Abstract
Please cite this paper as: Gonzales et al. (2012) Rate of introduction of a low pathogenic avian influenza virus infection in different poultry production sectors in the Netherlands. Influenza and Other Respiratory Viruses DOI: 10.1111/j.1750‐2659.2012.00348.x.
Background Targeted risk‐based surveillance of poultry types (PT) with different risks of introduction of low pathogenic avian influenza virus (LPAIv) infection may improve the sensitivity of surveillance.
Objective To quantify the rate of introduction of LPAIv infections in different PT.
Methods Data from the Dutch LPAIv surveillance programme (2007–2010) were analysed using a generalised linear mixed and spatial model.
Results Outdoor‐layer, turkey, duck‐breeder and meat‐duck, farms had a 11, 8, 24 and 13 times higher rate of introduction of LPAIv than indoor‐layer farms, respectively.
Conclusion Differences in the rate of introduction of LPAIv could be used to (re)design a targeted risk‐based surveillance programme.
Keywords: Avian Influenza, low pathogenic avian influenza, outdoor farming, poultry, relative risk
Introduction
Low pathogenic avian influenza virus (LPAIv) infections in poultry with H7 and H5 LPAIv subtypes are notifiable to the OIE. Hence, member states (MS) of the European Union (EU) have implemented surveillance programmes. 1 Guidelines for the implementation of these programmes recommend risk‐based sampling of the different poultry production sectors (layer chickens, broilers, ducks, turkeys, etc.) by suggested differences in the risk of introduction of a LPAIv infection (see Gonzales et al. 2 and references therein). However, quantitative information regarding the possible differences in risk between these poultry sectors [here referred to as poultry types (PT)] is sparse. Such information is important to optimise the design of risk‐based surveillance 3 and carry out quantitative evaluations of these programmes using for example scenario tree models. 3
In a previous study, 2 we observed a significantly higher risk of introduction of LPAIv infections on farms housing Anseriformes PT (duck, geese and game birds) compared with farms housing Galliformes PT (chicken breeders, broilers, layer chickens and turkeys), and no significant differences were observed among Galliformes PT. However, information on different farming systems such as outdoor (free‐range) or indoor farming of Galliformes PT, which could be an important risk factor, was not available.
Approximately 95% of the poultry farms in the Netherlands are chicken farms. These comprise of breeder farms (≈18%), broilers farms (≈31%) and indoor (≈35%)‐ and outdoor (≈10%)‐layer chicken farms. 4 The latter are expected to have a higher risk of infection with LPAIv, because outdoor farming is suggested to be a risk factor for the transmission of LPAIv from wild birds to poultry. 5 , 6 The objective of this study was to quantify the rate of introduction of a LPAIv infection for different PT in the Netherlands.
Methods
The Dutch LPAIv surveillance programme has been described elsewhere. 2 , 7 Briefly, all poultry farms, with the exception of outdoor‐layer farms, should be tested at least once a year. Outdoor‐layer farms are tested three to four times per year. 7 Surveillance data collected from 2007 to July 2010 was analysed. Farms were identified by their unique farm number (UBN) and PT [duck breeders, duck meat (meat production), turkeys, broilers, indoor‐layers, outdoor‐layers, pullets and broiler breeders]. Based on the sampling frequency (time interval between samplings), the time at risk of exposure to a LPAIv infection (“time at risk”) was calculated per PT. For PTs sampled once a year or once per production cycle, the age of the birds at the time of sampling was taken as the time at risk. For PTs sampled more than once per production cycle, the average sampling interval was taken as the time at risk. Positive cases were defined as follows: (i) farms with at least one seropositive animal – to any LPAIv strain – in both the screening test (IDEXX FLockCheck AI MultiS‐Screen or agar gel precipitation, which is only used for broilers) and the confirmatory test (haemagglutination inhibition test) or (ii) three or more positives in the screening test. Furthermore, only primary cases (excluding secondary spread detected by epidemiological tracing) were included.
