Abstract
Please cite this paper as: Van Poucke et al. (2013) Effect of receptor specificity of A/Hong Kong/1/68 (H3N2) influenza virus variants on replication and transmission in pigs. Influenza and Other Respiratory Viruses 7(2) 151–159.
Background Several arguments plead for an important role of pigs in human influenza ecology, including the similar receptor expression pattern in the respiratory tract of both species. How virus receptor binding specificity affects transmission in pigs, on the other hand, has not been studied so far.
Objectives Using recombinant viruses R1‐HK, which harbored all genes from the original pandemic virus A/Hong Kong/1/68 (H3N2), and R2‐HK, which differed by L226Q and S228G mutations in the hemagglutinin and conversion to an avian‐virus‐like receptor specificity, we assessed the role of receptor specificity on (i) replication in porcine respiratory explants, (ii) pig‐to‐pig transmission, and (iii) replication and organ tropism in pigs.
Results In nasal, tracheal, and bronchial explants, we noticed a 10‐ to 100‐fold lower replication of R2‐HK compared with R1‐HK. In the lung explants, the viruses replicated with comparable efficiency. These observations correlated with the known expression level of Siaα2,3‐galactose in these tissues. In the pathogenesis study, virus titers in the respiratory part of the nasal mucosa, the trachea, and the bronchus were in line with the ex vivo results. R2‐HK replicated less efficiently in the lungs of pigs than R1‐HK, which contrasted with the explants results. R2‐HK also showed a pronounced tropism for the olfactory part of the nasal mucosa. Transmissibility experiments revealed that pig‐to‐pig transmission was abrogated when the virus obtained Siaα2,3‐galactose binding preference.
Conclusions Our data suggest that Siaα2,6‐galactose binding is required for efficient transmission in pigs.
Keywords: A/Hong Kong/1/68, pathogenesis, pigs, receptor specificity, respiratory explants, transmission
Introduction
Introduction of a new influenza virus to which the human population has no or little preexisting immunity and that is capable of transmitting from humans to humans poses a pandemic threat. Such a virus can arise from genetic reassortment between avian and mammalian influenza viruses or by the adaptation of a non‐human influenza virus. 1 Although aquatic birds are the major influenza virus reservoir, pigs are supposed to play an important role in human influenza ecology. Swine have been postulated to be either an intermediate host for the transmission of avian influenza viruses (AIVs) to humans or a mixing vessel for avian and mammalian viruses. 2 , 3 , 4 This is based on the observation that most, if not all, avian virus subtypes can replicate in swine. 5 , 6 Furthermore, both terminal sialic acid (Sia)α2,3‐galactose and Siaα2,6‐galactose, the preferred virus receptors of avian and human influenza viruses respectively, were shown to be present in the porcine trachea. 7 More recent research has confirmed the abundant expression of Siaα2,6‐galactose on the epithelial lining of the porcine respiratory tract, but Siaα2,3‐galactose was detected only sparsely in the upper respiratory tract. 8 , 9 , 10 Hence, the receptor distribution pattern in the respiratory tract of pigs shows extensive similarity with that of humans. 11 , 12 More detailed pathogenesis studies have shown that although pigs support replication of many AIVs, the titers reached in the respiratory tract are modest. 13 , 14 , 15 , 16 Also, low and highly pathogenic AIVs could not transmit from pig to pig, while swine‐adapted viruses could under the same experimental conditions. 5 , 13 , 14
A large body of data highlights the importance of the viral hemagglutinin (HA) glycoprotein for the host range restriction. Thus, the pandemic “Hong Kong” H3N2 influenza virus emerged by reassortment of the then circulating human H2N2 virus with an avian H3 influenza virus and the avian HA showed a switch in receptor binding specificity shortly after the introduction of the virus in the human population in 1968. 17 , 18
Residues 226 and 228 in the HA of H3N2 influenza virus strains have been shown to determine receptor binding specificity. When the HA harbors leucine at 226 and serine at 228, the virus binds Siaα2,6‐galactose (human‐type receptor), whereas glutamic acid at 226 and glycine at 228 preferentially recognize Siaα2,3‐galactose (avian‐type receptor). 19 , 20 In cultures of human tracheobronchial epithelial cells, recombinant viruses with these mutations displayed differences in cell tropism, similar to the ones observed with wild‐type avian and human influenza viruses. 21 , 22 Roberts and colleagues used a pair of recombinant influenza viruses with L226Q/S228G substitutions in the A/Victoria/3/75 (H3N2) backbone to examine their transmissibility in ferrets. They observed a lack of transmission of the mutant virus with avian‐virus‐like receptor specificity. 23
Global surveillance of influenza viruses in bird, swine, and human populations is considered a key factor in the early detection of viruses with pandemic potential. A lack of detained knowledge on molecular determinants of viral host range restriction, especially in pigs, hampers predictions of the pandemic potential of emerging influenza viruses as illustrated by the 2009 pandemic. 24 Therefore, we aimed to determine whether L226Q/S228G mutations in the HA of A/Hong Kong/1/68 (H3N2) would affect (i) replication potential in porcine respiratory explants, (ii) replication potential and organ tropism in pigs, and (iii) pig‐to‐pig transmission.
