Abstract
Please cite this paper as: Gillard et al. (2012) An assessment of prime‐boost vaccination schedules with AS03A‐adjuvanted prepandemic H5N1 vaccines: a randomized study in European adults. Influenza and Other Respiratory Viruses DOI: 10.1111/j.1750‐2659.2012.00349.x.
Background Long‐term persistence of immune response and safety of an H5N1 prepandemic influenza vaccine adjuvanted with AS03 (an α‐tocopherol oil‐in‐water emulsion‐based adjuvant system) was evaluated using various prime‐boost schedules that mimicked potential pandemic scenarios (NCT00430521).
Methods Five hundred and twelve healthy adults aged 18–60 years received primary vaccination with one or two doses (0, 21 days schedule) of the A/Vietnam/1194/2004 H5N1 vaccine followed by a booster dose (A/Vietnam/1194/2004 or A/Indonesia/05/2005 strain) six or twelve months later across eight randomized groups. Immunogenicity results by hemagglutination inhibition [HI] assay, microneutralization assay, and the cell‐mediated immune response (CMI) are reported here for the four groups boosted at Month 12.
Results A one‐dose‐adjuvanted primary administration followed 12 months later by a single‐adjuvanted booster dose containing a heterologous vaccine strain met or exceeded all US and European criteria for both strains. Increasing the interval between the first and second dose (from 21 days to 12 months) resulted in stronger cross‐reactive immune responses against the A/Indonesia/05/2005 strain. The HI antibody response against the two strains persisted for 6 months after the booster dose irrespective of the booster vaccine’s strain. The neutralizing antibody responses and the CMI observed in the study population paralleled the HI immune response. Overall, the vaccine had a clinically acceptable safety profile.
Conclusion The H5N1 vaccine in this study allowed for flexibility in the time interval between primary and booster vaccination and the use of a heterologous strain without impacting the strength of the humoral and cellular immune response to both vaccine strains.
Keywords: Adjuvant, AS03, H5N1, influenza, prepandemic, prime‐boost
Introduction
Since its re‐emergence in 2003, the H5N1 avian influenza virus has caused sporadic cases in humans with higher morbidity and mortality rates than observed previously for influenza; 1 as per the latest update from the World Health Organization (WHO), as of March 07, 2011, 528 laboratory‐confirmed cases and 311 deaths because of H5N1 infection have been recorded worldwide. 2 Despite the emergence of the H1N1 2009 pandemic influenza virus, the threat posed by the H5N1 avian influenza virus persists and this highly pathogenic virus has been identified as one of the potential candidates to cause a future influenza pandemic. 3 , 4
Immunization is considered to be the best prophylactic method of mitigating influenza pandemic‐related morbidity and mortality. 5 , 6 In addition to acceptable safety and immunogenicity profiles, many vaccine developers regard cross‐clade protection (the ability to induce immune responses against strains genetically distinct from the strain used to make the antigen) and dose sparing (the ability to induce immune responses with only a small mass of antigen) as key aspects of pandemic influenza vaccine development. Both of these characteristics could be enhanced by adjuvants. 7 , 8 The Strategic Advisory Group of Experts (SAGE) of the World Health Organization (WHO) has also acknowledged the importance of influenza vaccines formulated with oil‐in‐water‐based adjuvants in parallel with un‐adjuvanted vaccines. 9
An inactivated, split‐virion recombinant H5N1 influenza [3·75 μg hemagglutinin (HA) with AS03 adjuvant system (an α‐tocopherol oil‐in‐water emulsion‐based adjuvant system)] developed by GlaxoSmithKline (GSK) Biologicals has been found to induce strong immune responses against both vaccine homologous as well as heterologous strains, with a clinically acceptable safety profile in different populations. 10 , 11 , 12 , 13
This study (NCT00430521) was conducted with the aim to further evaluate the immunogenicity and safety of this AS03‐adjuvanted H5N1 vaccine using various prime‐boost schedules in a set‐up that mimicked potential pandemic scenario. Healthy adults aged 18–60 years received primary vaccination with one or two doses of the H5N1 vaccine following different vaccination schedules followed by a booster dose six or twelve months later. The immunogenicity data from subjects who received a booster dose at Month 6 have been presented previously. 14
The results presented in this manuscript focus on the study objectives to evaluate the humoral immune response in terms of hemagglutination inhibition (HI) antibody titers, neutralizing antibody titers, and cell‐mediated immune response (CMI) in subjects who received a booster dose at Month 12 and persistence of humoral immune response in these subjects 6 months later at Month 18. The data on the comparison of the humoral immune response (in terms of both HI and neutralizing antibody titers) against vaccine homologous and heterologous strains induced by the booster following priming with one or two vaccine doses are also presented here.
Materials and methods
Study design and subjects
Adults aged between 18 and 60 years at the time of study start, without history of previous vaccination with any investigational pandemic influenza vaccine or with any inactivated or live seasonal influenza vaccine within 2 or 4 weeks before study start, respectively were enrolled in Germany after obtaining written informed consent to be randomized into eight groups as described previously (Figure 1). 14
Figure 1.
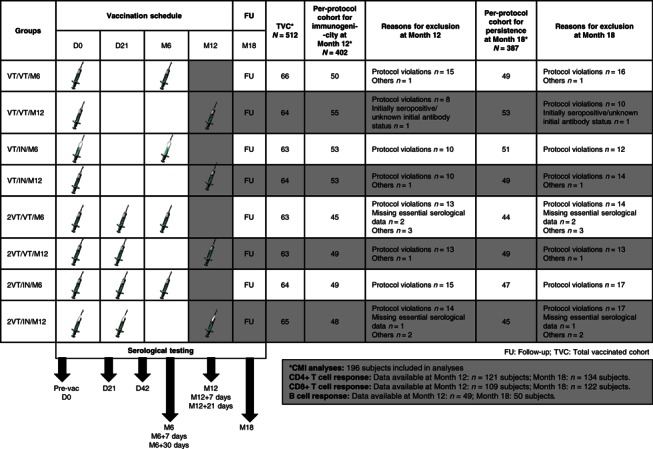
Study design diagram + CONSORT. Explanation for the study design: In the primary study, subjects were randomized into 8 study groups to receive one or two doses (21 days apart) of AS03A‐adjuvanted H5N1 vaccine with either A/Vietnam/1194/2004 strain (VT) or A/Indonesia/05/2005 strain (IN). A homologous or heterologous booster dose (VT or IN) was administered to 4 study groups at Month 6 and the remaining 4 study groups at Month 12. Blood samples were drawn before vaccination, at Days 21 and 42, Month 6 (+7 and +30 days), Month 12 (+7 and +21 days), and at Month 18. This manuscript presents data from groups that were boosted at Month 12. Group names: VT/VT/M12: A/Vietnam/1194/2004 vaccine at Day 0; A/Vietnam/1194/2004 vaccine at Month 12. VT/IN/M12: A/Vietnam/1194/2004 vaccine at Day 0; A/Indonesia/05/2005 vaccine at Month 12. 2VT/VT/M12: A/Vietnam/1194/2004 vaccine at Days 0 and 21; A/Vietnam/1194/2004 vaccine at Month 12. 2VT/IN/M12: A/Vietnam/1194/2004 vaccine at Days 0 and 21; A/Indonesia/05/2005 vaccine at Month 12.
