Abstract
Please cite this paper as: Hollmeyer et al. (2012) Review: interventions to increase influenza vaccination among healthcare workers in hospitals. Influenza and Other Respiratory Viruses 7(4), 604–621.
Annual influenza vaccination rates among hospital healthcare workers (HCW) are almost universally low despite recommendations from WHO and public health authorities in many countries. To assist in the development of successful vaccination programmes, we reviewed studies where interventions aimed to increase the uptake of influenza vaccination among hospital HCW. We searched PUBMED from 1990 up to December 2011 for publications with predetermined search strategies and of pre‐defined criteria for inclusion or exclusion. We evaluated a large number of ‘intervention programmes’ each employing one or more ‘intervention components’ or strategies, such as easy access to vaccine or educational activities, with the goal to raise influenza vaccine uptake rates in hospital HCW during one influenza season. Included studies reported results of intervention programmes and compared the uptake with the season prior to the intervention (historical control) or to another intervention programme within the same season that started from the same set of baseline activities. Twenty‐five studies performed in eight countries met our selection criteria and described 45 distinct intervention programmes. Most studies used their own facility as historical control and evaluated only one season. The following elements were used in intervention programmes that increased vaccine uptake: provision of free vaccine, easy access to the vaccine (e.g. through mobile carts or on‐site vaccination), knowledge and behaviour modification through educational activities and/or reminders and/or incentives, management or organizational changes, such as the assignment of personnel dedicated to the intervention programme, long‐term implementation of the strategy, requiring active declination and mandatory immunization policies. The number of these components applied appeared to be proportional to the increase in uptake. If influenza uptake in hospital HCW is to be increased on sustained basis, hospital managers need to be committed to conduct a well‐designed long‐term intervention programme that includes a variety of co‐ordinated managerial and organizational elements.
Keywords: Health personnel, influenza vaccine, vaccination rates
Introduction
Influenza infections in hospital healthcare workers (HCW) may lead to nosocomial outbreaks, 1 , 2 particularly in immunocompromised patients, 3 and staff shortages with associated disruption of services. 4 HCW have been implicated in the transmission of influenza to inpatients. 5 , 6 When antigenically well matched, seasonal influenza immunization has been efficacious in HCW. 7 , 8 Increased uptake by HCW has also been shown to reduce morbidity and mortality in geriatric long‐term‐care patients 9 , 10 , 11 , 12 and may have a protective effect for hospitalized patients, 13 , 14 making vaccination of HCW particularly important for this group as the efficacy of the vaccine is lower in the elderly and immunocompromised than in younger healthy adults. 15 , 16 Although many national public health institutions and the World Health Organization (WHO) have recommended seasonal influenza vaccination for HCW for many years, low uptake is widespread. In our first review article on influenza vaccine uptake by HCW, we identified two major reasons for non‐receipt: lack of convenient access to vaccination and lingering misconceptions or lack of knowledge about influenza and the vaccine. 17 In the current review, we analyse interventions used to increase the uptake of influenza vaccination among HCW, to assist public health policy makers, hospital managers and infection control practitioners in the development of successful vaccination programmes.
Methods
Published articles were identified searching PUBMED computerized database from January 1990 to December 2011 with a combination of keyword and subject heading searches using the following terms: ‘influenza’, ‘health personnel’, ‘vaccination’, ‘influenza vaccines’, ‘hospitals’ (see the exact search strategy in Annex 1). We also checked the reference lists of relevant publications, including reviews and meta‐analyses of interventions aimed at increasing influenza vaccination rates among HCW. The following selection criteria for inclusion were applied: the study implemented and evaluated a strategy aimed at increasing seasonal influenza vaccination uptake among HCW; the study population included HCW from acute care hospitals; the study compared the effect of the vaccination strategy against a historical or concurrent control; the study described all activities that were carried out before (historical control) and after the start of the vaccination strategy; the publication was published in English, French or German.
For the review of the selected studies, we evaluated the impact of intervention programmes implemented during an influenza season, which we have designated one ‘observation season’. Each intervention programme includes a set of individual intervention components, which are specific interventions used to raise influenza vaccine uptake rates in HCW, such as free vaccine, worker education and improved access. Some studies implemented and compared more than one set of intervention components in the same year in different facilities or settings. These were called intervention programme arms. The ‘baseline season’ is the season in the year prior to the introduction of an intervention programme evaluated by the study. An eligible study provided information about the vaccine uptake in the baseline season and the implementation phase as well as the components used to raise vaccine uptake.
According to the design and duration of interventions, we grouped the selected studies into four categories:
-
A)
Studies that implemented and evaluated one intervention programme in one observation season (1 year before‐and‐after studies). The baseline vaccine uptake served as a comparison group for the only implemented intervention programme. Effects are given as the percentage point difference.
-
B)
Studies that implemented and evaluated identical and/or distinct intervention programmes over consecutive observation seasons within the same facility (extended before‐and‐after studies). The baseline vaccine uptake served as a comparison group for the first intervention programme, and the uptake rates of subsequent intervention programmes were compared with those implemented during the immediately previous season. Effects are given as the percentage point difference.
-
C)
Studies that implemented and evaluated distinct intervention programme arms in different settings/facilities/HCW groups during the same observation season and with at least one concurrent control strategy for comparison (before‐and‐after studies with control). In type C studies, the vaccination activities used in the baseline season (baseline programme) may or may not have been continued during the observation season. A randomization step may have assigned study sites to specific strategies. Thus, according to the specific set‐up of a study, vaccine uptake in an intervention programme implemented in an observation season was compared with (i) the baseline uptake before the observation season (historical control), (ii) the vaccine uptake of a control intervention programme arm during the same observation season. This control intervention programme arm was usually the baseline programme (continued for another season) or the intervention programme arm with the fewest additional components. Effects are given as the percentage point difference and as the ratio of the odds to be vaccinated in the intervention group in comparison with the control group.
-
D)
Studies evaluating an intervention programme that was implemented consistently for more than 10 observation seasons. For these long‐term intervention programmes, vaccine uptake during the baseline season served as the only comparison. Effects are given as the percentage point difference.
Based on the study analysis, activities used to increase vaccine uptake included 10 intervention components that can be grouped into one of three categories:
Access related
1. Free vaccine: vaccination offered at no cost to the recipient HCW;
2. Flexible and worksite vaccine delivery: provision of convenient access to influenza vaccine at the worksite during different working shifts, typically by using mobile vaccination carts;
Knowledge and behaviour related
3. Education material: dissemination of information to increase awareness regarding the significance of influenza in the healthcare setting and the importance, safety and effectiveness of the vaccine for HCW. Methods included posters, pamphlets, mass mailings, newsletters, flyers;
4. Education sessions: for example, in‐service meetings, presentations and lectures;
5. Reminders: distribution of information indicating time and place of vaccine administration. Reminders were delivered verbally, by email and/or on paper;
6. Incentives: for example, distributed during a vaccination fair, gift incentives after vaccination, coupons for the hospital cafeteria, a raffle for a free vacation;
Management and policy related
7. Assignment of dedicated staff: assignment of one or more – mostly specially trained – staff member who organizes and promotes influenza vaccination among peers;
8. Feedback: information to HCW regarding the total rates of vaccine uptake to further motivate vaccine uptake;
9. Signed declination statements: requirement from HCW to sign a statement when the vaccine is declined, for reasons other than medical contraindications such as a religious belief or philosophical reasons; failure to be vaccinated does not result in termination of the contract;
10. Mandatory vaccination: requirement from HCW to receive influenza vaccination as a prerequisite for continued employment, unless exemptions based on medical or religious reasons were granted.
As the effectiveness and specific nature of interventions is very much dependent on institutional and cultural settings, as well as on different baseline approaches, our review did not attempt to estimate the overall magnitude of effect of a single intervention component.
Results
Our initial search in PUBMED yielded 423 published articles. In addition, 58 articles were identified through checking the reference lists of relevant publications. Of these 481 articles, we eliminated 289 articles because they addressed another study question and/or an infectious agent other than seasonal influenza. Other reasons for exclusion were that the study population did not include HCW in acute care hospitals (n = 91) or that the article did not provide control data or information about the activities implemented before and during the vaccination strategy (n = 77). Following the selection process, we identified 24 published articles describing 25 studies that met our inclusion criteria. These eligible studies evaluated the effectiveness of 45 multiple‐component intervention programmes. For the inventory of intervention components, we identified 14 type A, 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 four type B, 32 , 33 , 34 , 35 five type C 36 , 37 , 38 , 39 , 40 and two type D studies. 13 , 32 Altogether, A, B and C studies evaluated 43 intervention programmes or programme arms.