Materials and methods
Animals
Six‐ to 8‐week‐old pigs were obtained from a commercial herd serologically negative for influenza. All animals were tested for influenza virus antibodies before the start of the experiment by hemagglutination inhibition (HI) assay, by a competitive anti‐influenza A nucleoprotein enzyme immunoassay (Idexx Laboratories, Hoofddorp, Netherlands), and by immunoperoxidase monolayer assay (IPMA). Swine/Belgium/1/98 (H1N1), swine/Flanders/1/98 (H3N2), and swine/Gent/7625/99 (H1N2) were used in the HI and IPMA. Pigs were housed in separate high‐efficiency particulate air‐filtered isolation units. At arrival, they were treated intramuscularly with ceftiofur (Naxcel®, Pfizer‐1 ml/20 kg body weight).
All experiments were authorized by the Ethical and Animal Welfare Committee of the Faculty of Veterinary Medicine, Ghent University.
Viruses
The recombinant viruses R1‐HK and R2‐HK have been described previously. 19 They were generated using the eight plasmids reverse genetic system described by Hoffmann et al. 25 and site‐directed mutagenesis to introduce the amino acid substitutions. R1‐HK harbors all eight original genes of the pandemic human virus A/Hong Kong/1/68 (H3N2). R2‐HK differs from R1‐HK solely by the L226Q/S228G mutations in the HA, which lead to a switch in receptor specificity from preferential binding of Siaα2,6‐galactose (R1‐HK) to preferential recognition of Siaα2,3‐galactose (R2‐HK). 19 Virus stocks were prepared in MDCK cells, and their infectivity was determined by plaque titration in MDCK cells. The low pathogenic AIV duck/Italy/3139‐1/06 (H3N2) was provided by Dr. I. Capua. Swine/Flanders/1/98 represents a European human‐like H3N2 swine influenza virus. The HA and NA genes of the swine H3N2 virus lineage are derived from descendants of A/Hong Kong/1/68 and the six gene fragments coding for internal and non‐structural proteins from the avian‐like H1N1. The amino acids at locations 226 and 228 are identical to those of R1‐HK.
Porcine respiratory explants systems
Porcine nasal (NE), tracheal (TE), bronchial (BE), and lung (LE) explants were prepared as described elsewhere. 9 The replication potential of R1‐HK, R2‐HK, duck/Italy/3139‐1/06 (dk/It/06), and swine/Flanders/1/98 (sw/Fl/1/98) inoculated at three different doses [104, 105, and 106 plaque forming units (PFU)] was assessed by determining the virus titers in supernatant at 1, 24, and 48 hours post inoculation. Virus titrations were performed on MDCK cells in 96‐well plates followed by an immunocytochemical staining with monoclonal antibody HB‐65 against viral nucleoprotein, and virus titers were calculated by the method of Reed and Muench. 26
Transmission studies
For each of the recombinant viruses, we used six directly inoculated (donor) pigs and six contact pigs. The six donor pigs of each group were housed in a separate isolation unit and inoculated intranasally with 106 PFU of R1‐HK or R2‐HK on day 0, as described earlier. 5 Two days after primary inoculation, six contact pigs were introduced into each group. The housing allowed both direct and airborne contact between inoculated and sentinel animals. Virus shedding was monitored by collecting nasal swabs from 0 through 9 days post inoculation (pi) or post contact (pc). Nasal swabs were put into 1 ml of transport medium and used for virus titration in MDCK cells. Blood samples for serological examination were collected at 14 and 28 dpi/dpc. In the group of R2‐HK contact pigs, blood was collected at 17 instead of 28 dpc. Transmission was defined by detection of virus from the nasal swabs and/or seroconversion in contact animals.