A generalised linear mixed model (GLMM) with a binomial error distribution and a cloglog link was used for the analysis. Assuming that β is the rate of introduction of infection onto a farm of a specific PT, then the probability of infection p in a given time interval t is 1−exp(−βt). Following this reasoning, we modelled p as p ij = 1−exp(−βj t ij y), which upon linearisation gives log(−log (1−p ij)) = log βj + log t ij + log y. In this model, the status of farm i of poultry type j (p ij) is the binary response variable, log βj is the regression coefficient of the explanatory variable PT, “time at risk” in months (log t ij) is the offset, and logy (year of surveillance) is the grouping variable (random effect). Indoor‐layer chickens were the reference category; therefore, the exponent of the model intercept log β0 represents the rate of introduction of LPAIv onto indoor‐layer chicken farms per month. For a different PT, this rate is the exponent of the sum of log β0 and the corresponding regression coefficient log βj. The exponent of log βj of each PT was interpreted as the relative risk (RR) of introduction of LPAIv. The fit of the model was assessed by residual analysis. The GLMM was performed using the library lme4 of the statistical software R. 8
Because of lack of positive results, broiler data could not be modelled with a GLMM. Instead, we estimated the 95% (one‐sided) Fisher’s exact confidence interval (CI) of the rate of introduction.
To identify risks associated with the geographical location of the farms, we carried out a spatial analysis using a spatial scan statistic to assess the presence of geographical clusters of LPAIv‐infected farms under the Bernoulli probability model assumption. 9 This analysis was carried out using the software SatScan version 9.1.1. 10
Results
The surveillance results are summarised in Table 1. Almost all seropositive results appeared to be single introductions. Exceptions were the following: (i) six positive turkey farms detected in 2007, which were positive to LPAIv of H1N5 subtype and (ii) three seropositive layer farms (one indoor‐ and two outdoor‐layers) detected in 2010, which were positive to LPAIv of H6N1 subtype. Five of the seropositive turkey farms and two of the seropositive layer farms (one indoor and one outdoor) were secondary cases linked to a primary outbreak. We excluded these secondary cases from the statistical analysis.
Table 1.
Total number of poultry farms and total number of samplings (one farm per sampling) taken from 2007 to July 2010 in the Netherlands. Farms are categorised by poultry type, in addition the average frequency of sampling, the average time at risk (in months) of exposure to infection and the total number of seropositive detections is given
| Year | Poultry type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Duck breeders | Duck meat | Turkeys | Indoor‐layers | Outdoor‐layers | Pullets | Broiler breeders | Broilers | Total | |
| 2007 | |||||||||
| Farms* | 12 | 44 | 87 | 802 | 272 | 261 | 256 | 719 | 2453 |
| No of samplings | 19 | 46 | 300 | 1057 | 652 | 261 | 256 | 811 | 3402 |
| Frequency | 1·6 | 1·0 | 3·4 | 1·3 | 2·4 | 1·0 | 1·0 | 1·1 | 1·4 |
| Time_risk | 9·8 | 1·2 | 3·7 | 10·4 | 6·3 | 3·7 | 8·9 | 1·2 | |
| Positive | 2 | 0 | 6** | 0 | 3 | 1 | 0 | 0 | 12 |
| 2008 | |||||||||
| Farms | 12 | 42 | 70 | 714 | 295 | 250 | 249 | 775 | 2407 |
| No of samplings | 22 | 45 | 248 | 952 | 830 | 250 | 249 | 908 | 3504 |
| Frequency | 1·8 | 1·1 | 3·5 | 1·3 | 2·8 | 1·0 | 1·0 | 1·2 | 1·5 |
| Time_risk | 8·8 | 1·2 | 3·7 | 10·3 | 5·2 | 3·7 | 8·9 | 1·2 | |
| Positive | 1 | 1 | 0 | 1 | 4 | 0 | 1 | 0 | 8 |
| 2009 | |||||||||
| Farms | 13 | 56 | 68 | 678 | 286 | 239 | 240 | 808 | 2388 |
| No of samplings | 13 | 62 | 210 | 841 | 796 | 239 | 240 | 899 | 3300 |
| Frequency | 1·0 | 1·1 | 3·1 | 1·2 | 2·8 | 1·0 | 1·0 | 1·1 | 1·4 |
| Time_risk | 10·3 | 1·2 | 3·7 | 10·9 | 5·6 | 3·7 | 8·9 | 1·2 | |
| Positive | 0 | 0 | 1 | 2 | 7 | 0 | 0 | 0 | 10 |
| 2010 | |||||||||
| Farms | 9 | 27 | 60 | 351 | 227 | 231 | 236 | 547 | 1688 |
| No of samplings | 11 | 27 | 115 | 408 | 444 | 231 | 236 | 570 | 2042 |
| Frequency | 1·2 | 1·0 | 1·9 | 1·2 | 2·0 | 1·0 | 1·0 | 1·0 | 1·2 |
| Time_risk | 5·6 | 1·2 | 3·7 | 5·6 | 3·6 | 3·7 | 8·9 | 1·2 | |
| Positive | 0 | 0 | 1 | 6*** | 9*** | 0 | 0 | 0 | 16 |
*Farm population each year of surveillance. All farms in the Netherlands were sampled at least once each year.