Study vaccine
The A/Vietnam/1194/2004 vaccine (Prepandrix™, a trade mark of GlaxoSmithKline group of companies) contained 3·75 μg HA of the A/Vietnam/1194/2004‐like NIBRG‐14 clade 1 strain per dose (National Institute for Biological Standards and Control Potters Bar, UK) adjuvanted with AS03A [an α‐tocopherol oil‐in‐water emulsion‐based adjuvant system (11·86 mg tocopherol)]. The A/Indonesia/05/2005 vaccine contained 3·75 μg HA of the A/Indonesia/05/2005‐like IBCDC‐RG2 clade 2.1 strain per dose [Centers for Disease Control and Prevention (CDC) Atlanta, USA] adjuvanted with AS03. The study vaccines were developed and manufactured by GSK Biologicals (the antigens were manufactured in Rixensart, Belgium and the adjuvant in Dresden, Germany) as described earlier. 11 , 12 The vaccines (0·5 ml) were administered intramuscularly into the deltoid of the non‐dominant arm.
Laboratory assays
Hemagglutination inhibition antibody titers against the A/Vietnam/1194/2004 and A/Indonesia/05/2005 strains were assessed at GSK Biologicals Central Laboratory using standard assay methods (cutoff for HI: ≥1:10) modified to utilize an equine erythrocyte suspension instead of an avian erythrocyte suspension, as described previously. 15 HI antibody titers were determined using the method described by the WHO Collaborating Centre for influenza, CDC, Atlanta, USA (1991). Antibody titers were measured on thawed frozen sera with a standardized and comprehensively validated micromethod using four hemagglutination‐inhibiting units (4 HA units) of the appropriate antigens and a 0·5% (horse) erythrocyte suspension. Non‐specific serum inhibitors were removed by heat treatment and receptor‐destroying enzyme. Starting with a dilution of 1:10, a dilution series (by a factor of 2) was prepared up to an end dilution of 1:20480. The titration end‐point was taken as the highest dilution step that showed complete inhibition (100%) of hemagglutination. All assays were performed in duplicate. Immunological assessments were made in terms of the geometric mean titers (GMT), seroprotection rate (SPR), seroconversion rate (SCR), and seroconversion factor (SCF).
The viral microneutralization assay was performed at GSK Biologicals Central laboratory as described previously. 12 The thawed frozen sera was subjected to heat treatment at 56°C for 30 minutes and then tested in triplicate. The assay used a standardized amount of virus mixed with serial twofold dilutions of serum samples to allow binding of the antibodies to the virus. The mixture of virus and anti‐serum was added to a defined amount of Madin‐Darby Canine Kidney (MDCK) cell cultures and incubated for 7 days at 33°C. After the incubation period, virus replication was visualized by hemagglutination of chicken red blood cells. The 50% neutralization titer of a serum was calculated by the Reed and Muench method. 16 The assay cutoff was 1:28.
Cell‐mediated immune response in terms of CD4+ and CD8+ T cells was evaluated by Intracellular Cytokine assay (ICS) as described previously. 17 Influenza vaccine antigens were used to re‐stimulate influenza‐specific T cells to produce cytokines and/or express activation markers and enumerated by Cytokine Flow‐Cytometry (CFC) following conventional immunofluorescence labeling of cellular phenotype markers and for intracellular cytokines production. Results were expressed as a frequency of cytokine(s)‐positive CD4+ or CD8+ T cells within the CD4+ or CD8+ T cell subpopulation.
Cell‐mediated immune response in terms of split H5N1‐specific memory B cells was evaluated by enzyme‐linked immunosorbent spot assay as described previously. 17 The results were expressed as a frequency of influenza‐specific antibody‐secreting plasma cells within the IgG‐producing plasma cells.
Immunogenicity assessments
Serum samples were collected before vaccination (Day 0), at Day 21, Day 42 (for groups receiving two primary doses), at Months 6, 12, and 18, as well as 7 and 21 days after booster dose at Months 6 and 12. This article details only serological analyses of groups boosted at Month 12, thus at the following time points: Days 0, 21, and 42 (for groups receiving two primary doses), Month 12, Month 12 + 7 days, Month 12 + 21 days, and Month 18.
The assessment of immune responses for immunogenicity was based on the seroconversion rate (SCR: percentage of subjects with pre‐vaccination titer <1:10 and post‐vaccination titer ≥1:40, or pre‐vaccination titer >1:10 and at least fourfold increase in post‐vaccination titer), seroprotection rate (SPR: percentage of subjects with a post‐vaccination titer ≥1:40) and seroconversion factor (SCF: post‐vaccination fold increase in GMTs) in terms of HI antibodies against the vaccine homologous and heterologous strains and on the SCR (at least a fourfold increase in post‐vaccination titer) in terms of neutralizing antibodies against the vaccine homologous and heterologous strains.
The outcome measures of immune responses included evaluation based on the immunogenicity criteria for pandemic influenza vaccines in adults as required by the Committee for Medicinal Products for Human Use (CHMP; point estimates for HI antibody SCR: >40%, SPR: >70% and SCF: >2·5) and Center for Biologics Evaluation and Research [CBER; lower limit of 95% confidence interval (CI) for HI antibody for SCR: ≥40% and SPR: ≥70%]. 5 , 6 Descriptive assessments were made for CMI in terms of frequencies of H5N1‐specific CD4+ and CD8+ T cells and memory B cells at the different time points.
Safety and reactogenicity assessments
The subjects used diary cards to record solicited local and general adverse events up to 7 days following the booster dose; unsolicited adverse events were recorded up to 30 days following the booster dose.