Of the selected studies, 16 (64%) were conducted in the USA, 13 , 18 , 20 , 21 , 22 , 24 , 25 , 26 , 30 , 31 , 32 , 34 , 35 , 36 , 40 six (24%) in Europe 19 , 27 , 28 , 29 , 37 , 39 and one each in Korea, 33 Singapore 38 and Brazil. 23 All but three studies 25 , 34 , 40 were carried out in individual hospitals and 14 (56%) in university hospitals. 13 , 20 , 22 , 23 , 24 , 27 , 28 , 29 , 31 , 32 , 33 , 37 , 39 , 40 The study populations of 17 studies (65%) were explicitly described as ‘healthcare workers’. However, four of the studies addressed hospital employees in general, 27 , 34 , 35 , 38 two non‐physician employees, 25 , 40 one medical residents 20 and one only HCW with direct patient contact. 24 Most of the studies ascertained vaccination status either through records of the vaccine provider or a self‐administered questionnaire for HCW.
Eight (32%) of 25 studies reported that their baseline programme activities only included the provision of vaccine at no cost. 20 , 21 , 22 , 23 , 27 , 28 , 32 All studies implemented intervention programmes that included more than one of our 10 identified intervention components. On average, a programme included five components. Among the 45 intervention programmes (among all A–D studies) reviewed, the four most commonly utilized components were free vaccine (42 programmes), educational material (39 programmes), reminders (35 programmes), and flexible and worksite vaccine delivery (31 programmes). As a general rule, the vaccine uptake after one intervention season (compared with the baseline uptake) tended to increase with the number of components included in the intervention programme (r 2 = 0·25), where each additional component was associated with an average increase in vaccine uptake of 5–6% (Figure 1).
Figure 1.
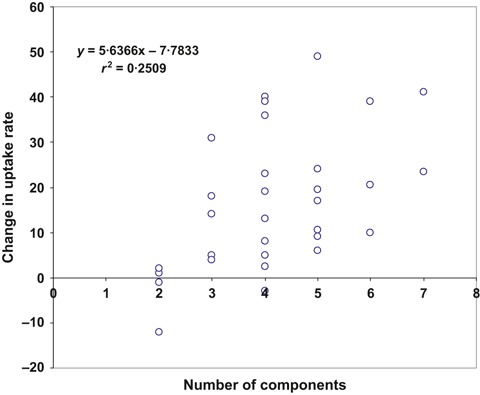
Illustration of the change in percentage points in vaccine uptake among healthcare workers after one intervention season compared with the uptake before the intervention seasons as a function of the number of intervention components used in the respective type A and B studies (n = 43).
Of the 25 studies reviewed, six (24%) were tailored to address‐specific findings of previous pre‐intervention surveys or studies that identified barriers to uptake, for example, information coming from a survey among the hospital’s HCW on knowledge about influenza or the vaccine, or on self‐reported reasons to receive or not receive influenza vaccine. 18 , 27 , 28 , 33 , 37 , 40 For example, one study associated with an increase from 21% to 38% based its educational sessions on survey information. 18 In another example, the pre‐intervention survey demonstrated important racial disparities that could then be addressed in the intervention programme. 40
Type A and B studies (1 year and extended before‐and‐after studies)
Fourteen studies implemented one intervention programme during one season (type A) and four implemented intervention programmes during two, four, four and five consecutive seasons, respectively (type B) (1, 2), 32 , 33 , 34 , 35 for a total of 29 intervention programmes. The 18 studies had a median baseline vaccination rate of 29% (range, 2%–73%), including three (17%) with a baseline of <10%. 23 , 26 , 27 Among the 29 intervention programmes, the median increase in vaccine uptake was 17%, ranging from −12% to 49%, compared with the prior season. Eight intervention programmes raised the uptake by more than 30% after 1 year. 20 , 22 , 23 , 24 , 26 , 33 , 35 , 38 The programme that experienced a decrease of 12% 32 attributed the decline to a vaccine shortage that impeded timely provision of vaccine to all HCW. Vaccine uptake of the 14 intervention programmes of type A started at a median of 23% (range, 2%–73%) and increased to a median of 45% (range, 4·5%–99%; Figure 1). Three of the four type B studies started at 54% (Figure 1), and all four reached high or very high uptake levels (78%, 98%, 98% and 99%). In general, the addition of more components led to substantial increases in type B studies.
Table 1.
Type A studies (n = 14) with one intervention programme in one season (no. 1–14). The column on increase in uptake indicates the difference after one observation season compared with the baseline season
| Programme | Author (year of publication) | Place of study | Tailored strategy | Components used before and continued during intervention programme | Intervention components added to previous season | Study population at baseline | Vaccine uptake before intervention | Uptake after one observation season | Increase in uptake after one season |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Begue (1998) 18 | New Orleans, USA | Yes | Free vaccine, educational material, flexible and worksite delivery, reminders | Educational sessions | 1100 | 21% | 38% | 17% |
| 2 | Bertin (2007) 30 | Cleveland, USA | No | Free vaccine, educational material, reminders, incentives, declination statement (paper forms), feedback | Declination statement (intranet‐based) | 20 170 | 38% | 55% | 17% |
| 3 | Gaughan (2010) 31 | Maywood, USA | No | Free vaccine, educational material, flexible and worksite delivery, reminders | Mandatory vaccination | 7484 | 73% | 99% | 26% |
| 4 | de Juanes (2007) 19 * | Madrid, Spain | No | Free vaccine, educational material, reminders | Flexible and worksite delivery | 5718 | 21% | 40% | 19% |
| 5 | Fedson (1996) 20 | Virginia, USA | No | Free vaccine | Flexible and worksite delivery, assignment of dedicated staff | 65 | 63% | 94% | 31% |
| 6 | Girasek (1990) 21 | New York, USA | No | Free vaccine | Educational material and sessions, reminders | 102 | 9–11%** | 30–36%*** | 21–25% |
| 7 | Hall (1998) 22 | Kentucky, USA | No | Free vaccine | Educational material, reminders, flexible and worksite delivery | 2358 | 34% | 83% | 49% |
| 8 | Lopes (2008) 23 | Sao Paulo, Brazil | No | Free vaccine | Educational material and sessions, flexible delivery | 20 000 | 6% | 45% | 39% |
| 9 | Llupia (2010) 29 | Barcelona, Spain | No | Free vaccine, educational material, flexible and worksite delivery | Reminders, incentives, feedback | 4783 | 24% | 37% | 13% |
| 10 | McCullers (2006) 24 | Memphis, USA | No | Free vaccine, educational campaign | Reminders, flexible and worksite delivery, assignment of dedicated staff, feedback | 702 | 45% | 86% | 41% |
| 11 | Ribner (2008) 25 | Atlanta, USA | No | Free vaccine, educational material, flexible and worksite delivery, feedback | Reminders, incentives, declination statement | 9214 | 43% | 67% | 24% |
| 12 | Shannon (1993) 26 | Lawrence, USA | No | Free vaccine, educational programmes | Incentives, flexible and worksite delivery, assignment of dedicated staff | 1500 | 5% | 44% | 39% |
| 13 | Smedley (2002) 27 | Southhampton, UK | Yes | Free vaccine | Educational material and sessions, reminders | 6706 | 2% | 4·5% | 2·5% |
| 14 | Tapiainen (2005) 28 | Basel, Switzerland | Yes | Free vaccine | Educational material and sessions, flexible and worksite delivery | 554 | 19% | 24% | 5% |
*This study carried out an intervention measure in the previous season that continued the components already used in the year before and increased vaccine uptake from 16% to 21%.
**9% among nurses, 11% among physicians.
***30% among nurses, 36% among physicians.
Table 2.