Pathogenesis study
Two separate groups, of five pigs each, were inoculated intranasally with 106 PFU of R1‐HK or R2‐HK as described for the transmission study. From 1 until 5 dpi, one pig from each group was euthanized. The following samples were collected for virus titration: nasal mucosa respiratory part, nasal mucosa olfactory part, tonsils, trachea, bronchus (at the level of the bifurcation), bulbus olfactorius, and lung. Five different areas of the lung were collected and titrated separately: (i) apical + cardiac lobe right (ii) apical + cardiac lobe left (iii) diaphragmatic lobe right (iv) diaphragmatic lobe left, and (v) accessory lung lobe. All tissue samples for virus titration were weighed and put in phosphate‐buffered saline containing 10 IU/ml penicillin and 10 μg/ml streptomycin to prepare 20% tissue homogenates.
Serological examination
Antibody titers against the homologous viruses were determined by hemagglutination inhibition (HI), virus neutralization (VN), and IPMA. Antibodies against the viral NP were detected by a competitive anti‐influenza A nucleoprotein enzyme immunoassay (NP‐ELISA). HI and VN assays were performed as described earlier. 27 Sera from the R2‐HK group were tested in parallel HI assays with chicken and horse erythrocytes, while sera from R1‐HK were tested with chicken erythrocytes only, because the R1‐HK virus did not agglutinate the horse erythrocytes. In short, the sera were heat inactivated (56°C,30′), pre‐treated with receptor‐destroying enzyme, and adsorbed onto either chicken or horse erythrocytes. Sera were incubated with four hemagglutinating units of virus for 1 hour and then left to hemagglutinate 0·5% chicken erythrocytes or 1% horse erythrocytes during 1 or 2 hours at room temperature, respectively. In the VN assay, twofold serum dilutions were incubated with 102 TCID50 of the homologous virus during 1 hour. Next, 100 μl of MDCK cells was added at a concentration of 300 000 cells/ml, and after 24 hours, the plates were fixed. Lack of infection was confirmed by immunocytochemical staining. For the IPMA, monolayers of MDCK cells were inoculated with 103 TCID50 per well and fixed after 24 hours. They were incubated with twofold dilutions of the swine sera followed by incubation with goat anti‐swine peroxidase. 5 The influenza A virus antibody test kit®, purchased from IDEXX Laboratories and carried out following the manufacturer’s instructions, was a blocking ELISA. If NP antibodies are present in the serum sample to be tested, they will prevent the conjugate from binding with the nucleoprotein that is adsorbed to the ELISA plate and color development will be blocked. Starting dilutions of the sera in the serological assays were 1:2 in VN, 1:5 in IPMA, and 1:10 in HI and NP‐ELISA. All sera were tested in duplicate. Antibody titers were expressed as the reciprocal of the highest serum dilutions that completely inhibited virus replication in MDCK cells (VN assay), that stained influenza infected MDCK cells (IPMA), and that inhibited hemagglutination (HI assay). Results from the NP‐ELISA were expressed as S/N ratio. The S/N response is the ratio of the sample optical density (OD) reading to the kit negative control OD reading.
Lectin histochemistry
The pronounced tropism of R2‐HK for the olfactory part of the nasal mucosa triggered us to study the receptor expression in this tissue. Siaα2,6‐galactose distribution was examined using a digoxigenin labeled Sambucus nigra agglutinin (SNA). Two different isoforms of the Maackia amurensis agglutinin, MAA‐I and MAA‐II, were applied to identify the α2,3‐linked Sia moiety of the receptor. Staining was developed with New Fuchsin. 9
Data analyses
Replication differences between viruses in the explants were compared by a paired t‐test. P < 0·05 was considered significant. The average virus excretion in the nasal swabs from each group of pigs was determined by calculating the area under the curve (AUC). To compare the AUC between groups, the two sample Mann–Whitney test was used and P < 0·05 was considered significant. All analyses were performed with Prism5, version 5·0c.