Intensity of solicited symptoms was graded on a standard scale of (0–3), where Grade 1 symptoms were defined as those noticeable but not interfering with normal activities, Grade 2 symptoms were defined as those that caused sufficient discomfort to interfere with normal activities, and Grade 3 symptoms were defined as those that prevented normal activities [Grade 3 redness and swelling: diameter >100mm; Grade 3 fever: temperature >39°C (>102·2°F)]. Serious adverse events (SAEs) and a subset of adverse events that include both autoimmune diseases and other inflammatory and/or neurologic disorders that may or may not have an autoimmune etiology (pIMDs) occurring throughout the study period were also recorded.
Statistical analyses
The analyses of immunogenicity at Month 12 and Month 18 were performed on the per‐protocol cohort for immunogenicity and per‐protocol cohort for persistence, respectively, and the analyses of safety were performed on the total vaccinated cohort (TVC). The per‐protocol cohort for immunogenicity included all subjects who met all protocol‐defined eligibility criteria and procedures and for whom data were available at Month 12; the per‐protocol cohort for persistence included all subjects who met all protocol‐defined eligibility criteria and procedures and for whom data were available at Months 12 and 18. The TVC included all vaccinated subjects for whom data were available.
Results
Study population
The study was concluded on October 20, 2008. Of the 512 subjects who received primary vaccination, 449 completed the study through Month 18. The number of subjects included in the per‐protocol cohort for immunogenicity at Month 12 and in the per‐protocol cohort for persistence at Month 18 along with the reasons for elimination are presented in Figure 1. A total of 196 subjects who received a booster dose at Month 12 were included in the CMI analyses. Of these, data for CD4+ response were available for 121 subjects at Month 12 and 134 subjects at Month 18, data for CD8+ response were available for 109 subjects at Month 12 and 122 subjects at Month 18. For B cell immune response, data were available for 49 and 50 subjects, at the respective time points.
Overall, the median age of subjects at Month 18 was 34·0 years (range: 18–60 years). The male to female ratio was 44·4%:55·6%, and all except three subjects (99·2%) were Caucasian. The demographic characteristics were similar across all groups.
Immunogenicity
HI antibody immune response post‐booster at Month 12
The HI antibody responses against the two strains prior to booster vaccination at Month 12, 7, and 21 days after the booster dose and at Month 18 are presented in Table 1.
Table 1.
Immune response in terms of HI antibodies against vaccine (A) heterologous A/Indonesia/05/2005 strain (B) homologous A/Vietnam/1194/2004 strain [CBER/CHMP criteria] (per‐protocol cohort for immunogenicity)
| Immune response [CHMP criteria] | Time point | Nn | VT/VT/M12 | Nn | VT/IN/M12 | Nn | 2VT/VT/M12 | Nn | 2VT/IN/M12 |
|---|---|---|---|---|---|---|---|---|---|
| (A) A/Indonesia/05/2005 | |||||||||
| Value or % (95% CI) | |||||||||
| Geometric mean titer | PRE | 55 | 5·0 (5·0–5·0) | 53 | 5·0 (5·0–5·0) | 49 | 5·0 (5·0–5·0) | 48 | 5·0 (5·0–5·0) |
| D21 | 55 | 6·4 (5·2–7·9) | 53 | 7·0 (5·6–8·7) | 49 | 5·4 (5·0–5·8) | 48 | 5·8 (5·0–6·6) | |
| D42 | – | – | – | – | 43 | 18·1 (12·4–26·4) | 42 | 29·4 (18·1–47·7) | |
| M12 | 52 | 5·8 (5·0–6·8) | 52 | 5·9 (5·1–6·8) | 41 | 5·7 (5·0–6·5) | 44 | 8·4 (6·4–11·1) | |
| M12 + 7 | 50 | 97·8 (66·5–143·8) | 51 | 196·2 (128·6–299·2) | 40 | 106·5 (66·7–170·0) | 43 | 239·4 (149·9–382·2) | |
| M12 + 21 | 50 | 139·3 (92·0–211·0) | 51 | 420·0 (283·8–621·6) | 40 | 191·9 (121·6–303·0) | 43 | 624·9 (469·3–831·9) | |
| M18 | 49 | 56·6 (35·5–90·2) | 45 | 139·3 (85·7–226·6) | 40 | 121·3 (76·6–192·0) | 39 | 385·9 (252·9–588·6) | |
| Seroconversion rate [point estimate >40%] | D21 | 55 | 5·5 (1·1–15·1) | 53 | 9·4 (3·1–20·7) | 49 | 0·0 (0·0–7·3) | 48 | 2·1 (0·1–11·1) |
| D42 | – | – | – | – | 43 | 41·9 (27·0–57·9) | 42 | 50·0 (34·2–65·8) | |
| M12 | 52 | 3·8 (0·5–13·2) | 52 | 3·8 (0·5–13·2) | 41 | 0·0 (0·0–8·6) | 44 | 11·4 (3·8–24·6) | |
| M12 + 7 | 50 | 80·0 (66·3–90·0) | 51 | 86·3 (73·7–94·3) | 40 | 85·0 (70·2–94·3) | 43 | 90·7 (77·9–97·4) | |
| M12 + 21 | 50 | 84·0 (70·9–92·8) | 51 | 96·1 (86·5–99·5) | 40 | 90·0 (76·3–97·2) | 43 | 100 (91·8–100) | |