Type B studies (n = 4) with distinct or identical intervention programmes in consecutive years in the same facility (no. 1–15). For intervention programmes 1, 3, 7 and 12 the column on increase in uptake indicates the comparison in uptake to the baseline season; for the other intervention programmes the column indicates the increase to the intervention programme of the previous year
| Programme | Author (year of publication) | Place of study | Tailored strategy | Components used before and continued during intervention programme | Intervention components added to previous season | Study population at baseline | Vaccine uptake before intervention | Uptake after one observation season | Increase in uptake after one season |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Babcock (2010) 34 season 1 | St Louis, USA | No | Free vaccine, educational material and sessions, incentives, flexible and worksite delivery | Feedback, declination statement | ∼26 000 | 54% | 71% | 17% |
| 2 | Babcock (2010) 34 season 2 | St Louis, USA | No | See interventionabove | Mandatory vaccination | 25 980 | 71% | 98·4% | 27·4% |
| 3 | Poland (2005)*; season 1 of 4 | Rochester, USA | No | Free vaccine | Flexible andworksite delivery | ∼25 000 | 54% | 42% | −12% |
| 4 | Poland (CDC) (2005) 32 ; season 2 of 4 | Rochester, USA | No | See intervention programme 1 of 4 | No additional components compared to season 1 | ∼25 000 | 42% | 43% | 1% |
| 5 | Poland (2005); season 3 of 4 | Rochester, USA | No | See intervention programme 2 of 4 | Reminders, incentives | ∼25 000 | 43% | 56% | 13% |
| 6 | Poland (2005); season 4 of 4 | Rochester, USA | No | See intervention programme 3 of 4 | Educational material, assignment of dedicated staff | ∼25 000 | 56% | 76·5% | 20·5% |
| 7 | Rakita** (2010) 35 ; season 1 | Seattle, USA | No | Free vaccine, educational material and sessions, reminders, incentives, flexible and worksite delivery, assignment of dedicated staff | Mandatory vaccination | 4703 | 54% | 97·6% | 43·6% |
| 8–11 | Rakita (2010) 35 ; seasons 2–5 | Seattle, USA | No | See intervention above | No additional components compared to season 1 | 4742–4967 | 97·6% | 98·5%–98·9% | 0·9%–1·3% |
| 12 | Song*** (2006) 33 ; season 1 of 4 | Seoul, Republic of Korea | Yes | Vaccine supply at low cost | Educational material, reminders | 1·096 | 23% | 25% | 2% |
| 13 | Song (2006) 33 ; season 2 of 4 | Seoul, Republic of Korea | Yes | See intervention programme 1 of 4 | No additional components compared to season 1 | 1114 | 25% | 24% | −1% |
| 14 | Song (2006) 33 ; season 3 of 4 | Seoul, Republic of Korea | Yes | See intervention programme 2 of 4 | Flexible and worksite delivery | 1114 | 24% | 42% | 18% |
| 15 | Song (2006) 33 ; season 4 of 4 | Seoul, Republic of Korea | Yes | See intervention programme 3 of 4 | Free vaccine | 1131 | 42% | 78% | 36% |
*This peer vaccination programme at the Mayo Clinic (USA) added and evaluated intervention components during four consecutive influenza seasons. During the first intervention (2000/01), vaccination shortages and delays prevented many HCW from receiving vaccination.
**This first mandatory vaccination programme in the USA evaluated the same multi‐component intervention measures during five consecutive influenza seasons. Because of a national vaccine shortage, only 29·5% of employees were vaccinated prior to the first intervention season. We have therefore considered the vaccination rate of the previous season (54% in 2003/04) as the baseline rate.
***During four consecutive seasons, this study added two components in the first season, none in the second and each one in the third and fourth season.
For reasons stated above, we were unable to analyse components independently. Consequently, we describe the characteristics of the individual components without formal analysis:
Free vaccine
In all but one study, influenza vaccine was offered free of charge at baseline before the start of an intervention programme. Those who offered free vaccine as their only strategy at baseline had a median coverage of 19% before new interventions were added. One type B study in Singapore started with giving vaccines at low‐cost and implemented different components during 3 years. In the fourth year, free vaccine was added as the only new component of the intervention programme, and this led to a jump in uptake from 42% to 78%. 33
Flexible and worksite vaccine delivery
Twenty‐four (83%) of the 29 intervention programme seasons included the organization of flexible and worksite vaccine delivery using mobile carts or a similar approach to reduce inconvenience barriers to vaccination. These intervention programmes were associated with an average difference in vaccine uptake of 20% (range, −12%–49%), whereas the four programmes not using this component raised vaccine uptake on average by only 9% (range, −1%–23%). All of the seven intervention programmes that increased coverage by more than 30% used, among other intervention component, a flexible and worksite vaccine delivery. 20 , 22 , 23 , 24 , 26 , 33 , 35 The drop in coverage experienced by one intervention programme happened during a time of vaccine shortage, as mentioned above. 32 Two intervention programmes that used mobile carts or same‐service area vaccination as the only newly added intervention component to the previous season’s programme had increases of 18% and 19%, respectively. 19 , 33
Educational material/educational sessions
All but four intervention programmes 20 , 32 included the use of educational material as a means to improve HCW vaccination rates. Contents addressed the importance of nosocomial influenza, its mode of transmission, the seriousness of influenza especially for high‐risk patients, the rationale for vaccinating HCW and the effectiveness and safety of the vaccine. One innovative study involved HCW themselves who described in a humorous way their vaccination experience in weekly ‘peer‐to‐peer’ online messages combined with photographs of vaccinated HCW. 29 This prompted a discussion on patient safety in the HCW social networks which, after many years of stagnation, was associated with an increase in vaccination rates from 24% to 37%.
Reminders
Twenty‐one (72%) of 29 intervention programmes included reminders to HCW for vaccination. Methods varied from sending printed newsletters, email notices, personal letters inserted with the pay check, to posters located throughout the institution.
Incentives
Different forms of incentives were incorporated as a component in 14 (48%) intervention programmes, including free food, movie tickets or health books that were distributed during a vaccination fair. One multi‐year study reported that the addition to the previous season of only incentives as a single intervention component raised vaccine uptake from 43% to 56% after it had dropped or stagnated in the previous 2 years. 32
Assignment of dedicated staff
Nine (31%) intervention programmes utilized specially trained or otherwise enlisted staff members dedicated to organizing and promoting influenza vaccination among HCW. Examples included (i) one nurse who was assigned personal responsibility for the vaccination programme and provided vaccine to HCW at their worksites; 20 (ii) an employee health nurse who made appointments with each hospital department to stress the importance and safety of the vaccine and to ensure on‐site vaccination at a time convenient to each shift; 26 and (iii) the introduction of ‘influenza vaccine champions’ (i.e. employee health and infection control staff members) who conducted grand rounds on the subject, distributed notices to all employees by e‐mail, attended meetings with nursing supervisors, established a telephone hotline and answered questions at the vaccination clinics. 32 Three of the six intervention programmes with a 30% increase above the baseline 20 , 24 , 26 assigned one nurse the responsibility to raise vaccination rates, resulting in almost universal vaccination uptake in one programme conducted at a general medicine clinic (94%). 20 In one study, supervisors were instructed to contact unvaccinated personnel individually and urge them to get vaccinated. 24
Feedback
Six (21%) of 29 intervention programmes provided regular feedback to HCW regarding their overall vaccination rates, 24 , 25 , 29 , 30 , 34 for example, by providing periodic updates during staff meetings or in frequented areas of the hospitals. 24 , 25 Intervention programmes that included feedback were associated with an increase between 44% and 76% in vaccine uptake.
Signed declination statement
Three intervention programmes included the use of a signed declination statement that required HCW to sign a form when the vaccine is refused, meanwhile ensuring that HCW receive appropriate information regarding the rationale and benefits of vaccination. These three programmes were associated with an increase in uptake from 43% at baseline to 67% (24% increase 25 ), 54%–71% (17% increase 34 ) and 38%–55% (17% increase 30 ). All three programmes had some similarities: they had tried other components in previous seasons, but felt to have reached a limit; were supported by top management; and combined the policy with other concurrent strategies, such as appointment of a special implementation team, additional educational efforts and extensive communication to and with staff. One used an intranet‐based tool requiring HCW to document vaccination or declination for medical or other reasons with the dual use to educate staff. 30 In consequence, the specific contribution of the declination statement alone is unclear.
Mandatory vaccination
Three studies in the USA evaluated intervention programmes that involved mandatory influenza vaccination policies with resulting vaccination rates of >98%. 31 , 34 , 35 Similar to the programmes that had introduced a declination statement, the policy of mandatory vaccination was accompanied by a number of other components. In 2004, a hospital in Seattle was the first medical centre to make annual influenza vaccination a mandatory requirement of every employee by making it a condition for employment. 35 In this study, previous campaigns with a large number of intervention components had been used, but vaccine uptake had remained between 30% and 54%. The multi‐component intervention programme was then able to increase vaccine uptake to 97·6%, and to sustain rates of more than 98% during the four subsequent influenza seasons after the addition of a mandatory vaccination policy. The second study that introduced the requirement of a declination statement for non‐vaccines was associated with an increase from 54% to 71% in the first season. 34 In the second season, mandatory vaccination was implemented and achieved a 98·4% vaccination rate among approximately 26 000 employees. 34 Both studies reported that having strong and visible leadership support as well as extensive communication was crucial in improving uptake rates successfully and in overcoming objections raised by HCW. In the third study, vaccine uptake increased from 73% to 99·2% when mandatory vaccination was added to other strategies. 31 In all three programmes, exemptions for medical or religious reasons were possible. Employees who were granted an exemption were required or encouraged to wear a surgical mask during the influenza season. Only a very small proportion of employees (0·03%–0·15%) were terminated or left the medical centre as a result of the policy.
Type C studies (before‐and‐after studies with control)
Five multi‐arm type C studies 36 , 37 , 38 , 39 , 40 implemented 14 intervention programmes (Table 3). In three studies, 36 , 38 , 40 the baseline approach was continued in at least one site during the intervention season, which allowed comparison of post‐intervention vaccination rates of interventions to concurrent control measures.
Table 3.