**These farms were all infected with Low pathogenic avian influenza virus (LPAIv) H1N5. Five of these farms were secondary cases, which were removed for the statistical analysis.
***One indoor‐layer and two outdoor‐layer farms were infected with LPAIv H6N1. Two of these (one indoor‐ and one outdoor‐layer farm) were secondary cases and were removed from the statistical analysis.
No significant (P > 0·17) geographical clusters were found in the spatial analysis (Figure 1). The rate of introduction of LPAIv onto indoor‐layer farms was 3·5 × 10−4 per month. The rate of introduction of the LPAIv infection onto outdoor‐layer, duck breeder, duck meat and turkey farms was 11, 24, 13 and 8 times – significantly (P < 0·05) – higher than onto indoor‐layer farms, respectively (Table 2). No significant differences were observed between the relative risk (RR) of introduction onto chicken breeders and pullet farms compared with indoor‐layer farms. The CI of the rate of introduction of LPAIv onto broiler farms indicated no significant difference with the rate estimated for indoor layers (Table 2).
Figure 1.
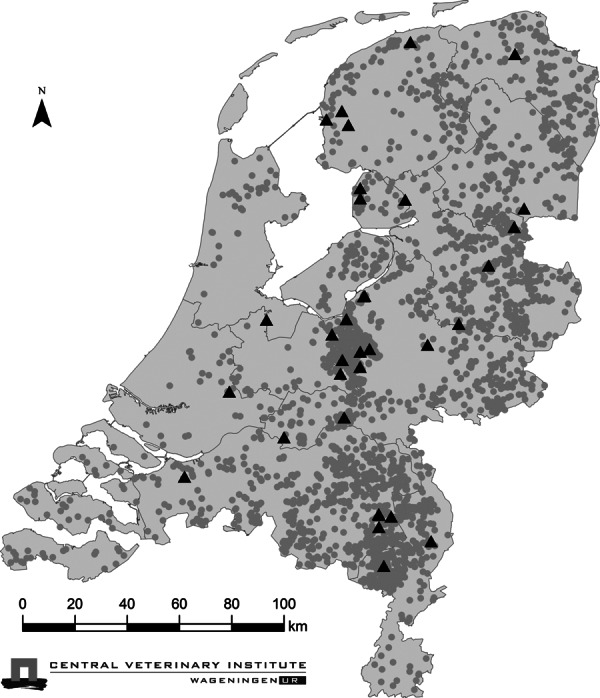
Location of poultry farms in the Netherlands. Poultry farms with birds sampled that were serologically positive to Low pathogenic avian influenza virus in the study period of 2007 to July 2010 are shown as black triangles (only primary introductions), and negative farms are shown as smaller grey circles. No significant (P > 0·17) spatial clustering was detected.
Table 2.
