
- 8.McCallum DE, Byrne P, Bruera E. How children die in hospital. J Pain Symptom Manage 2000;20:417–23. 10.1016/S0885-3924(00)00212-8 [DOI] [PubMed] [Google Scholar]
- 9.Ramnarayan P, Craig F, Petros A, et al. . Characteristics of deaths occurring in hospitalised children: changing trends. J Med Ethics 2007;33:255–60. 10.1136/jme.2005.015768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein D, Brill JE. A history of pediatric critical care medicine. Pediatr Res 2005;58:987–96. 10.1203/01.PDR.0000182822.16263.3D [DOI] [PubMed] [Google Scholar]
- 11.Widger K, Seow H, Rapoport A, et al. . Children’s end-of-life health care use and cost. Pediatrics 2017;139:e20162956 10.1542/peds.2016-2956 [DOI] [PubMed] [Google Scholar]
- 12.Plunkett A, Parslow RC. Is it taking longer to die in paediatric intensive care in England and Wales? Arch Dis Child 2016;101:798–802. 10.1136/archdischild-2015-309592 [DOI] [PubMed] [Google Scholar]
- 13.Hain R, Devins M, Hastings R, et al. . Paediatric palliative care: development and pilot study of a ‘Directory’ of life-limiting conditions. BMC Palliat Care 2013;12:43 10.1186/1472-684X-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noyes J, Edwards RT, Hastings RP, et al. . Evidence-based planning and costing palliative care services for children: novel multi-method epidemiological and economic exemplar. BMC Palliat Care 2013;12:18 10.1186/1472-684X-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Together for short lives. Children’s palliative care definitions. http://www.togetherforshortlives.org.uk/assets/0000/4089/CPC_definitions.pdf
- 16.MacNamara-Goodger K, Feudtner C. History and epidemiology : Goldman A, Hain R, Liben S, Oxford textbook of palliative care for children. 2nd edn Oxford: Oxford University Press, 2012. [Google Scholar]
- 17.Hain R, Heckford E, McCulloch R. Paediatric palliative medicine in the UK: past, present, future. Arch Dis Child 2012;97:381–4. 10.1136/archdischild-2011-300432 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell S, Morris A, Bennett K, et al. . Specialist paediatric palliative care services: what are the benefits? Arch Dis Child 2017;102:923–9. 10.1136/archdischild-2016-312026 [DOI] [PubMed] [Google Scholar]
- 19.Rowse V. Home-based palliative care for children: the case for funding. Paediatr Nurs 2006;18:20–4. 10.7748/paed.18.7.20.s22 [DOI] [PubMed] [Google Scholar]
- 20. National Institute for Health and Care Excellence (NICE). End of life care for infants, children and young people with life-limiting conditions: planning and management. https://www.nice.org.uk/guidance/ng61
- 21.Villanueva G, Murphy MS, Vickers D, et al. . End of life care for infants, children and young people with life limiting conditions: summary of NICE guidance. BMJ 2016;355:i6385 10.1136/bmj.i6385 [DOI] [PubMed] [Google Scholar]
- 22. Scottish Government. Strategic framework for action on palliative and end of life care. 2015. http://www.gov.scot/Topics/Health/Quality-Improvement-Performance/peolc/SFA
- 23. Welsh Government. End of life care delivery plan. 2014. http://gov.wales/topics/health/nhswales/plans/end-of-life-care/?lang=en
- 24. Department of Health, Social Services and Public Safety, Northern Ireland. Living matters, dying matters: a palliative and end of life care strategy for adults in Northern Ireland. www.dhsspsni.giv.uk/publications/living-matters-dying-matters-strategy-2010
- 25.Mason J. Qualitative Researching. London: SAGE Publishing, 2002. [Google Scholar]
- 26.Crotty M. The foundations of social research: meaning and perspective in the research process. Sydney: Allen & Unwin, 1998. [Google Scholar]
- 27.Ormston R, Spencer L, Barnard M. The foundations of qualitative research In: Ritchie J, Lewis J, McNaughton Nicholls C, Ormston R, et al eds Qualitative research practice, 2013. [Google Scholar]
- 28.Schiller CJ. Critical realism in nursing: an emerging approach. Nurs Philos 2016;17:88–102. 10.1111/nup.12107 [DOI] [PubMed] [Google Scholar]
- 29.Walsh D, Evans K. Critical realism: an important theoretical perspective for midwifery research. Midwifery 2014;30:e1–e6. 10.1016/j.midw.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 30.Connelly J. A realistic theory of health sector management. The case for critical realism. J Manag Med 2000;14:262–71. [DOI] [PubMed] [Google Scholar]
- 31.Ragin C, Becker H. What is a case?Exploring the foundations of social inquiry. Cambridge University Press, 1992. [Google Scholar]
- 32.Bhaskar R. A realist theory of science. Brighton: Harvester, 1975. [Google Scholar]
- 33.Pawson R, Tilley N. Realistic evaluation. London: Sage Publications, 1997. [Google Scholar]
- 34.Clark AM, MacIntyre PD, Cruickshank J. A critical realist approach to understanding and evaluating heart health programmes. Health 2007;11:513–39. 10.1177/1363459307080876 [DOI] [PubMed] [Google Scholar]
- 35.Sayer A. Realism and social science. London: Sage Publications, 2000. [Google Scholar]
- 36.Hain RD. Palliative care in children in Wales: a study of provision and need. Palliat Med 2005;19:137–42. 10.1191/0269216305pm967oa [DOI] [PubMed] [Google Scholar]
- 37.Hain RW. Palliative care in children: Who needs it, and why? Acta Paediatrica. 100, 2011:4. [Google Scholar]