Study type C (n = 5; before‐and‐after studies with control). Type C studies conducted more than one intervention programme during the same observation season. In the second to the last column, the vaccination rates before and after the 14 interventions were used to calculate odds ratios and 95% confidence intervals, showing the effect to improve vaccination rates compared with the historical control (at baseline). The odds ratios in the last column show the effect of intervention programmes (n = 7) in comparison with concurrent control measures, that is, where the baseline measures were continued 36 , 38 , 40
| Programme | Author (year of publication) | Place of study | Tailored strategy | Components used before and continued during intervention programme | Components added to previous season in intervention (IG) and control group (CG) | Study population | Baseline vaccination rate | Coverage after one season | Increase after one season | OR (95% CI) cf. to historical control | OR (95% CI) cf. to concurrent control |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Doratotaj (2008)* 36 | Cleveland, USA | No | Free vaccine, educational sessions, reminders | CG: no additional components (baseline approach) | 200 | 34% | 38% | 4% | 1·19 (0·77–1·83) | NA |
| 2 | Doratotaj (2008) 36 | Cleveland, USA | No | See campaign at baseline | IG: incentives | 200 | 34% | 39% | 5% | 1·24 (0·81–1·91) | 1·04 (0·68–1·59) |
| 3 | Doratotaj (2008) 36 | Cleveland, USA | No | See campaign at baseline | IG: educational material | 200 | 34% | 42% | 8% | 1·41 (0·92–2·15) | 1·18 (0·78–1·80) |
| 4 | Doratotaj (2008) 36 | Cleveland, USA | No | See campaign at baseline | IG: educational material, incentives | 200 | 34% | 44·5% | 10·5% | 1·56 (1·02–2·38) | 1·31 (0·86–1·99) |
| 5 | Harbarth(1998)** 37 | Geneva, Switzerland | Yes | Free vaccine, reminders | CG: educational material | 4356 | 9% | 23% | 14% | 3·02 (2·66–3·43) | NA |
| 6 | Harbarth(1998) 37 | Geneva, Switzerland | Yes | See campaign at baseline | IG: educational material and sessions, flexible and worksite delivery | 1076 | 13% | 37% | 24% | 3·93 (3·15–4·91) | NA |
| 7 | Lee (2007)*** 38 | Singapore | No | Free vaccine, educational material, incentives | CG: no additional components (baseline approach) | 5946 | 57% | 61% | 4% | 1·19 (1·11–1·28) | NA |
| 8 | Lee (2007) 38 | Singapore | No | See campaign at baseline | IG: flexible and worksite delivery | 5946 | 57% | 97% | 40% | 24·39 (20·82–28·72) | 20·50 (17·5–24·1 |
| 9 | Sartor (2004) † 39 | Marseille, France | No | Free vaccine, reminders | CG: educational material | 2349 | 7% | 4% | −3% | 0·55 (0·42–0·73) | NA |
| 10 | Sartor (2004) 39 | Marseille, France | No | See campaign at baseline | IG: educational material, flexible and worksite delivery | 2216 | 7% | 26,5% | 19·5% | 4·79 (3·95–5·81) | NA |
| 11 | Zimmerman (2009)†† 40 | Pittsburgh, USA | Yes | Free vaccine, educational material, reminders, feedback | CG: no additional components (baseline approach) | 1247 | 32% | 34·5% | 2·5% | 1·12 (0·94–1·33) | NA |
| 12 | Zimmerman (2009) 40 | Pittsburgh, USA | Yes | See campaign at baseline | IG: incentives | 3904 | 32% | 38% | 6% | 1·30 (1·19–1·43) | 1·16 (1·02–1·33) |
| 13 | Zimmerman (2009) 40 | Pittsburgh, USA | Yes | See campaign at baseline | IG: flexible and worksite delivery | 2461 | 30% | 39% | 9% | 1·49 (1·32–1·68) | 1·21 (1·05–1·40) |
| 14 | Zimmerman (2009) 40 | Pittsburgh, USA | Yes | See campaign at baseline | IG: incentives, flexible and worksite delivery | 6500 | 31% | 41% | 10% | 1·55 (1·44–1·66) | 1·32 (1·16–1·50) |
*In this study at an urban tertiary care hospital, four groups consisting of randomly selected HCW of different professional categories were simultaneously exposed to distinct sets of intervention components. Beyond the usual annual vaccination campaign, the intervention groups were randomly assigned to either receive a raffle ticket offer to win a vacation, an educational letter explaining the importance of influenza vaccination for HCW, or both the raffle ticket offer and the educational letter. The control group received only components that were already offered during the annual vaccination campaign (educational posters, newsletters, T‐shirts, buttons, department meetings and open access for long hours at multiple influenza vaccination sites).
**This study provided several intervention components at three departments with high‐risk patients for nosocomial influenza only, while basic components (education, reminders) were applied in the entire institution. Vaccination rates were then compared between these two areas.
***The reporting rate of employees attending worksite vaccine delivery was 86·9% versus 70·4% at vaccination booths (OR, 2·77; 95% CI, 2·29–3·37). However, only 24% of all employees were reached by worksite vaccination arrangements.
†The table provides the vaccination rates achieved by the employee health unit among all HCW (upper row) and by the mobile cart programme among those who had not been vaccinated by the health unit or otherwise (lower row).
††This study involved non‐physician employees in a large healthcare system in the USA. Of the 11 participating facilities, four had incentives including a grocery store gift card and lottery to win a paid day off (second row), while two had mobile vaccination carts only, (third row) three facilities had both incentives and carts (forth row), and two control sites had neither (first row).
Similar to the type A studies, flexible and worksite delivery appeared to lead to a substantial increase in uptake. In all five programmes that involved flexible and worksite delivery alone 38 , 39 , 40 or in combination with either educational sessions 37 or incentives, 40 HCW in the intervention group were more likely to receive the vaccine than those in the historical or concurrent control groups. Three of these intervention programmes resulted in a substantial increase (19·5%–40%) compared with the uptake at baseline. 37 , 38 , 39 One of these studies reported that the intervention programme that included a mobile cart vaccination programme increased vaccine uptake by 20% in the first year compared with a decline of 3% among those HCW who only had the opportunity to receive vaccine at inconvenient locations. 39 A later analysis of reasons for non‐acceptance of influenza vaccine found that inconvenient access was one of the main barriers for vaccination. A study from Switzerland implemented an intervention programme that included educational material and reminders in the entire institution, but in three departments with high‐risk patients, the authors also organized on‐site vaccination and educational conferences to address the issues that were identified in a previous survey as main reasons for non‐receipt of vaccination. 37 In the departments where these additional intervention components were applied, the mean increase in uptake over baseline was significantly higher compared with that observed in the rest of the institution (24% versus 14% increase, respectively; P < 0·001). In a study from Singapore employees who were offered vaccine at their worksites were significantly more likely to be vaccinated compared with those employees who only attended the usual vaccination booths (40% versus 4% increase from 57% baseline respectively; P < 0·001). 38
In two studies where only the provision of incentives was added to the baseline vaccination activities, 36 , 40 only one 40 was associated with a significant increase in uptake. The latter compared the rates for educational material and reminders only (arm 1, continued baseline approach), addition of incentives (arm 2), use of mobile vaccination carts (arm 3) or both incentives and mobile carts (arm 4) with the baseline uptake. Although increases in uptake rate were small (5–10%), arms 2–4 significantly increased the vaccination rates compared with the pre‐intervention baseline season because of the large number of HCW involved. The increase in arm 1 (2·5%) was not significant. The other study, a randomized controlled trial, showed no statistical difference in uptake rates between the continued baseline programme, the use of incentives (a raffle), educational letters and both combined. 36 However, the announcement of an influenza vaccine shortage during the intervention season of this study may have prevented a number of HCW from vaccine receipt as they potentially believed they were not a priority group for vaccination.
Type D studies
Two long‐term studies conducted in the USA had observation periods of 12 and 18 years (Table 4). 13 , 32 Although these studies started at low baseline uptake levels (<25% and 4%), eventually they achieved coverage rates of approximately two‐thirds of the HCW. In the 12‐year long study, providing regular feedback was a prominent part of a comprehensive and long‐term strategy that was associated with an increase in vaccination rates from 4% at baseline to 67% as well as a significant decrease in nosocomial influenza among patients over time. 13 The 18‐year study established a ‘mobile vaccination cart programme’ that pre‐scheduled vaccination times for all HCW, streamlined documentation of vaccination and trained nurses to educate HCW about additional methods for preventing nosocomial influenza transmission, such as proper hand hygiene. 32 The study authors attributed the increase in vaccine uptake to 65% to ‘the cumulative impact of ongoing education, communication and access’.
Table 4.