| M18* | 48 | 70·8 (55·9–83·0) | 44 | 88·6 (75·4–96·2) | 40 | 85·0 (70·2–94·3) | 39 | 94·9 (82·7–99·4) | |
| Seroprotection rate [point estimate >70%] | PRE | 55 | 0·0 (0·0–6·5) | 53 | 0·0 (0·0–6·7) | 49 | 0·0 (0·0–7·3) | 48 | 0·0 (0·0–7·4) |
| D21 | 55 | 5·5 (1·1–15·1) | 53 | 9·4 (3·1–20·7) | 49 | 0·0 (0·0–7·3) | 48 | 2·1 (0·1–11·1) | |
| D42 | – | – | – | – | 43 | 41·9 (27·0–57·9) | 42 | 50·0 (34·2–65·8) | |
| M12 | 52 | 3·8 (0·5–13·2) | 52 | 3·8 (0·5–13·2) | 41 | 0·0 (0·0–8·6) | 44 | 11·4 (3·8–24·6) | |
| M12 + 7 | 50 | 80·0 (66·3–90·0) | 51 | 86·3 (73·7–94·3) | 40 | 85·0 (70·2–94·3) | 43 | 90·7 (77·9–97·4) | |
| M12 + 21 | 50 | 84·0 (70·9–92·8) | 51 | 96·1 (86·5–99·5) | 40 | 90·0 (76·3–97·2) | 43 | 100 (91·8–100) | |
| M18 | 49 | 69·4 (54·6–81·7) | 45 | 86·7 (73·2–94·9) | 40 | 87·5 (73·2–95·8) | 39 | 97·4 (86·5–99·9) | |
| Seroconversion factor [point estimate >2·5] | D21 | 55 | 1·3 (1·0–1·6) | 53 | 1·4 (1·1–1·7) | 49 | 1·1 (1·0–1·2) | 48 | 1·2 (1·0–1·3) |
| D42 | – | – | – | – | 43 | 3·6 (2·5–5·3) | 42 | 5·9 (3·6–9·5) | |
| M12 | 52 | 1·2 (1·0–1·4) | 52 | 1·2 (1·0–1·4) | 41 | 1·1 (1·0–1·3) | 44 | 1·7 (1·3–2·2) | |
| M12 + 7 | 50 | 19·6 (13·3–28·8) | 51 | 39·2 (25·7–59·8) | 40 | 21·3 (13·3–34·0) | 43 | 47·9 (30·0–76·4) | |
| M12 + 21 | 50 | 27·9 (18·4–42·2) | 51 | 84·0 (56·8–124·3) | 40 | 38·4 (24·3–60·6) | 43 | 125·0 (93·9–166·4) | |
| M18* | 48 | 10·3 (6·6–16·0) | 44 | 25·1 (15·8–39·8) | 40 | 21·3 (13·2–34·4) | 39 | 44·5 (27·9–71·0) | |
| (B) A/Vietnam/1194/2004 | |||||||||
| Value or % (95% CI) | |||||||||
| Geometric mean titer | PRE | 55 | 5·1 (4·9–5·4) | 53 | 5·0 (5·0–5·0) | 49 | 5·2 (4·8–5·6) | 48 | 5·0 (5·0–5·0) |
| D21 | 55 | 18·4 (12·6–27·0) | 53 | 20·3 (13·1–31·5) | 49 | 16·7 (11·3–24·7) | 48 | 18·9 (12·6–28·2) | |
| D42 | – | – | – | – | 43 | 179·1 (117·2–273·6) | 42 | 228·1 (141·8–367·1) | |
| M12 | 52 | 8·4 (6·4–11·0) | 52 | 9·9 (7·3–13·5) | 41 | 14·2 (10·1–20·1) | 44 | 17·4 (11·6–25·9) | |
| M12 + 7 | 50 | 235·9 (153·7–362·1) | 51 | 354·3 (244·7–513·0) | 40 | 257·7 (162·4–409·0) | 43 | 358·2 (228·7–561·1) | |
| M12 + 21 | 50 | 352·6 (229·9–540·8) | 51 | 662·2 (458·3–956·6) | 40 | 441·0 (279·5–695·9) | 43 | 942·2 (703·1–1262·7) | |
| M18 | 49 | 76·1 (47·1–123·1) | 45 | 115·8 (71·9–186·3) | 40 | 149·3 (93·0–239·6) | 39 | 337·5 (219·5–518·9) | |
| Seroconversion rate [point estimate >40%] | D21 | 55 | 41·8 (28·7–55·9) | 53 | 43·4 (29·8–57·7) | 49 | 30·6 (18·3–45·4) | 48 | 43·8 (29·5–58·8) |
| D42 | – | – | – | – | 43 | 90·7 (77·9–97·4) | 42 | 90·5 (77·4–97·3) | |
| M12 | 52 | 11·5 (4·4–23·4) | 52 | 19·2 (9·6–32·5) | 41 | 22·0 (10·6–37·6) | 44 | 38·6 (24·4–54·5) | |
| M12 + 7 | 50 | 90·0 (78·2–96·7) | 51 | 96·1 (86·5–99·5) | 40 | 92·5 (79·6–98·4) | 43 | 93·0 (80·9–98·5) | |
| M12 + 21 | 50 | 92·0 (80·8–97·8) | 51 | 98·0 (89·6–100) | 40 | 95·0 (83·1–99·4) | 43 | 100 (91·8–100) | |
| M18* | 48 | 68·8 (53·7–81·3) | 44 | 68·2 (52·4–81·4) | 40 | 72·5 (56·1–85·4) | 39 | 87·2 (72·6–95·7) | |
| Seroprotection rate [point estimate >70%] | PRE | 55 | 0·0 (0·0–6·5) | 53 | 0·0 (0·0–6·7) | 49 | 0·0 (0·0–7·3) | 48 | 0·0 (0·0–7·4) |
| D21 | 55 | 41·8 (28·7–55·9) | 53 | 43·4 (29·8–57·7) | 49 | 32·7 (19·9–47·5) | 48 | 43·8 (29·5–58·8) | |
| D42 | – | – | – | – | 43 | 90·7 (77·9–97·4) | 42 | 90·5 (77·4–97·3) | |
| M12 | 52 | 11·5 (4·4–23·4) | 52 | 19·2 (9·6–32·5) | 41 | 22·0 (10·6–37·6) | 44 | 38·6 (24·4–54·5) | |
| M12 + 7 | 50 | 90·0 (78·2–96·7) | 51 | 96·1 (86·5–99·5) | 40 | 92·5 (79·6–98·4) | 43 | 93·0 (80·9–98·5) | |
| M12 + 21 | 50 | 92·0 (80·8–97·8) | 51 | 98·0 (89·6–100) | 40 | 95·0 (83·1–99·4) | 43 | 100 (91·8–100) | |
| M18 | 49 | 75·5 (61·1–86·7) | 45 | 82·2 (67·9–92·0) | 40 | 85·0 (70·2–94·3) | 39 | 97·4 (86·5–99·9) | |
| Seroconversion factor [point estimate >2·5] | D21 | 55 | 3·6 (2·5–5·2) | 53 | 4·1 (2·6–6·3) | 49 | 3·2 (2·2–4·8) | 48 | 3·8 (2·5–5·6) |
| D42 | – | – | – | – | 43 | 34·4 (22·7–52·2) | 42 | 45·6 (28·4–73·4) | |
| M12 | 52 | 1·6 (1·3–2·1) | 52 | 2·0 (1·5–2·7) | 41 | 2·7 (1·9–3·9) | 44 | 3·5 (2·3–5·2) | |
| M12 + 7 | 50 | 45·9 (29·8–70·7) | 51 | 70·9 (48·9–102·6) | 40 | 49·4 (31·0–78·6) | 43 | 71·6 (45·7–112·2) | |
| M12 + 21 | 50 | 68·6 (44·5–105·8) | 51 | 132·4 (91·7–191·3) | 40 | 84·5 (53·3–133·9) | 43 | 188·4 (140·6–252·5) | |
| M18* | 48 | 10·2 (6·4–16·1) | 44 | 12·0 (7·3–19·8) | 40 | 10·2 (6·2–16·8) | 39 | 18·6 (11·1–31·2) | |
Nn, Number of subjects with available results; CI, Confidence Interval.