Rate and Relative Risk (RR), with accompanying 95% confidence interval, of introduction of a Low pathogenic avian influenza virus infection onto poultry farms. Indoor‐layer farms were considered as the reference category
| Poultry type | Rate/month* | RR** | ||||
|---|---|---|---|---|---|---|
| Mean | LCL*** | UCL*** | Mean | LCL | UCL | |
| Broiler breeders | 1·0 × 10−4 | 1·4 × 10−5 | 7·8 × 10−4 | 0·3 | 0·0 | 2·4 |
| Pullets | 2·5 × 10−4 | 3·2 × 10−5 | 1·9 × 10−3 | 0·7 | 0·1 | 5·7 |
| Indoor‐layers | 3·5 × 10−4 | 1·5 × 10−4 | 8·1 × 10−4 | 1·0 | ||
| Outdoor‐layers | 3·9 × 10−3 | 2·1 × 10−3 | 7·4 × 10−3 | 11·1 | 4·9 | 25·2 |
| Turkeys | 2·7 × 10−3 | 7·9 × 10−4 | 9·3 × 10−3 | 7·7 | 2·0 | 29·3 |
| Duck meat | 4·5 × 10−3 | 5·9 × 10−4 | 3·4 × 10−2 | 12·8 | 1·6 | 103·6 |
| Duck breeders | 8·6 × 10−3 | 2·5 × 10−3 | 3·0 × 10−2 | 24·5 | 6·4 | 94·1 |
| Broilers† | 0·0 | 0·0 | 8·1 × 10−4 | 0·0 | ||
*Limits of the confidence interval. Lower confidence limit (LCL) and upper confidence limit (UCL).
**The exponent of the model intercept β0 is the rate of introduction onto Indoor‐layers. This rate for a different PT, say Broiler breeders = exp(β0 + βbreeders) and the variance (var) = exp [var (β0) + var (βbreeders) + 2cov (β0βbreeders)]. Confidence intervals were estimated using the normal approximation = mean ± 1·96*sqrt (var).
***The RR was obtained by exponentiation of the model parameters. E.g. the RR for breeders = exp (βbreeders). Confidence intervals were obtained by the normal approximation using the estimated variance of βbreeders.
†One‐sided 95% confidence interval of the rate of introduction per month were estimated. Here the number of farms (samplings) times the months at risk (3719 farm‐months at risk) in the study period (2007–July 2010) was taken as the denominator.
Discussion
Our analysis shows that outdoor‐layer farms, duck (breeders and meat) farms and turkey farms have a significantly higher risk of introduction of the LPAIv infection compared with indoor‐layer farms. Duck breeders have the highest risk. This could be related to (i) their higher susceptibility to infection with LPAIv of wild‐bird origin (ducks, geese and swans) than chickens, 11 (ii) their long production cycle (time of exposure) and (iii) their higher exposure to LPAIv from a contaminated environment and/or contact with wild waterfowl. The latter could also be the reason for the higher risk observed in outdoor‐layer than indoor‐layer farms.
In the Netherlands, turkeys are raised indoors and despite the small population of turkey farms, we observed a higher risk of introduction of the LPAIv infection to turkeys than indoor layers. This higher risk might be partly associated with the apparent higher susceptibility of turkeys to LPAIv infections than chickens. 12 We also observed a significantly higher risk of introduction onto duck meat farms. This was surprising because this PT is kept indoors and has a short production cycle (6 weeks). Higher susceptibility of ducks than chickens to LPAIv infections could be one reason for the observed higher risk. 11 On the other hand, there also might be flaws in biosecurity on indoor poultry farms enabling introduction. Future research should focus on unravelling the mechanisms of introduction of LPAIv on farms that house their birds inside.
The risk of introduction of LPAIv onto broiler breeder and pullet farms appeared to be low but was not significantly different from indoor‐layer farms. For broiler farms, not a single introduction of the LPAIv infection was detected in the study period. This suggests that this PT has a low risk of introduction. However, because of the short lifespan of broilers, combined with testing only once a year, the rate of introduction onto broiler farms was not significantly different from indoor‐layer farms.
The estimated rates of introduction of LPAIv in each PT provide parameters for risk analysis and evaluation of surveillance programmes. 3 However, it should be noted that seasonal differences could be expected. 5 We did not include season in this study because some PTs such as indoor‐layers and broiler breeders, whose production cycle is longer than a year, were sampled only once, and in most seropositive cases, no virus was isolated.