Study type D. Type D studies implemented and assessed long‐term intervention programmes with an observation period of more than 10 years
| Programme | Author (year of publication) | Place of study | Tailored strategy | Components used before and continued during intervention programme | Intervention components added to baseline approach | Study population at baseline | Baseline vaccination rate | Coverage after one observation season | Coverage after several observation seasons | Increase after several seasons |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nichol (CDC) (2005) 32 | Minneapolis, USA | No | Free vaccine | Educational material, reminders, flexible and worksite delivery, assignment of dedicated staff | 3177 | <25% | Not indicated | 65% (after 18 years) | 40% (after 18 years) |
| 2 | Salgado (2004) 13 | Charlottville, USA | No | Free vaccine, educational material*, reminders | Flexible and worksite delivery, feedback | Not indicated | 4% | 26% | 67% (after 12 years) | 63% (after 12 years) |
*Summary of CDC guidelines used as annual memorandum.
Discussion
Few methodologically rigorous studies have been published on how to successfully and sustainably raise influenza vaccine uptake rates among HCW; however, several insights emerged from our review of the available literature. We found in our limited analysis that programmes using a larger number of intervention components achieved higher vaccine coverage. Among specific strategies reported to have high success rates, the provision of free vaccine seems to be indispensable. The most effective intervention, however, appears to be a mandatory vaccination policy for healthcare workers. The three programmes that used this strategy achieved nearly universal coverage. While most studies reviewed were implemented during a single season, we found evidence that sustained efforts lead to high and sustained vaccination uptake rates.
Provision of free vaccine was used in almost all programmes at baseline, but was formally evaluated in one study where it appeared to be the crucial component to substantially improve vaccine uptake after other strategies before showed either zero or a lesser success. 33 This finding is consistent with results of a meta‐analysis of studies that have investigated self‐reported reasons for non‐receipt of the vaccine where inconvenient access has been identified as one of the major obstacles, particularly for physicians. 17
Other useful intervention components included flexible and worksite vaccine delivery, the assignment of staff dedicated to take responsibility for the programme, and provision of educational materials. Regarding the latter, an important aspect of designing educational material is that nurses and physicians will likely need to be targeted in different ways. 21 , 41 This is supported by several studies that found that the vaccination rate of nurses was significantly lower than that of physicians, 19 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 and research indicating large differences in attitudes and knowledge concerning influenza vaccination between nurses and physicians. 37 , 41 , 42 , 50 , 51 , 52 , 53 , 54 , 55 Educational messages will likely need to be conveyed in many different ways to increase the likelihood and frequency of encounters. A recent meta‐analysis on interventions for adult immunization programmes supports the notion of the potential positive effects incentives may have. 56 Understanding knowledge and attitudes prior to the intervention has been used to tailor intervention programmes and shape the contents of educational activities to local needs and was also assessed by several studies. Although some of these tailored studies reported a substantial increase in uptake, their success could not be linked directly to the conducted survey. 18 , 33 , 37 Because local conditions, peer opinion, cultural, institutional and logistical factors will all lead to differences in knowledge and behaviour and because reasons for refusal of the vaccine cover a wide and diverse spectrum, the use of a pre‐intervention survey makes intuitive sense.
Making vaccination a mandatory condition of initial and continued HCW employment is likely to be the most controversial, but also successful method for increasing uptake. 57 , 58 , 59 , 60 , 61 , 62 A recent survey among US HCW who reported working at a facility where vaccination was required by their employer, 98·1% were vaccinated. 63 Insufficient vaccine uptake levels have prompted numerous healthcare facilities in the USA to institute mandatory programmes for their HCW to protect patients. 62 , 64 , 65 Moreover, several US professional societies recommended that influenza vaccination of HCW be made mandatory, 66 and several studies showed support for this policy among HCW in the USA and medical students in Germany. 67 , 68 , 69 , 70 However, the vaccination mandate in the USA has met considerable resistance. HCW protested against the implementation of a vaccination requirement and made a successful legal challenge on the basis that the hospital had violated the terms of their contracts. 57 , 60 , 61 , 71 Opponents claim that a mandate violates HCW personal autonomy and right to make medical decisions concerning their body themselves and that it may ‘alienate staff and damage morale’, undermine trust and negatively affect employee–employer relationship. 58 , 60 , 61 , 62 , 71 , 72 Commentators who support mandatory vaccination assume the failure of voluntary vaccination strategies and argue that the benefits for patients outweigh burdens and risks of vaccination on behalf of the HCW and that the restrictions of HCW autonomy and freedom of choice are therefore ethically justified, unless a valid medical contraindication exists. 62 , 66 , 68 , 73 , 74 , 75 , 76 From their perspective, mandatory programmes meet the professional values and codes of ethics adopted by HCW, that is, to do no harm and to act in patients’ best interests. 58 , 77 However, while the prevention of harm to others is a potential reason for the limitation of autonomy, mandatory measures are only justified under certain conditions. 71 , 75 , 78 , 79 , 80 Successful programmes presented in this review have made substantial organizational and educational efforts prior to the start of the mandatory policy suggesting that a mandatory programme must not be used as the easy, administrative magic bullet, but needs at least contemporaneous or even better antecedent implementation of a multifaceted programme using other elements described in this review to maximize chances for a ‘friendly reception’ of the policy by staff.
One alternative or compromise may be represented by a recently published study that implemented a ‘multifaceted patient safety programme’ combining the institutional responsibility to protect patients from nosocomial infections with the HCWs’ right for vaccine declination for any reason. 81 The programme, which was associated with a vaccination rate of 96%, required all employees with direct patient contact 1 to choose between vaccination and an ‘appropriate non‐vaccine alternative’, that is, either to wear a surgical mask or to exclude patient contact. Other authors have also made the case for a ‘combined approach’ using ‘opt‐out’ declination statements in conjunction with the exclusion of unvaccinated HCW from work in defined areas where the most vulnerable patients are cared for, such as ICUs, oncology, transplantation. 82 , 83
There is evidence, however, that sustained efforts of voluntary vaccination programmes are capable of leading to high vaccination rates nevertheless. Two institutions in the USA who observed results of their interventions for 12 and 18 years, respectively, were both able to increase vaccination uptake rates from moderately or very low levels to two‐thirds of their targeted health personnel. 13 , 32 Moreover, experience from the USA indicates that on a country‐wide scale, it requires many years to raise influenza vaccination rates substantially. In 1989, the national vaccination coverage among HCW was still at the 10% level, but was estimated at 34% in 1997, 44% in 2003 and at 63·5% in 2011. 16 , 55 , 63 , 84 These long‐standing efforts would indicate a degree of management support for the programme, a likely factor in success not explicitly measured in any of the studies reviewed.
Finally, one unique intervention approach not included in our review was tried by the MOH of Germany that conducted a nationwide, low‐cost, 2‐year vaccination programme targeted to all hospitals in the country. 41 The material included posters, pamphlets and briefing notes for mass mailings and a presentation for informational sessions. An evaluation of a convenience sample of 20 hospitals showed that the uptake rate increased only in those who used the material, albeit by <10% points indicating that such an approach can effectively complement and support efforts in individual hospitals to raise influenza vaccination uptake rates.
The typical study evaluated one intervention programme with multiple intervention components in one facility during a single season and the studies reported a wide variety of interventions. Consequently, our retrospective analysis has a number of limitations. First, the reporting of intervention studies is likely subject to publication bias as studies without an increase in vaccination rate after an intervention are less likely to be published. Second, although we attempted to make interventions more comparable by grouping them into distinct components, it is clear that neither individual intervention components nor intervention programmes can be standardized and any comparisons should be made with caution. Intervention components were not mutually exclusive and often one activity entailed elements of two different components. For example, worksite delivery of vaccines was often combined with education on‐site. Furthermore, some single activities had overlapping purposes, so it was difficult to assign them to one intervention component. For example, there may have been reminders that repeated educational messages. Third, each component may be implemented in a number of different ways. For example, the provision of educational material may differ in wording, in the way it is presented, its appropriateness for the target audience, where and how and in which number it is presented or delivered, and of course other often intangible factors. Lastly, comparison of the effect of components also may vary depending on the type of comparison group, if a control group was included in the design, or if settings were randomized. For example, before‐and‐after studies without comparable control group are logistically easier but do not control for any influence from outside the intervention programme, such as vaccine shortage or changes in awareness over time.