*Month 12 time point as the pre‐booster reference point. Bold values in (A) did not meet CBER criteria for M12 +7 and M18 time points.
Group names: VT/VT/M12: A/Vietnam/1194/2004 vaccine at Day 0; A/Vietnam/1194/2004 vaccine at Month 12. VT/IN/M12: A/Vietnam/1194/2004 vaccine at Day 0; A/Indonesia/05/2005 vaccine at Month 12. 2VT/VT/M12: A/Vietnam/1194/2004 vaccine at Days 0 and 21; A/Vietnam/1194/2004 vaccine at Month 12. 2VT/IN/M12: A/Vietnam/1194/2004 vaccine at Days 0 and 21; A/Indonesia/05/2005 vaccine at Month 12.
The persistence of humoral immune response against the vaccine homologous A/Vietnam/1194/2004 strain and heterologous A/Indonesia/05/2005 strain was low prior to the booster dose at Month 12 (GMT against the two strains were <18 and <9, respectively).
A rapid and high HI antibody response against both strains following the booster dose was observed in all study groups. The CHMP criteria and the more stringent CBER criteria were met and exceeded as early as 7 days after the booster dose. Figure 2A presents the reverse cumulative curves for HI antibody response for the subjects who received a single primary dose and a heterologous booster dose at Month 12 compared with those who received two homologous primary doses and a homologous booster dose at Month 12, at all time points.
Figure 2.
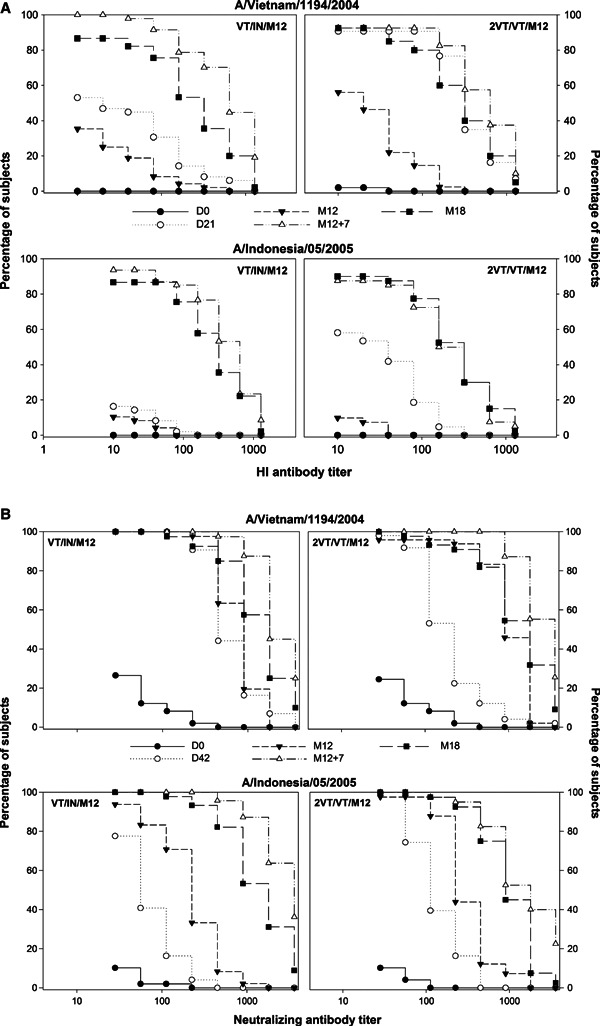
(A) Reverse cumulative curves for HI antibody response against the A/Vietnam/1194/2004 and A/Indonesia/05/2005 strains at all time points (per‐protocol cohort for immunogenicity). (B) Reverse cumulative curves for neutralizing antibody response against the A/Vietnam/1194/2004 and A/Indonesia/05/2005 strains at all time points (per‐protocol cohort for immunogenicity). For group names see Figure 1.
Twenty‐one days after booster vaccination at Month 12, the highest HI immune responses against the A/Vietnam/1194/2004 strain and the A/Indonesia/05/2005 strain were observed in subjects who received two primary doses followed by a heterologous booster dose (GMTs: 942·2 and 624·9, respectively; SPR/SCR: 100·0% for both strains).
Immune response against the A/Indonesia/05/2005 strain following booster vaccination was higher in subjects who received a single primary dose followed by a heterologous booster at Month 12 compared with those subjects who received two primary doses 21 day apart followed by a homologous booster (GMTs: 420·0 versus 191·9). The breadth of the immune response was greater with heterologous booster vaccination as compared to homologous booster vaccination, irrespective of whether one or two doses of primary vaccination were given. Even a single primary vaccine dose allowed for heterologous boosting after an interval of 12 months.
The CHMP and CBER criteria in terms of HI antibody response for the A/Vietnam/1194/2004 and A/Indonesia/05/2005 strains were met and exceeded 21 days after the booster dose at Month 12 in other groups, irrespective of whether the booster vaccine strain was homologous or heterologous to the primary vaccination and independent of whether the subjects received one or two primary doses.
HI antibody persistence at Month 18
The HI antibody response against the two strains persisted 6 months after the booster dose irrespective of the vaccine strain of the booster dose (although no longer meeting the regulatory guidance criteria); persistence of HI antibody response was marginally higher in subjects who received the two‐dose primary vaccination compared with those who received a single primary dose and highest in those who received two primary doses followed by a heterologous booster dose (GMTs of 385·9 and 337·5 against the vaccine heterologous and homologous strains, respectively).
Neutralizing antibody response
Pre‐booster neutralizing antibody response against the A/Indonesia/05/2005 strain at Month 12 tended to be higher in subjects who received two primary doses compared with those who received a single primary dose (GMTs of 197·5/234·6 versus 124·7/106·5; although SCRs were comparable – 100%/100% versus 98·0%/100%). For the A/Vietnam/1194/2004 strain, pre‐booster values were within the same range across all vaccine groups.
A highly cross‐reactive neutralizing antibody response was observed after the booster dose at Month 12 + 7 days (GMTs: 936·9–2556·6; SCRs: 97·6–100%) and subsequently at Month12 + 21 days (GMTs: 1301·5–4602·2; SCRs: 97·5–100%) irrespective of whether the booster vaccine strain was homologous or heterologous to the primary vaccination and independent of whether the subjects received one or two primary doses. Figure 2B presents the reverse cumulative curves for neutralizing antibody response for the subjects who received a single primary dose and a heterologous booster dose at Month 12 compared with those who received two homologous primary doses and a homologous booster dose at Month 12, at all time points.