If a LPAIv of an H5 or H7 subtype infects a farm and later spreads to other farms before detection, the risk of mutation to a highly pathogenic virus would be increased. 13 Hence, frequent sampling of high risk PTs may contribute to reduce the risk of transmission between farms. Our study supports the need of sampling outdoor layers and turkeys with a higher frequency than indoor layers, which is currently carried out in the Netherlands. However, despite the higher sampling frequency, secondary spread may occur. Simulation models could be used to optimise the current surveillance programme. 14
Acknowledgements
We gratefully acknowledge the Dutch Animal Health Service (GD Deventer) for providing the surveillance data (screening tests). We thank Eric de Kluijver (Central Veterinary Institute part of WUR) for providing the results of the confirmatory tests. This study was supported by the Foundation for Economic Structure Strengthening (FES), in the Netherlands: FES programme on Avian Influenza.
References
- 1. European Commission . Commission Decision 2007/268/EC of 13 April 2007 on the implementation of surveillance programmes for avian influenza in poultry and wild birds to be carried out in the Member States and amending Decision 2004/450/EC. OJEU 2007; 115:2003. [Google Scholar]
- 2. Gonzales JL, Elbers ARW, Bouma A, Koch G, de Wit JJ, Stegeman JA. Low‐pathogenic notifiable avian influenza serosurveillance and the risk of infection in poultry – a critical review of the European Union active surveillance programme (2005–2007). Influenza Other Respir Viruses 2010; 4:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin PAJ, Cameron AR, Greiner M. Demonstrating freedom from disease using multiple complex data sources: 1: a new methodology based on scenario trees. Prev Vet Med 2007; 79:71–97. [DOI] [PubMed] [Google Scholar]
- 4. PVE . Livestock, Meat and Eggs in the Netherlands. Zoetermeer: Product Board for Livestock, Meat and Eggs, 2011. Available at http://www.pve.nl (Accessed 15 August 2011). [Google Scholar]
- 5. Halvorson DA, Kelleher CJ, Senne DA. Epizootiology of avian influenza: effect of season on incidence in sentinel ducks and domestic turkeys in Minnesota. Appl Environ Microbiol 1985; 49:914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koch G, Elbers ARW. Outdoor ranging of poultry: a major risk factor for the introduction and development of High‐Pathogenicity Avian Influenza. NJAS 2006; 54:179–194. [Google Scholar]
- 7. European Commission . SANCO/10110/2008. Survey Programme for Avian Influenza in Poultry and Wild Birds: the Netherlands, Brussels: European Commission, 2008. Available at http://ec.europa.eu/food/animal/diseases/eradication/program2008/2008_vetprog_avian_influenza/2008_vetprog_avian_influenza_the_netherlands.pdf (accessed 30 July 2011). [Google Scholar]
- 8. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: Foundation for Statistical Computing; 2005. Available at http://www.r‐project.org (accessed 5 January 2011). [Google Scholar]
- 9. Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods 1997; 26:1481–1496. [Google Scholar]
- 10. Kulldorff M. SatScan. Software for the Spatial, Temporal, and Space‐Time Scan Statistics. Boston, USA: Information Management Services Inc., 2011. Available at http://www.satscan.org/techdoc.html (accessed 5 July 2011). [Google Scholar]
- 11. Mundt E, Gay L, Jones L, Saavedra G, Tompkins S, Tripp R. Replication and pathogenesis associated with H5N1, H5N2, and H5N3 low‐pathogenic avian influenza virus infection in chickens and ducks. Arch Virol 2009; 154:1241–1248. [DOI] [PubMed] [Google Scholar]
- 12. Tumpey TM, Kapczynski DR, Swayne DE. Comparative susceptibility of chickens and turkeys to avian influenza A H7N2 virus infection and protective efficacy of a commercial avian influenza H7N2 virus vaccine. Avian Dis 2004; 48:167–176. [DOI] [PubMed] [Google Scholar]
- 13. Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine 2007; 25:5637–5644. [DOI] [PubMed] [Google Scholar]
- 14. Graat EAM, de Jong MCM, Frankena K, Franken P. Modelling the effect of surveillance programmes on spread of bovine herpesvirus 1 between certified cattle herds. Vet Microbiol 2001; 79:193–208. [DOI] [PubMed] [Google Scholar]