In conclusion, the authors believe that vaccination of HCW is a key part of a strategy to prevent influenza in groups who are most at risk of complications. The reviewed literature suggests that while no single component is capable of raising influenza vaccination rates in HCW rapidly and to a relevant degree, except perhaps mandatory vaccination, a comprehensive, well‐supported, well‐staffed and well‐planned, multifaceted vaccination intervention programme can raise uptake rates substantially and sustainably. Indeed, it seems likely that in such a multifaceted programme, the individual components described in this review would support each other and perhaps have a synergistic effect. A successful programme would contain as many elements as possible (Text box 1); however, in resource‐limited settings, hospital managers might want to focus on two components that seem to be most effective in rapidly raising vaccination rates. First, flexible access to free vaccination is key to overcome time‐ and access‐related barriers to vaccine uptake. Second, the approach for a successful HCW vaccination programme requires culturally sensitive education on the risk of influenza and the overall benefits of vaccination, tailored to specific professional characteristics. Periodic surveys can help to identify specific motivators and barriers of HCW vaccine receipt and to tailor programmes accordingly. Programmes that attain higher vaccination rates can expect in turn to reduce the risk of nosocomial influenza infections and related healthcare costs. 13 , 14 It is also worth noting that predictor studies have found consistently that ‘history of influenza vaccination’ is perhaps the most reliable factor associated with vaccine receipt in the next season. 43 , 45 , 48 , 85 , 86 While these previously vaccinated HCW represent ‘low hanging fruit’ for an intervention programme, it also means that HCW who have rejected the vaccine in previous years may be particularly resistant. Programmes aiming for high rates of coverage will need to target this group of HCW. Hospital managers who consider influenza vaccination uptake rates in their employees as a quality marker for their facility should be prepared to commit the required human and financial resources to meet this goal.
Text box 1. Elements suggested for a successful HCW vaccination programme
1. Commitment of and strong support by the hospital’s top management;
2. Pre‐intervention information collection to identify important barriers to vaccination with a consequent adjustment (tailoring) of the intervention programme to the gained experience from the pre‐intervention investigations (e.g. profession, gender, race sensitive);
3. Provision of free vaccine;
4. Organization of easily accessible vaccine, for example, through flexible and worksite delivery;
5. Organization of several activities belonging to the components educational material, education session, reminders, incentives;
6. Management optimization, such as (i) assignment of (one or more) dedicated staff to organize and actively promote the measure, and/or (ii) giving feedback of vaccination uptake rates during the preparation phase for the influenza season;
7. In a well‐prepared setting: requirement of all HCW to become vaccinated against influenza with the possibility to opt‐out by signing a declination statement;
8. Continuation of the assessment – planning – intervention cycle for several years.
Disclaimer
The first author is a staff member of the World Health Organization and alone is responsible for the views expressed in this publication, and these views do not necessarily represent the decisions or policies of the World Health Organization.
Funding
In 2002, the Swiss Federal Office of Public Health supported the WHO Global Influenza Programme (GIP) in its endeavour to increase influenza vaccination coverage globally, particularly in high‐risk groups and healthcare workers. This support (fund of $33 500) included contributions to several activities aimed at increasing influenza vaccination coverage, including the assessment of factors influencing vaccination rates among healthcare workers.
Acknowledgements
We are grateful to Simon Derpmann, Gregory Poland, David Scales, Mikiko Senga, Jason Sigurdson, Sebastian Stein and the Swiss Federal Office of Public Health for their support.
© 2012 Blackwell Publishing Ltd. The World Health Organization retains copyright and all other rights in the manuscript of this article as submitted for publication
Annex 1: Search strategy for review of interventions to increase influenza vaccination among healthcare workers in hospitals
To assist in the development of successful vaccination programmes, we reviewed studies where interventions aimed to increase the uptake of influenza vaccination among hospital healthcare workers (HCW). For the inventory of intervention studies, published articles were identified searching PUBMED computerized database from January 1990 to December 2011 with a combination of keyword and subject heading searches using the following terms: ‘influenza’, ‘health personnel’, ‘vaccination’, ‘influenza vaccines’, ‘hospitals’. We also checked the reference lists of relevant publications, including reviews and meta‐analyses of interventions aimed at increasing influenza vaccination rates among HCW. The exact search strategy included the following steps (Figure A1):
Figure A1.
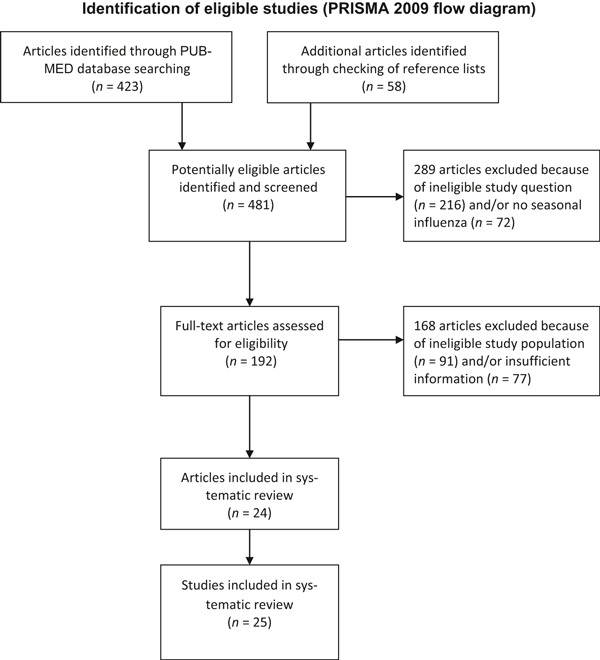
Identification of eligible studies (PRISMA 2009 Flow Diagram).
Step 1
(‘Influenza, Human’[Mesh] OR ‘Influenza B virus’[Mesh] OR ‘Influenza A virus’[Mesh] OR ‘Influenzavirus B’[Mesh] OR ‘Influenzavirus A’[Mesh] OR influenza*[TIAB] OR ‘flu’[TIAB] OR ‘grippe’[TIAB] OR ‘seasonal influenza’[Text Word] OR ‘flu’[Text Word]).
Step 2
(‘Health Personnel’[MeSH] OR ‘Allied Health personnel’[MeSH] OR ‘Caregivers’[MeSH] OR ‘Physicians’[MeSH] OR ‘Medical Staff, Hospital’[MeSH] OR ‘Health Personnel’[tw] OR ‘Healthcare workers’[tw] OR ‘Healthcare worker’[tw] OR ‘Medical Staff ‘[tw] OR ‘General Practitioners’[tw] OR Caregivers [tw] OR ‘Nurses’[MeSH] OR ‘Nurses’ Aides’[MeSH] OR ‘Nurse Practitioners’[MeSH] OR ‘Nurses’ [tw] OR ‘Nurse’ [tw] OR ‘Midwives’[tw] OR ‘Nurse Practitioners’ [tw] OR Nursing Staff [tw] OR ‘Students, Medical’[MeSH] OR ‘Students, Nursing’[MeSH] OR ‘Medical students’ [tw] OR ‘Nursing students’[tw]).
Step 3
(‘Hospital Units’[Mesh] OR ‘Hospitals’[Mesh] OR ‘hospital’ [TIAB] OR ‘hospitals’ [TIAB]).
Step 4
(‘Vaccination’[Mesh] OR ‘immunization’ [TIAB] OR ‘immunisation’ [TIAB]) OR ‘Vaccines’[Mesh] OR vaccination [TIAB] OR vaccines [TIAB] OR vaccine [TIAB]).
Step 5
(‘Influenza Vaccines’[Mesh] OR ‘Influenza Vaccines’ [TIAB] OR ‘Flu Vaccines’ [TIAB] OR ‘Influenza Vaccine’ [TIAB] OR ‘Flu vaccine’ [TIAB]).
Combinations
| Combo #1 – Step1 and Step2 and Step3 and Step4 | 489 |
| Combo #2 – Step2 and Step3 and Step5 | 359 |
| Combo #1 or Combo #2 | 491 |
Limits
| Publication Date from 1990/01/01 to 2011/12/31 and English, French, German | 423 |
Additional articles
| Additional articles (n = 58) that were identified through checking of the reference lists of relevant publications | 481 |
Included employees who accessed patient care areas or worked within six feet of patients during influenza season.