The highest cross‐reactive immune response at Months 12 and 18 was observed in subjects who received two primary doses followed by a heterologous booster dose (Month 12: GMTs: 4602·2; SCRs: 100%; Month 18: 1708·9; SCRs: 79·5%). The neutralizing antibody results parallel the HI antibody response induced by the vaccine regimens.
Cell‐mediated immune response at Month 12 and Month 18
Figure 3A, B presents the data on CMI at Months 12 and 18. For CMI response in terms of frequencies of specific CD4+ T cells, pre‐booster values against the A/Vietnam/1194/2004 strain at Month 12 showed a quite high individual dispersion.
Figure 3.
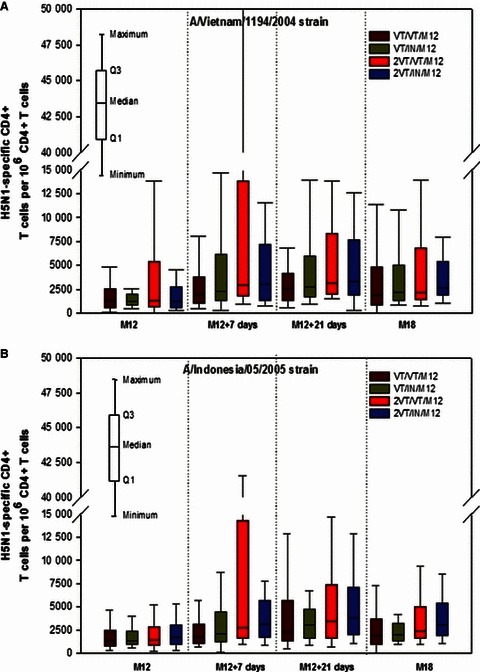
(A, B) Cell‐mediated immune response at Months 12 and 18 (per‐protocol cohort for immunogenicity). For group names see Figure 1.
For both A/Vietnam/1194/2004 and A/Indonesia/05/2005 strains, pre‐booster values were within the same range across all vaccine groups. An increase in CMI responses after the booster dose at Month 12 (M12 + 7 days) was observed in all groups against both strains. A sustained increase in values against both strains was observed 21 days after the booster dose (M12 + 21 days). Point estimates for peak T cell‐mediated responses appeared to be higher in subjects who received the two‐dose primary vaccination compared with those who received the one‐dose primary vaccination, as evident from Figure 3. At Month 18, T cell‐mediated immune responses against both strains were similar for all groups.
For CMI response in terms of CD8+ T cells, no antigen‐specific CD8+ T cell response was observed in any of the groups with the assay used in this study. For CMI response in terms of H5N1‐specific memory B cells against both strains, pre‐vaccination response was low and varied substantially from one individual to another (A/Vietnam/1194/2004 strain: 0‐9162; A/Indonesia/05/2005 strain: 0‐8221). The vaccine‐induced immune response against both strains persisted up to Month 12. The distribution of immune response was quite large, and no difference was detected at Month 12 between subjects who received the one‐ or two‐dose primary vaccination regimens. An increase in immune response was observed after the booster dose, especially 21 days after vaccination; the immune response appeared to wane up to Month 18, although it was still higher than that observed before vaccination (Figure 4).
Figure 4.
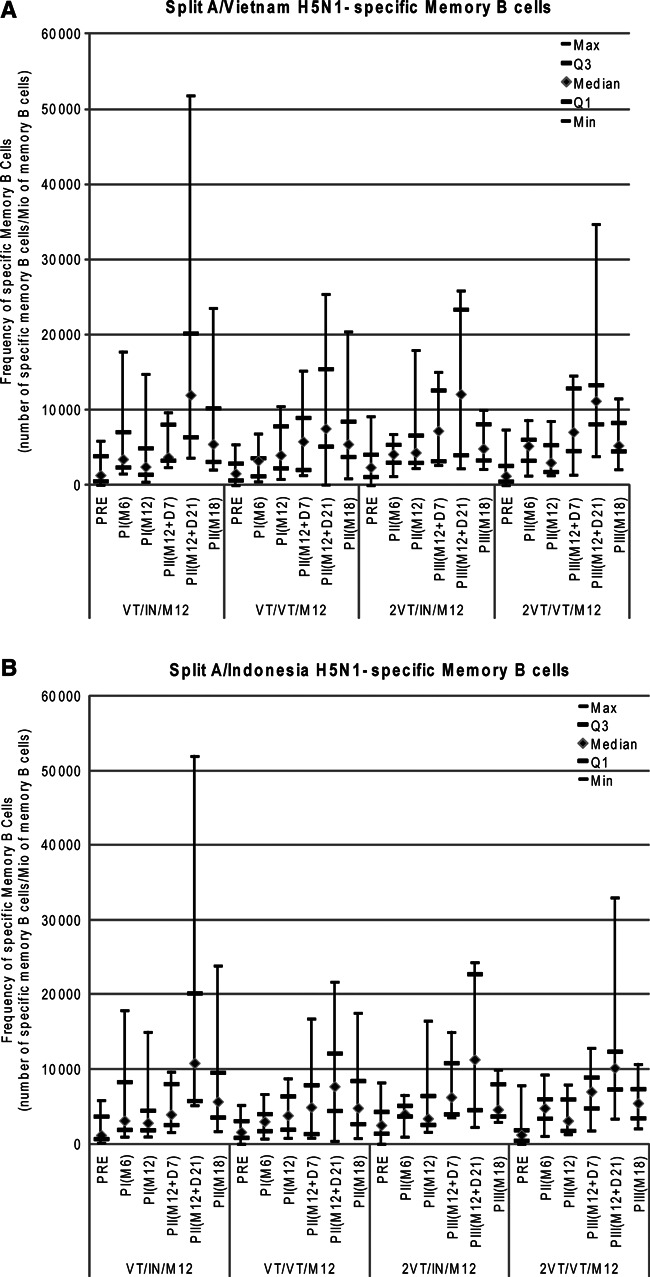
(A, B) Split H5N1‐specific memory B cell response against the A/Vietnam/1194/2004 and A/Indonesia/05/2005 strains at all time points (per‐protocol cohort for immunogenicity). For group names see Figure 1.