Results
Replication in porcine respiratory explants
Results from replication studies in porcine respiratory explants are shown in Figure 1. Although there was a clear dose‐dependent effect, virus replication in NE and TE was comparable, it was poor for dk/It/06, moderate for R2‐HK, high for R1‐HK, and highest for sw/Fl/1/98. In BE, R1‐HK and sw/Fl/1/98 showed equally high virus titers (P = 0·92). Replication of R2‐HK and dk/It/06 in BE was also increased compared with NE and TE, but remained significantly different from R1‐HK (P = 0·008 and P = 0·010) and sw/Fl/98 (P = 0·019 and P = 0·009) respectively when 106 PFU was administrated. In LE, in contrast, virus titers did not significantly differ between the four viruses at the inoculation doses of 106 and 105 PFU.
Figure 1.
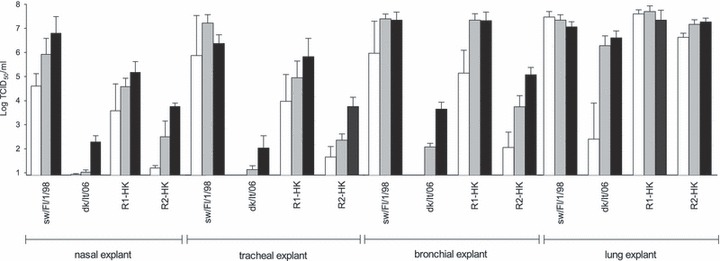
Virus yield in the supernatant of porcine respiratory explants at 48 hpi. Three different inoculation doses were assessed for each virus: 104 (white bars), 105 (gray bars) and 106 plaque forming units (black bars). Bars show the mean and standard deviation of 4 repeats. Swine/Flanders/1/98 (sw/Fl/1/98) and duck/Italy/3139‐1/06 (dk/It/06) represent a swine‐adapted and non‐swine‐adapted influenza virus, respectively.
Transmission studies
An overview of the nasal virus shedding in all groups is depicted in Figure 2. After inoculation with R1‐HK, all pigs shed virus in nasal swabs until six dpi. Virus titers showed little individual variation between pigs, resulting in an average AUC of 23·1. The six contact animals in this group also excreted virus, starting at 1–3 dpc. The duration of shedding was similar to that of the inoculated pigs, but the average AUC for the contact group was only 17·4. This was because of the very low AUC values in three of six pigs.
Figure 2.
Nasal virus shedding of R1‐K (left panel) and R2‐HK (right panel) influenza viruses of inoculated and sentinel pigs. The virus titers are shown as mean ± standard deviation. R2‐HK was undetectable in nasal swabs of all contact pigs. The numbers in italics represent the area under the curve for each group of pigs.
All R2‐HK‐inoculated pigs were positive for virus excretion. Their excretion profile was similar to that of R1‐HK‐inoculated pigs in terms of start and duration of viral shedding, but the average AUC was only 15·7 (significantly lower than in the R1‐HK‐inoculated group). Four of six pigs showed a remarkable drop in virus excretion at 2 and 3 dpi. By 4 dpi, the nasal shedding in all six pigs had increased again to levels between 3·8 and 5·3 log10 TCID50/ml. In contrast, none of the contact animals in the R2‐HK group had detectable virus titers in nasal swabs.
Pathogenesis study
Of 11 tissues analyzed for virus titers, only one was negative during five subsequent days for R1‐HK compared with four negative tissues for R2‐HK. The bulbus olfactorius tested negative for both R1‐HK and R2‐HK, while the tonsil, the right diaphragmatic, and the left diaphragmatic lung lobes were also negative for R2‐HK. With the exception of the olfactory part of the nasal mucosa, the average virus titers obtained for R2‐HK were at least 20‐fold lower than for R1‐HK. The virus titers obtained for R2‐HK were particularly low in bronchus and lungs, with the exception of those in the accessory lung lobe (Figure 3).
Figure 3.
Replication of R1‐HK (gray bars) and R2‐HK (black bars) influenza virus in various parts of the respiratory tract and in the bulbus olfactorius of pigs during five subsequent days post inoculation. Each bar represents the virus titer of an individual pig. The thin dashed line represents the detection limit.