References
- 1. Voirin N, Barret B, Metzger MH, Vanhems P. Hospital‐acquired influenza: a synthesis using the Outbreak Reports and Intervention Studies of Nosocomial Infection (ORION) statement. J Hosp Infect 2009; 71:1–14. [DOI] [PubMed] [Google Scholar]
- 2. Salgado CD, Farr BM, Hall KK, Hayden FG. Influenza in the acute hospital setting. Lancet Infect Dis 2002; 2:145–155. [DOI] [PubMed] [Google Scholar]
- 3. Malavaud S, Malavaud B, Sandres K et al. Nosocomial outbreak of influenza virus A (H3N2) infection in a solid organ transplant department. Transplantation 2001; 72:535–537. [DOI] [PubMed] [Google Scholar]
- 4. Sartor C, Zandotti C, Romain F et al. Disruption of services in an internal medicine unit due to a nosocomial influenza outbreak. Infect Control Hosp Epidemiol 2002; 23:615–619. [DOI] [PubMed] [Google Scholar]
- 5. Evans ME, Hall KL, Berry SE. Influenza control in acute care hospitals. Am J Infect Control 1997; 25:357–362. [DOI] [PubMed] [Google Scholar]
- 6. Horcajada JP, Pumarola T, Martinez JA et al. A nosocomial outbreak of influenza during a period without influenza epidemic activity. Eur Respir J 2003; 21:303–307. [DOI] [PubMed] [Google Scholar]
- 7. Wilde JA, McMillan JA, Serwint J, Butta J, O’Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA 1999; 281:908–913. [DOI] [PubMed] [Google Scholar]
- 8. Scheifele DW, Bjornson G, Johnston J. Evaluation of adverse events after influenza vaccination in hospital personnel. CMAJ 1990; 142:127–130. [PMC free article] [PubMed] [Google Scholar]
- 9. Potter J, Stott DJ, Roberts MA et al. Influenza vaccination of health care workers in long‐term‐care hospitals reduces the mortality of elderly patients. J Infect Dis 1997; 175:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carman WF, Elder AG, Wallace LA et al. Effects of influenza vaccination of health‐care workers on mortality of elderly people in long‐term care: a randomised controlled trial. Lancet 2000; 355:93–97. [DOI] [PubMed] [Google Scholar]
- 11. Hayward AC, Harling R, Wetten S et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ 2006; 333:1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burls A, Jordan R, Barton P et al. Vaccinating healthcare workers against influenza to protect the vulnerable‐‐is it a good use of healthcare resources? A systematic review of the evidence and an economic evaluation Vaccine 2006; 24:4212–4221. [DOI] [PubMed] [Google Scholar]
- 13. Salgado CD, Giannetta ET, Hayden FG, Farr BM. Preventing nosocomial influenza by improving the vaccine acceptance rate of clinicians. Infect Control Hosp Epidemiol 2004; 25:923–928. [DOI] [PubMed] [Google Scholar]
- 14. Weinstock DM, Eagan J, Malak SA et al. Control of influenza A on a bone marrow transplant unit. Infect Control Hosp Epidemiol 2000; 21:730–732. [DOI] [PubMed] [Google Scholar]
- 15. Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature. Ann Intern Med 1995; 123:518–527. [DOI] [PubMed] [Google Scholar]
- 16. Fiore AE, Shay DK, Broder K et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58:1–52. [PubMed] [Google Scholar]
- 17. Hollmeyer HG, Hayden F, Poland G, Buchholz U. Influenza vaccination of health care workers in hospitals‐‐a review of studies on attitudes and predictors. Vaccine 2009; 27:3935–3944. [DOI] [PubMed] [Google Scholar]
- 18. Begue RE, Gee SQ. Improving influenza immunization among healthcare workers. Infect Control Hosp Epidemiol 1998; 19:518–520. [DOI] [PubMed] [Google Scholar]
- 19. de Juanes JR, Garcia dC, Arrazola MP, Jaen F, Sanz MI, Gonzalez A. Influenza vaccination coverage among hospital personnel over three consecutive vaccination campaigns (2001–2002 to 2003–2004). Vaccine 2007; 25:201–204. [DOI] [PubMed] [Google Scholar]
- 20. Fedson DS. Influenza vaccination of medical residents at the University of Virginia: 1986 to 1994. Infect Control Hosp Epidemiol 1996; 17:431–433. [DOI] [PubMed] [Google Scholar]
- 21. Girasek DC. Increasing hospital staff compliance with influenza immunization recommendations. Am J Public Health 1990; 80:1272–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hall KL, Holmes SS, Evans ME. Increasing hospital employee participation in an influenza vaccine program. Am J Infect Control 1998; 26:367–368. [DOI] [PubMed] [Google Scholar]
- 23. Lopes MH, Sartori AM, Mascheretti M et al. Intervention to increase influenza vaccination rates among healthcare workers in a tertiary teaching hospital in Brazil *. Infect Control Hosp Epidemiol 2008; 29:285–286. [DOI] [PubMed] [Google Scholar]
- 24. McCullers JA, Speck KM, Williams BF, Liang H, Mirro J Jr. Increased influenza vaccination of healthcare workers at a pediatric cancer hospital: results of a comprehensive influenza vaccination campaign. Infect Control Hosp Epidemiol 2006; 27:77–79. [DOI] [PubMed] [Google Scholar]
- 25. Ribner BS, Hall C, Steinberg JP et al. Use of a mandatory declination form in a program for influenza vaccination of healthcare workers. Infect Control Hosp Epidemiol 2008; 29:302–308. [DOI] [PubMed] [Google Scholar]
- 26. Shannon SC. Community hospitals can increase staff influenza vaccination rates. Am J Public Health 1993; 83:1174–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smedley J, Palmer C, Baird J, Barker M. A survey of the delivery and uptake of influenza vaccine among health care workers. Occup Med (Lond) 2002; 52:271–276. [DOI] [PubMed] [Google Scholar]
- 28. Tapiainen T, Bar G, Schaad UB, Heininger U. Influenza vaccination among healthcare workers in a university children’s hospital. Infect Control Hosp Epidemiol 2005; 26:855–858. [DOI] [PubMed] [Google Scholar]
- 29. Llupia A, Garcia‐Basteiro AL, Olive V et al. New interventions to increase influenza vaccination rates in health care workers. Am J Infect Control 2010; 38:476–481. [DOI] [PubMed] [Google Scholar]
- 30. Bertin M, Scarpelli M, Proctor AW et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37. [DOI] [PubMed] [Google Scholar]
- 31. Gaughan BA. The successful implementation of mandatory seasonal influenza vaccination for health care workers. Am J Infect Control 2010; 38:e51. [Google Scholar]
- 32. Centers for Disease Control and Prevention (CDC) . Interventions to increase influenza vaccination of health‐care workers‐‐California and Minnesota. MMWR Morb Mortal Wkly Rep 2005; 54:196–199. [PubMed] [Google Scholar]
- 33. Song JY, Park CW, Jeong HW, Cheong HJ, Kim WJ, Kim SR. Effect of a hospital campaign for influenza vaccination of healthcare workers. Infect Control Hosp Epidemiol 2006; 27:612–617. [DOI] [PubMed] [Google Scholar]
- 34. Babcock HM, Gemeinhart N, Jones M, Dunagan WC, Woeltje KF. Mandatory influenza vaccination of health care workers: translating policy to practice. Clin Infect Dis 2010; 50:459–464. [DOI] [PubMed] [Google Scholar]
- 35. Rakita RM, Hagar BA, Crome P, Lammert JK. Mandatory influenza vaccination of healthcare workers: a 5‐year study. Infect Control Hosp Epidemiol 2010; 31:881–888. [DOI] [PubMed] [Google Scholar]
- 36. Doratotaj S, Macknin ML, Worley S. A novel approach to improve influenza vaccination rates among health care professionals: a prospective randomized controlled trial. Am J Infect Control 2008; 36:301–303. [DOI] [PubMed] [Google Scholar]
- 37. Harbarth S, Siegrist CA, Schira JC, Wunderli W, Pittet D. Influenza immunization: improving compliance of healthcare workers. Infect Control Hosp Epidemiol 1998; 19:337–342. [DOI] [PubMed] [Google Scholar]
- 38. Lee HY, Fong YT. On‐site influenza vaccination arrangements improved influenza vaccination rate of employees of a tertiary hospital in Singapore. Am J Infect Control 2007; 35:481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sartor C, Tissot‐Dupont H, Zandotti C, Martin F, Roques P, Drancourt M. Use of a mobile cart influenza program for vaccination of hospital employees. Infect Control Hosp Epidemiol 2004; 25:918–922. [DOI] [PubMed] [Google Scholar]
- 40. Zimmerman RK, Nowalk MP, Lin CJ et al. Factorial design for improving influenza vaccination among employees of a large health system. Infect Control Hosp Epidemiol 2009; 30:691–697. [DOI] [PubMed] [Google Scholar]
- 41. Leitmeyer K, Buchholz U, Kramer M et al. Influenza vaccination in German health care workers: effects and findings after two rounds of a nationwide awareness campaign. Vaccine 2006;24:7003–7008. [DOI] [PubMed] [Google Scholar]