Safety and reactogenicity
Data on the solicited local and general symptoms following the booster dose are presented in Figure 5A, B, respectively. Pain at injection site (89·1–95·4% of subjects) was the most frequently reported solicited local symptom across all groups (overall). The occurrence of Grade 3 solicited local symptoms was infrequent; Grade 3 injection site pain was reported for 5–8 subjects (7·7–12·5% of subjects) across the four groups.
Figure 5.
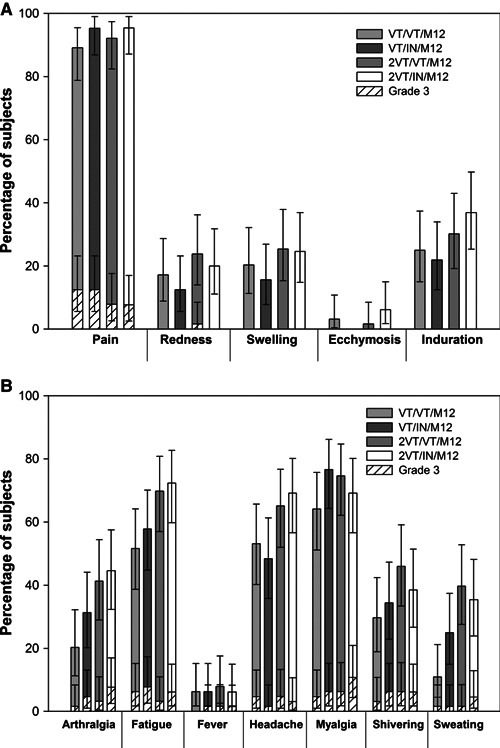
(A) Solicited local symptoms reported during the 7‐day post‐vaccination follow‐up period after Month 12 booster (total vaccinated cohort). (B) Solicited general symptoms reported during the 7‐day post‐vaccination follow‐up period after Month 12 booster (total vaccinated cohort). Error bars indicate the upper and lower limits of the 95% confidence intervals (CIs). For group names see Figure 1.
The most frequently reported solicited general symptoms were fatigue, headache, and myalgia (51·6–72·3%; 48·4–69·2%; 64·1–76·6% of subjects, respectively); the observed rates of Grade 3 symptoms showed an increasing trend after the booster dose as compared to after the primary vaccination. The pattern of reactogenicity did not change markedly between the different vaccination schedules nor following the primary versus booster vaccination.
Overall, at least one unsolicited adverse event was reported for 10 subjects who received the booster dose at Month 12. No pIMDs were recorded during the study period. Among subjects who received the booster dose at Month 12, 10 reported SAEs during the entire study period. One subject in Group VT/VT/M12 was diagnosed with non‐Hodgkin’s lymphoma, 47 days after the second vaccine dose; given the time interval from the last dose, the investigator assessed that causality could not be ruled out and the event was reported as related. No fatalities were reported during the entire study period.
Discussion
This article completes the first publication related to this study, which reported the immunogenicity results for subjects who received one or two primary doses of A/Vietnam/1194/2004 vaccine followed by a booster dose with the heterologous A/Indonesia/05/2005 strain 6 months later 14 with data pertaining to a homologous or heterologous booster administered 1 year after the primary vaccination.
In this study, a single primary dose of A/Vietnam/1194/2004 vaccine followed 12 months later by a single‐adjuvanted booster with the heterologous A/Indonesia/05/2005 strain met the CHMP and CBER guidance criteria for HI immune responses against either strain. Thus, although a single primary dose of H5N1 vaccine may not be adequate to meet the European and US regulatory guidance criteria for pandemic influenza vaccines, the immune response induced is sufficient in the context of late boosting as it effectively allowed for booster responses against vaccine homologous and even heterologous strains. In fact, the immune response to the booster dose at Month 12 met and exceeded the European and US regulatory guidance criteria within 7 days after the booster dose.
Data from this study also showed that a single booster dose induced strong and rapid immune responses against the vaccine homologous strain, 12 months after a one‐dose primary vaccination thus indicating that the immune memory induced by a single primary dose persisted for at least up to 12 months. This adds to the previous report that HI immune memory persisted for up to 6 months after primary vaccination. 14
The different time intervals between the two‐dose primary vaccination (21 days apart), one‐dose primary vaccination, and booster vaccine doses (12 months apart) did not appear to have a negative impact on the magnitude of immune response against both strains following the second vaccine dose. On the contrary, increasing the interval between the two doses resulted in an even stronger humoral immune response against the A/Indonesia/05/2005 strain. Although the HI immune responses may be interpreted within the limitations of assay variability because of different evaluation time points, the neutralizing antibody immune responses confirmed the trend observed in the HI immune responses. This observation is in agreement with the previously published results of this study on subjects who were boosted 6 months after primary vaccination. 14 For the CMI response in terms of CD4+ T cells and memory B cells, strong immune responses against both strains were observed 21 days after booster vaccination. Additionally, persistence of immune response was observed up to Month 18 in all subjects irrespective of the number of primary vaccination doses and the interval between them. However, the observations for the memory B cell response should be interpreted taking into consideration the modest sample size. Thus, the H5N1 vaccine in this study showed that flexibility in the time interval between primary and booster vaccination and the use of a heterologous strain did not impact the strength of immune response to either vaccine strain.
The potential of the vaccine formulation to enhance the immune response not only against the vaccine homologous strain but also against a heterologous strain could be due to the ability of the adjuvant to promote CD4+ T cell responses: a larger, and possibly qualitatively different CD4+ memory T cell pool could provide better help to B cells, leading to a larger and potentially more diverse B memory pool. This is convergent with recent data indicating that (i) adjuvanted vaccines have been shown to change the clonal composition of antigen‐specific CD4+ T cell populations responding to vaccines, favoring the higher number of CD4+ T Cells and the selection of higher‐affinity T cells. Adjuvantation of the split‐antigen vaccine with AS03A resulted in a strong increase in the numbers of antigen‐specific B cells and CD4+ T cells. 18 (ii) the oil‐in‐water adjuvant MF59 quantitatively and qualitatively enhances functional antibody responses to HA‐based vaccines by improving both epitope breadth and binding affinity, demonstrating the added value of such adjuvants for influenza vaccines. 19
The trial design for this study ensured that the vaccination schedules and dosage mimic that in a potential pandemic scenario to deliberate on the following possibilities: (i) the strain in the prepandemic vaccine may differ from that in the pandemic vaccine received later on as the H5N1 virus continues to drift/evolve, (ii) the difficulty to implement an uniform interval between the two vaccine doses, and (iii) the existing vaccine production capacity may not be able to meet the demand of the large number of doses required.