Serological examination
The results of the serological assays in the R1‐HK and R2‐HK groups are shown in 1, 2, respectively. In the HI test performed with 0·5% chicken red blood cells (RBC), all R1‐HK‐inoculated and contact pigs had seroconverted by 14 dpi/dpc (titers 10–40). None of the 12 pigs of the R2‐HK group reacted in this assay. In contrast, in the HI test with 1% horse RBC, all R2‐HK‐inoculated pigs had antibody titers (10–40) by 14 dpi but none of the contact animals showed seroconversion. The VN test largely confirmed the results of the HI test, but antibody titers for the R2‐HK group were significantly lower than for the R1‐HK group, even by 28 dpi. One of the six contact pigs in the R2‐HK group showed seroconversion in both the NP‐ELISA and IPMA. The IPMA gave the highest number of seropositive animals in both R1‐HK and R2‐HK groups by 14 dpi.
Table 1.
Antibody responses in pigs directly inoculated with R1‐HK virus and in contact pigs
| Assay | Number of serologically positive pigs and range of antibody titers (in parentheses) | |||||
|---|---|---|---|---|---|---|
| Directly inoculated pigs Days post inoculation | Contact pigs Days post contact | |||||
| 0 | 14 | 28 | 0 | 14 | 28 | |
| HI (chicken RBC) | 0 (<10) | 6 (10–40) | 6 (10–40) | 0 (<10) | 6 (10–40) | 6 (10–40) |
| VN | 0 (<2) | 6 (24–64) | 6 (12–64) | 0 (<2) | 6 (16–96) | 6 (32–64) |
| NP‐ELISA | 0 (≥0·6) | 2 (0·565–0·508) | 6 (0·584–0·445) | 0 (≥0·6) | 4 (0·529–0·427) | 5 (0·524–0·377) |
| IPMA | 0 (<5) | 6 (1280–5120) | 6 (1280–10240) | 0 (<5) | 6 (640–5120) | 6 (1280–5120) |
RBC, red blood cells.
The number of positive pigs among the total of six pigs is shown. Range of antibody titers in positive pigs are expressed as the reciprocal of the serum dilutions for virus neutralization, immunoperoxidase monolayer assay, and HI assays and as S/N ratio for the NP‐ELISA. S/N ratios ≥0·6 are considered negative.
Table 2.
Antibody responses in pigs directly inoculated with R2‐HK virus and in contact pigs
| Assay | Number of serologically positive pigs and range of antibody titers (in parentheses) | |||||
|---|---|---|---|---|---|---|
| Directly inoculated pigs Days post inoculation | Contact pigs Days post contact | |||||
| 0 | 14 | 28 | 0 | 14 | 17 | |
| HI (chicken RBC) | 0 (<10) | 0 (<10) | 0 (<10) | 0 (<10) | 0 (<10) | 0 (<10) |
| HI (horse RBC) | 0 (<10) | 6 (10–20) | 5 (20–40) | 0 (<10) | 0 (<10) | 0 (<10) |
| VN | 0 (<2) | 4 (2–6) | 6 (3–12) | 0 (<2) | 0 (<2) | 0 (<2) |
| NP‐ELISA | 0 (≥0·6) | 6 (0·581–0·250) | 6 (0·538–0·343) | 0 (≥0·6) | 1 (0·458) | 1 (0·565) |
| IPMA | 0 (<5) | 6 (640–2560) | 6 (1280–5120) | 0 (<5) | 1 (320) | 1 (640) |
RBC, red blood cells.
The number of positive pigs among the total of six pigs is shown. Range of antibody titers in positive pigs are expressed as the reciprocal of the serum dilutions for virus neutralization, immunoperoxidase monolayer assay, and HI assays and as S/N ratio for the NP‐ELISA. S/N ratios ≥0·6 are considered negative.
Lectin histochemistry
There was strong binding of both MAA‐I and MAA‐II to the epithelial lining of the olfactory part of the nasal mucosa (indicated by the arrows in Figure 4A,B), indicating the presence of both N and O‐linked Siaα2,3‐linked glycans. Siaα2,6‐galactose was not detected in any of the epithelial cell layers as there was no binding by SNA (Figure 4C).