- 42. Esposito S, Tremolati E, Bellasio M et al. Attitudes and knowledge regarding influenza vaccination among hospital health workers caring for women and children. Vaccine 2007; 25:5283–5289. [DOI] [PubMed] [Google Scholar]
- 43. Bautista D, Vila B, Uso R, Tellez M, Zanon V. Predisposing, reinforcing, and enabling factors influencing influenza vaccination acceptance among healthcare workers. Infect Control Hosp Epidemiol 2006; 27:73–77. [DOI] [PubMed] [Google Scholar]
- 44. Martinello RA, Jones L, Topal JE. Correlation between healthcare workers’ knowledge of influenza vaccine and vaccine receipt. Infect Control Hosp Epidemiol 2003; 24:845–847. [DOI] [PubMed] [Google Scholar]
- 45. Nichol KL, Hauge M. Influenza vaccination of healthcare workers. Infect Control Hosp Epidemiol 1997; 18:189–194. [DOI] [PubMed] [Google Scholar]
- 46. Wicker S, Rabenau HF, Doerr HW, Allwinn R. Influenza vaccination compliance among health care workers in a German university hospital. Infection 2009; 37:197–202. [DOI] [PubMed] [Google Scholar]
- 47. Christini AB, Shutt KA, Byers KE. Influenza vaccination rates and motivators among healthcare worker groups. Infect Control Hosp Epidemiol 2007; 28:171–177. [DOI] [PubMed] [Google Scholar]
- 48. Beguin C, Boland B, Ninane J. Health care workers: vectors of influenza virus? Low vaccination rate among hospital health care workers Am J Med Qual 1998; 13:223–227. [DOI] [PubMed] [Google Scholar]
- 49. Doebbeling BN, Edmond MB, Davis CS, Woodin JR, Zeitler RR. Influenza vaccination of health care workers: evaluation of factors that are important in acceptance. Prev Med 1997; 26:68–77. [DOI] [PubMed] [Google Scholar]
- 50. Mah MW, Hagen NA, Pauling‐Shepard K et al. Understanding influenza vaccination attitudes at a Canadian cancer center. Am J Infect Control 2005; 33:243–250. [DOI] [PubMed] [Google Scholar]
- 51. Saluja I, Theakston KD, Kaczorowski J. Influenza vaccination rate among emergency department personnel: a survey of four teaching hospitals. CJEM 2005; 7:17–21. [DOI] [PubMed] [Google Scholar]
- 52. Abramson ZH, Levi O. Influenza vaccination among primary healthcare workers. Vaccine 2008; 26:2482–2489. [DOI] [PubMed] [Google Scholar]
- 53. Mehta M, Pastor CA, Shah B. Achieving optimal influenza vaccination rates: a survey‐based study of healthcare workers in an urban hospital. J Hosp Infect 2008; 70:76–79. [DOI] [PubMed] [Google Scholar]
- 54. Valour F, Maulin L, Ader F et al. Vaccination against influenza: results of a study on vaccination coverage among health care workers in the Croix‐Rousse Hospital (Hospitals of Lyon). Med Mal Infect 2007; 37:51–60. [DOI] [PubMed] [Google Scholar]
- 55. King WD, Woolhandler SJ, Brown AF et al. Brief report: Influenza vaccination and health care workers in the United States. J Gen Intern Med 2006; 21:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stone EG, Morton SC, Hulscher ME et al. Interventions that increase use of adult immunization and cancer screening services: a meta‐analysis. Ann Intern Med 2002; 136:641–651. [DOI] [PubMed] [Google Scholar]
- 57. Finch M. Point: mandatory influenza vaccination for all heath care workers? Seven reasons to say “no” Clin Infect Dis 2006; 42:1141–1143. [DOI] [PubMed] [Google Scholar]
- 58. Anikeeva O, Braunack‐Mayer A, Rogers W. Requiring influenza vaccination for health care workers. Am J Public Health 2009; 99:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O’Neal DJ III. Point counterpoint: mandatory flu vaccination for health care workers. Am J Nurs 2010; 110:26. [DOI] [PubMed] [Google Scholar]
- 60. Lugo NR. Will carrots or sticks raise influenza immunization rates of health care personnel? Am J Infect Control 2007; 35:1–6. [DOI] [PubMed] [Google Scholar]
- 61. Isaacs D, Leask J. Should influenza immunisation be mandatory for healthcare workers? No. BMJ 2008; 337:a2140. [DOI] [PubMed] [Google Scholar]
- 62. Sullivan PL. Influenza vaccination in healthcare workers: should it be mandatory? Online J Issues Nurs 2010; 15:6. (Generic). [Google Scholar]
- 63. Centers for Disease Control and Prevention . Influenza vaccination coverage among health‐care personnel – United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077. [PubMed] [Google Scholar]
- 64. Kuehn BM. Mandatory influenza vaccination urged for clinicians, other health workers. JAMA 2010; 304:1545. [DOI] [PubMed] [Google Scholar]
- 65. Sullivan SJ, Jacobson R, Poland GA. Mandating influenza vaccination for healthcare workers. Expert Rev Vaccines 2009; 8:1469–1474. [DOI] [PubMed] [Google Scholar]
- 66. Poland GA. Mandating influenza vaccination for health care workers: putting patients and professional ethics over personal preference. Vaccine 2010; 28:5757–5759. [DOI] [PubMed] [Google Scholar]
- 67. deSante JE, Caplan A, Shofer F, Behrman AJ. Physician attitudes towards influenza immunization and vaccine mandates. Vaccine 2010; 28:2517–2521. [DOI] [PubMed] [Google Scholar]
- 68. Douville LE, Myers A, Jackson MA, Lantos JD. Health care worker knowledge, attitudes, and beliefs regarding mandatory influenza vaccination. Arch Pediatr Adolesc Med 2010; 164:33–37. [DOI] [PubMed] [Google Scholar]
- 69. Poland GA, Ofstead CL, Tucker SJ, Beebe TJ. Receptivity to mandatory influenza vaccination policies for healthcare workers among registered nurses working on inpatient units. Infect Control Hosp Epidemiol 2008; 29:170–173. [DOI] [PubMed] [Google Scholar]
- 70. Wicker S, Rabenau HF, Betz W, Lauer HC. Attitudes of dental healthcare workers towards the influenza vaccination. Int J Hyg Environ Health 2011; 215:482–486. [DOI] [PubMed] [Google Scholar]
- 71. Stewart AM. Mandatory vaccination of health care workers. N Engl J Med 2009; 361:2015–2017. [DOI] [PubMed] [Google Scholar]
- 72. Rice R. Is mandatory influenza vaccination for health care workers ethically permissible? JAAPA 2010; 23:56, 57. [PubMed] [Google Scholar]
- 73. Caplan A. Time to mandate influenza vaccination in health‐care workers. Lancet 2011; 378:310–311. [DOI] [PubMed] [Google Scholar]
- 74. Gilbert GL, Kerridge I, Cheung P. Mandatory influenza immunisation of health‐care workers. Lancet Infect Dis 2010; 10:3–5. [DOI] [PubMed] [Google Scholar]
- 75. van Delden JJ, Ashcroft R, Dawson A, Marckmann G, Upshur R, Verweij MF. The ethics of mandatory vaccination against influenza for health care workers. Vaccine 2008; 26:5562–5566. [DOI] [PubMed] [Google Scholar]
- 76. Lambert SB. Mandatory flu vaccination. Patient care drives mandatory vaccination. BMJ 2008; 337:a2588. [DOI] [PubMed] [Google Scholar]
- 77. Rea E, Upshur R. Semmelweis revisited: the ethics of infection prevention among health care workers. CMAJ 2001; 164:1447–1448. [PMC free article] [PubMed] [Google Scholar]
- 78. Marckmann G. Vaccination programs between individual autonomy and common welfare. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2008; 51:175–183. [DOI] [PubMed] [Google Scholar]
- 79. Ruderman C, Tracy CS, Bensimon CM et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics 2006; 7:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Verweij M. Individual and collective considerations in public health: influenza vaccination in nursing homes. Bioethics 2001; 15:536–546. [DOI] [PubMed] [Google Scholar]
- 81. Septimus EJ, Perlin JB, Cormier SB, Moody JA, Hickok JD. A multifaceted mandatory patient safety program and seasonal influenza vaccination of health care workers in community hospitals. JAMA 2011; 305:999–1000. [DOI] [PubMed] [Google Scholar]
- 82. McLennan S, Wicker S. Reflections on the influenza vaccination of healthcare workers. Vaccine 2010; 28:8061–8064. [DOI] [PubMed] [Google Scholar]
- 83. Wicker S, Rabenau HF, Gottschalk R, Krause G, McLennan S. Low influenza vaccination rates among healthcare workers Time to take a different approach. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2010; 53:1298–1303. [DOI] [PubMed] [Google Scholar]
- 84. Walker FJ, Singleton JA, Lu P, Wooten KG, Strikas RA. Influenza vaccination of healthcare workers in the United States, 1989‐2002. Infect Control Hosp Epidemiol 2006; 27:257–265. [DOI] [PubMed] [Google Scholar]
- 85. Stephenson I, Roper JP, Nicholson KG. Healthcare workers and their attitudes to influenza vaccination. Commun Dis Public Health 2002; 5:247–252. [PubMed] [Google Scholar]
- 86. Heimberger T, Chang HG, Shaikh M, Crotty L, Morse D, Birkhead G. Knowledge and attitudes of healthcare workers about influenza: why are they not getting vaccinated? Infect Control Hosp Epidemiol 1995; 16:412–415. [DOI] [PubMed] [Google Scholar]