In conclusion, the strong immune response against the vaccine homologous and heterologous strains induced by a booster dose 12 months after primary vaccination indicated that a single primary dose of the AS03A‐adjuvanted H5N1 vaccine not only induced sufficient immune response that allowed for late boosting but also induced the persistence of immune memory up to 12 months. In addition, the strength of the immune response to either vaccine strain was neither affected by the flexible dosing schedule of the AS03A‐adjuvanted H5N1 vaccine nor by the use of a heterologous strain as booster.
Financial disclosure
GlaxoSmithKline Biologicals was the funding source and was involved in all stages of the study conduct and analysis (ClinicalTrials.gov Identifier: NCT00430521). GlaxoSmithKline Biologicals also took in charge all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data, and the corresponding author had final responsibility to submit for publication.
Conflict of interest
Drs. Markus Knuf and Tino F. Schwarz were investigators in this study funded by GlaxoSmithKline. Drs. M. Knuf and T. F. Schwarz have received honoraria from GlaxoSmithKline as an investigator in vaccine trials, consultant, member of advisory boards unrelated to this study and/or as lecturers. All participating institutions received compensation for study involvement. Drs. Paul Gillard, Adrian Caplanusi, François Roman, Karl Walravens, Phillipe Moris and Mr. Mamadou Dramé are employees of GlaxoSmithKline Biologicals. F. Roman, P. Gillard, A. Caplanusi, P. Moris and K. Walravens report ownership of stock options.
Acknowledgements
All authors participated in the implementation of the study including substantial contributions to conception and design, the gathering of the data, or analysis and interpretation of the data. All authors were involved in the drafting of the article or revising it critically for important intellectual content, and final approval of the manuscript. We are grateful to the New York Medical College, New York for providing the vaccine virus reassortant and to the National Institute for Biological Standards and Control (NIBSC, UK) and Therapeutic Goods Administration (TGA) from the Australian Government for providing the reference standards. The authors are indebted to the participating study volunteers and their parents, clinicians, nurses, and laboratory technicians at the study sites as well as to the sponsor’s project staff for their support and contributions throughout the study. We are grateful to all teams of GSK Biologicals for their contribution to this study, especially Thierry Ollinger and Roger Bernhard from the clinical and serological laboratory teams, and Dr Claudius Meyer’s laboratory for the cellular immune response evaluation. Finally, the authors thank Conor Cahill for critically reviewing the manuscript and providing valuable input, Avishek Pal (GSK Biologicals) for providing medical writing services and Dr. Sophie Tambour and Dr. Santosh Mysore (XPE Pharma & Science, on behalf of GSK Biologicals) for editorial assistance and manuscript coordination. Prepandrix is a trade mark of the GlaxoSmithKline group of companies.
References
- 1. Melidou A, Gioula G, Exindari M, Chatzidimitriou D, Diza‐Mataftsi E. Influenza A (H5N1): an overview of the current situation. Eurosurveillance 2009; 145:220–226. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO, 6 May 2010. Global alert and response (GAR) Available at http://www.who.int/csr/disease/avian_influenza/country/cases_table_2011_03_07/en/index.html (Accessed 26 July 2011).
- 3. World Health Organization (WHO) . Antigenic and genetic characteristics of influenza A(H5N1) and influenza A(H9N2) viruses for the development of candidate vaccine viruses for pandemic preparedness, February 2011. Available at http://www.who.int/csr/disease/avian_influenza/guidelines/2011_02_h5_h9_vaccinevirusupdate.pdf (Accessed 23 May 2011). [PubMed]
- 4. Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus . Update on Avian Influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273. [DOI] [PubMed] [Google Scholar]
- 5. European Committee for Proprietary Medicinal Products (CHMP) . Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006). European Agency for the Evaluation of Medicinal Products, January 24, 2007.
- 6. US Food and Drug Administration (FDA) Guidance for Industry . Clinical data needed to support the licensure of pandemic influenza vaccines. US Food and Drug Administration May 2007. Available at http://www.fda.gov/cber/gdlns/panfluvac.htm (Accessed 10 March 2011).
- 7. World Health Organization (WHO) . Options for the Use of Human H5N1 Influenza Vaccines and the WHO H5N1 Vaccine Stockpile. Geneva, Switzerland: WHO scientific consultation, 2007. [Google Scholar]
- 8. Stephenson I, Gust I, Pervikov Y, Kieny MP. Development of vaccines against influenza H5. Lancet Infect Dis 2006; 6:458–460. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization . Pandemic (H1N1) 2009 briefing note 2. Global alert and response (GAR) July 13, 2009. Available at http://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090713/en/index.html (Accessed 16 March 2011).
- 10. Chu DW‐S, Hwang SJ, Lim FS et al. Immunogenicity and tolerability of an AS03A‐adjuvanted prepandemic influenza vaccine. A phase III study in a large population of Asian adults. Vaccine 2009; 27:7428–7435. [DOI] [PubMed] [Google Scholar]
- 11. Leroux‐Roels I, Borkowski A, Vanwolleghem T et al. Antigen sparing and cross‐reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580–589. [DOI] [PubMed] [Google Scholar]
- 12. Leroux‐Roels I, Bernhard R, Gerard P, Dramé M, Hanon E, Leroux‐Roels G. Broad clade 2 cross‐reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS ONE 2008; 3:e1665 499. Doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rümke HC, Bayas JM, de Juanes JR et al. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine 2008; 26:2378–2388. [DOI] [PubMed] [Google Scholar]
- 14. Schwarz TF, Horacek T, Knuf M et al. Single dose vaccination with AS03‐adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine 2009; 27:6284–6290. [DOI] [PubMed] [Google Scholar]
- 15. Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti‐H5 responses in human sera by HI using horse erythrocytes following MF59‐adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res 2004; 103:91–95. [DOI] [PubMed] [Google Scholar]
- 16. Reed LT, Muench H. A simple method of calculating fifty percent end point. Am J Hyg 1938; 27:493–498. [Google Scholar]
- 17. Moris P, van der Most R, Leroux‐roels I et al. H5N1 influenza vaccine formulated with AS03A induces strong cross‐reactive and polyfunctional CD4 T‐cell responses. J Clin Immunol 2011; 31:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malherbe L, Mark L, Fazilleau N, Heyzer‐Williams LJ, Heyzer‐Williams MG. Vaccine adjuvants alter TCR‐based selection thresholds. Immunity 2008; 28:698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khurana S, Verma N, Yewdell JW et al. MF59 adjuvant enhances diversity and affinity of antibody‐mediated immune response to pandemic influenza vaccines. Sci Transl Med 2011; 3:85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]