- 38.Baker JN, Levine DR, Hinds PS, et al. . Research priorities in pediatric palliative care. J Pediatr 2015;167:467–70. 10.1016/j.jpeds.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akard TF, Gilmer MJ, Friedman DL, et al. . From qualitative work to intervention development in pediatric oncology palliative care research. J Pediatr Oncol Nurs 2013;30:153–60. 10.1177/1043454213487434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kendall M, Harris F, Boyd K, et al. . Key challenges and ways forward in researching the “good death”: qualitative in-depth interview and focus group study. BMJ 2007;334:521 10.1136/bmj.39097.582639.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray SA, Kendall M, Carduff E, et al. . Use of serial qualitative interviews to understand patients’ evolving experiences and needs. BMJ 2009;339:b3702 10.1136/bmj.b3702 [DOI] [PubMed] [Google Scholar]
- 42.Gill P, Stewart K, Treasure E, et al. . Methods of data collection in qualitative research: interviews and focus groups. Br Dent J 2008;204:291–5. 10.1038/bdj.2008.192 [DOI] [PubMed] [Google Scholar]
- 43.Carter B, Coad J, Goodenough T. Department of Health. Community Children’s Nursing in England: An appreciativereview of CCNs, 2009. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_1248-98
- 44.Greig A, Taylor J, MacKay T. Doing research with children. A practical guide. London: SAGE, 2008:113–20. [Google Scholar]
- 45.Coad J, Gibson F, Horstman M, et al. . Be my guest! Challenges and practical solutions of undertaking interviews with children in the home setting. J Child Health Care 2015;19:432–43. 10.1177/1367493514527653 [DOI] [PubMed] [Google Scholar]
- 46.Lieblich A, Tuval-Mashiach R, Zilber T. Narrative research: reading, analysis and interpretation. London: Sage, 1998. [Google Scholar]
- 47.Bingley AF, Thomas C, Brown J, et al. . Developing narrative research in supportive and palliative care: the focus on illness narratives. Palliat Med 2008;22:653–8. 10.1177/0269216308089842 [DOI] [PubMed] [Google Scholar]
- 48.Burnard P, Gill P, Stewart K, et al. . Analysing and presenting qualitative data. Br Dent J 2008;204:429–32. 10.1038/sj.bdj.2008.292 [DOI] [PubMed] [Google Scholar]
- 49.Burnard P. A method of analysing interview transcripts in qualitative research. Nurse Educ Today 1991;11:461–6. 10.1016/0260-6917(91)90009-Y [DOI] [PubMed] [Google Scholar]
- 50.Kendall M, Murray SA, Carduff E, et al. . Use of multiperspective qualitative interviews to understand patients’ and carers’ beliefs, experiences, and needs. BMJ 2009;339:b4122 10.1136/bmj.b4122 [DOI] [PubMed] [Google Scholar]
- 51.Staniszewska S, Boardman F, Gunn L, et al. . The warwick patient experiences framework: patient-based evidence in clinical guidelines. Int J Qual Health Care 2014;26:151–7. 10.1093/intqhc/mzu003 [DOI] [PubMed] [Google Scholar]
- 52. Medical Research Council. MRC Ethics Guide: Medical research involving children. London: Medical Research Council, 2004. www.mrc.ac.uk [Google Scholar]
- 53.Shaw C, Brady L-M, Davey C. Guidelines for research with children and young people. London: National Children’s Bureau, 2011. [Google Scholar]
- 54.Calman L, Brunton L, Molassiotis A. Developing longitudinal qualitative designs: lessons learned and recommendations for health services research. BMC Med Res Methodol 2013;13:1–10. 10.1186/1471-2288-13-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hui D, De La Cruz M, Mori M, et al. . Concepts and definitions for ‘supportive care’ ‘best supportive care’ ‘palliative care’ and ‘hospice care’ in the published literature, dictionaries, and textbooks. Support Care Cancer 2013;21:659–85. 10.1007/s00520-012-1564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pastrana T, Jünger S, Ostgathe C, et al. . A matter of definition–key elements identified in a discourse analysis of definitions of palliative care. Palliat Med 2008;22:222–32. 10.1177/0269216308089803 [DOI] [PubMed] [Google Scholar]
- 57.Twamley K, Craig F, Kelly P, et al. . Underlying barriers to referral to paediatric palliative care services: knowledge and attitudes of health care professionals in a paediatric tertiary care centre in the United Kingdom. J Child Health Care 2014;18:19–30. 10.1177/1367493512468363 [DOI] [PubMed] [Google Scholar]
- 58.Monterosso L, Kristjanson LJ. Supportive and palliative care needs of families of children who die from cancer: an Australian study. Palliat Med 2008;22:59–69. 10.1177/0269216307084608 [DOI] [PubMed] [Google Scholar]
- 59.Morstad Boldt A, Yusuf F, Himelstein BP. Perceptions of the term palliative care. J Palliat Med 2006;9:1128–36. 10.1089/jpm.2006.9.1128 [DOI] [PubMed] [Google Scholar]
- 60.Crocker JC, Beecham E, Kelly P, et al. . Inviting parents to take part in paediatric palliative care research: a mixed-methods examination of selection bias. Palliat Med 2015;29:231–40. 10.1177/0269216314560803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunt A, Coad J, West E, et al. . The big study for life-limited children and their families: final research report. Bristol: Together for Short Lives, 2013. [Google Scholar]
- 62. World Health Organisation. Cancer: WHO definition of palliative care. http://www.who.int/cancer/palliative/definition/en/
- 63. Office for National Statistics. 2011 census glossary of terms office for National Statistics, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.