Figure 4.
Tissue binding of Maackia amurensis agglutinin I (A), Maackia amurensis agglutinin II (B) and Sambucus nigra agglutinin (C) in the porcine olfactory part of the nasal mucosa. Siaα2,3‐galactoseβ1,4‐glucosamine (A) was abundantly expressed on the epithelial lining, Siaα2,3‐galactoseβ1,3‐glucosamine (B) moderately (indicated by the arrows). Sia α2,6‐galactose (C) was not detected in any of the epithelial cell layers.
Discussion
Techniques such as reverse genetics, enabling the generation of large numbers of mutant or reassortant influenza viruses, have emphasized the need for reliable ex vivo tools to study these viruses and to predict their behavior in vivo. Because pigs play an important role in influenza ecology and zoonotic transmission, they are the species of choice to investigate the molecular determinants, both at viral and host levels, of inter‐ and intraspecies transmission. We have previously shown that replication efficiency of influenza viruses isolated from porcine, human, and avian hosts differs significantly in NE, TE, and BE but not in LE. 9 The restricted replication of AIVs in tissues of the upper respiratory tract was in line with the limited expression of Siaα2,3‐galactose in these tissues. 8 , 10 Using R1‐HK and R2‐HK viruses that differ solely by two amino acids in the receptor binding site, we have examined the usefulness of this ex vivo model of the porcine respiratory tract to assess replication differences between viruses with genetic modifications. The avian‐like R2‐HK replicated less efficiently than the human‐like R1‐HK in NE, TE, and BE. On the other hand, virus titers of R2‐HK were consistently higher than those of dk/It/06. This finding suggests that the two viruses could still differ in receptor binding properties or that the low replication level of the entirely AIV was not only attributed to a non‐optimal receptor binding tropism. A similar observation was the lower replication of R1‐HK compared with sw/Fl/1/98 in NE and TE, despite the human‐like tropism of both viruses. A reduction in the inoculation dose from 106 to 104 PFU resulted in a stronger drop of virus replication of R2‐HK than of R1‐HK, suggesting a lower minimal infectious dose of the latter virus. Roberts et al. 23 indeed showed that a similar A/Victoria/3/75 mutant virus with avian‐like receptor specificity required a 40‐fold higher infectious dose in ferrets than the wild‐type virus. To assess whether the replication differences observed in these explants were biologically relevant in vivo, we used the recombinant viruses for transmission and pathogenesis studies in pigs.
Although seroconversion was observed in one of six contact pigs of the R2‐HK group, our results indicate that a switch in the receptor preference of H3 from Siaα2,6‐galactose to Siaα2,3‐galactose was sufficient to block efficient transmission in pigs, as this virus was not isolated in any of the nasal swabs from contact animals. In previous studies in ferrets and guinea pigs, intraspecies transmission was also shown to be influenced by receptor binding preferences of viruses. 28 , 29 , 30 Tumpey et al. 28 showed that a switch toward an avian‐virus‐like receptor preference of the 1918 H1N1 pandemic virus abolished airborne transmission between ferrets. Pappas et al. 29 have also shown that an early isolate of the 1957 H2N2 pandemic with avian‐virus‐like receptor specificity did not efficiently spread between ferrets. A Q226L mutant with human‐virus‐like receptor specificity, which emerged during the course of their experiment, was transmissible even through respiratory droplets. When compared to R1‐HK, the nasal virus shedding of R2‐HK‐inoculated pigs was characterized by overall lower virus titers, a temporary drop at 2 and 3 dpi, and an identical duration. The lower AUC of R2‐HK, representing the overall viral load built up in the stable, could at least in part explain for the lack of transmission. To examine this possibility, we increased the inoculation dose of R2‐HK to 108 PFU in a preliminary experiment. As the AUC of R2‐HK was not significantly increased (AUC = 18) under these conditions, the hypothesis could not be confirmed. In literature, data on the role of the virus excretion level on transmission efficiency are contradicting. Mubareka et al. 31 showed that reduced virus shedding of a human H1N1 compared with a human H3N2 influenza virus was associated with less efficient transmission in guinea pigs. In another transmission study in ferrets, a human H3N2 and an avian H5N1 virus achieved similar peak mean titers in nasal washes but only the human virus transmitted efficiently. 32
The pathogenesis study confirmed the hampered replication potential of R2‐HK in porcine tissues, except for one particular region, the olfactory part of the nasal mucosa. The lower replication of R2‐HK could be the result of an imbalance between the receptor preference of HA and substrate specificity of NA. However, Scull et al. 21 showed that A/Victoria/3/75 with L226Q and S228G mutations in the HA was not significantly less fit than its wild‐type counterpart as both viruses infected similar numbers of human ciliated airway epithelium cells and replicated to comparable peak titers when incubated at 37°C. In the LE, we observed similar peak virus titers for R2‐HK and R1‐HK, indicating that the virus itself is replication‐competent. Another explanation for the limited R2‐HK replication could be a restricted binding of this virus to Sia receptors on target cells. This hypothesis is in agreement with our observations in the explants. R2‐HK preferably binds Siaα2,3‐galactose, and the expression of this linkage is absent or limited in NE, TE, and BE. 9 Still, the expected enhanced tropism of R2‐HK for the lungs, based on replication in LE and the Siaα2,3‐galactose expression in this organ, was not observed in vivo. This may result from a difference in exposure of the tissues to the inoculum. In the LE, virus is directly brought onto the tissue, while it must overcome several barriers to reach the LRT after intranasal inoculation. Furthermore, we demonstrated a difference in predilection for the olfactory part of the nasal mucosa between R2‐HK and R1‐HK. The preference of AIVs for this particular tissue was noticed before in pathogenesis studies in our laboratory, 5 unpublished data (De Vleeschauwer, Kyriakis). Here, we have shown a correlation with the absence of Siaα2,6‐galactose and the abundant expression of Siaα2,3‐galactose on the epithelial lining. Despite a lack of Siaα2,6‐galactose, swine/Belgium/1/98 (H1N1) could successfully infect this olfactory part of the nasal mucosa, 15 indicating that the SNA lectin may not stain all α2,6 variants or that another receptor mediates the endocytotic uptake of the virus.
In conclusion, our data suggest that Siaα2,6‐galactose binding is a prerequisite for efficient transmission of influenza viruses in pigs. This observation strengthens the resemblance between pigs and humans in terms of host range restrictions for influenza virus infections. The transmission efficiency was related to the overall replication level in vivo, for which the porcine explants can have a predictable value.
Acknowledgements
This work was financed by 7th Framework Programme FLUPIG (FP7‐GA 258084).
Special thanks go to Lieve Sys, Nele Dennequin, Melanie Bauwens, Zeger Van den Abeele and Kevin Fung for excellent technical support.
References
- 1. Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis 2006; 12:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Claas ECJ, Kawaoka Y, De Jong JC, Masurel N, Webster RG. Infection of children with avian‐human reassortant influenza virus from pigs in Europe. Virology 1994; 204:453–457. [DOI] [PubMed] [Google Scholar]
- 3. Ma W, Lager KM, Vincent AL, Janke BH, Gramer MR, Richt JA. The role of swine in the generation of novel influenza viruses. Zoonoses Public Health 2009; 56:326–337. [DOI] [PubMed] [Google Scholar]
- 4. Scholtissek C, Bürger H, Kistner O, Shortridge KF. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 1985; 147:287–294. [DOI] [PubMed] [Google Scholar]
- 5. De Vleeschauwer A, Van Poucke S, Braeckmans D, Van Doorsselaere J, Van Reeth K. Efficient transmission of swine‐adapted but not wholly avian influenza viruses among pigs and from pigs to ferrets. JID 2009; 200:1884–1892. [DOI] [PubMed] [Google Scholar]
- 6. Kida H, Ito T, Yasuda J et al. Potential for transmission of avian influenza viruses to pigs. J Gen Virol 1994; 75:2183–2188. [DOI] [PubMed] [Google Scholar]
- 7. Ito T, Couceiro JN, Kelm S et al. Molecular basis for the generation in pigs of influenza viruses with pandemic potential. J Virol 1998; 72:7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nelli RK, Kuchipudi SV, White GA, Perez BB, Dunham SP, Chang K. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. Vet Res 2010; 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K. Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J 2010; 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trebbien R, Larsen LE, Viuff BM. Distribution of sialic acid receptors and influenza A virus of avian and swine origin in experimentally infected pigs. Virol J 2011; 8:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yao L, Korteweg C, Hsueh W, Gu J. Avian influenza receptor expression in H5N1‐infected and noninfected human tissues. FASEB J 2008; 22:733–740. [DOI] [PubMed] [Google Scholar]
- 12. Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Influenza virus receptors in the human airway. Nature 2006; 440:435–436. [DOI] [PubMed] [Google Scholar]
- 13. Choi YK, Nguyen TD, Ozaki H et al. Studies of H5N1 influenza virus infection of pigs by using viruses isolated in Vietnam and Thailand in 2004. J Virol 2005; 79:10821–10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shortridge KF, Zhou NN, Guan Y et al. Characterisation of avian H5N1 influenza viruses from poultry in Hong Kong. Virol 1998; 252:331–342. [DOI] [PubMed] [Google Scholar]
- 15. De Vleeschauwer A, Atanasova K, Van Borm S et al. Comparative pathogenesis of an avian H5N2 and a swine H1N1 influenza virus in pigs. PLoS One 2009; 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lipatov AS, Kwon YK, Sarmento LV et al. Domestic pigs have low susceptibility to H5N1 highly pathogenic avian influenza viruses. PLoS Pathog 2008; 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian and equine H2 and H3 influenza virus isolates. Virology 1994; 205:17–23. [DOI] [PubMed] [Google Scholar]
- 18. Matrosovich M, Tuzikov A, Bovin N et al. Early alterations of the receptor‐binding properties of H1, H2 and H3 avian influenza virus hemagglutinins after their introduction into mammals. J Virol 2000; 74:8502–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matrosovich M, Matrosovich T, Uhlendorff J, Garten W, Klenk H. Avian‐virus‐like receptor specificity of the hemagglutinin impedes influenza virus replication in cultures of human airway epithelium. Virol 2007; 361:384–390. [DOI] [PubMed] [Google Scholar]
- 20. Vines A, Wells K, Matrosovich M, Castrucci MR, Ito T, Kawaoka Y. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J Virol 1998; 72:7626–7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scull MA, Gillim‐Ross L, Santos C et al. Avian influenza virus glycoproteins restrict virus replication and spread through human airway epithelium at temperatures of the proximal airways. PLoS Pathog 2009; 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk H. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. PNAS 2004; 101:4620–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roberts KL, Shelton H, Scull M, Pickles R, Barclay WS. Lack of transmission of a human influenza virus with avian receptor specificity between ferrets is not due to decreased virus shedding, but rather a lower infectivity in vivo . J Gen Virol 2011; 92:1822–1831. [DOI] [PubMed] [Google Scholar]
- 24. Garten RJ, Davis CT, Russel CA et al. Antigenic and genetic characteristics of swine‐origin 2009 A (H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. PNAS 2000; 97:6108–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reed LJ, Münch H. A simple method of estimating fifty per cent endpoints. Am J Hygiene 1938; 27:493–497. [Google Scholar]
- 27. De Vleeschauwer AR, Van Poucke SG, Karasin AI, Olsen CW, Van Reeth K. Cross‐protection between antigenically distinct H1N1 swine influenza viruses from Europe and North America. Influenza Other Respi Viruses 2010; 5:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tumpey TM, Maines TR, Van Hoeven N et al. A two‐amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 2007; 315:655–659. [DOI] [PubMed] [Google Scholar]
- 29. Pappas C, Viswanathan K, Chandrasekaran A et al. Receptor specificity and transmission of H2N2 subtype viruses isolated from the pandemic of 1957. PLoS One 2010; 5:e11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao Y, Zhang Y, Shinya K et al. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog 2009; 5:e1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mubareka S, Lowen AC, Steel J, Coates AL, Garcia‐Sastre A, Palese P. Transmission of influenza virus via aerosols and fomites in the guinea pig model. JID 2009; 199:858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maines TR, Chen L, Matsuoka Y et al. Lack of transmission of H5N1 avian‐human reassortant influenza viruses in a ferret model. PNAS 2006; 103:12121–12126. [DOI] [PMC free article] [PubMed] [Google Scholar]


