Abstract
Introduction
Liver disease is the third most common cause of premature mortality in the UK. Liver failure accelerates frailty, resulting in skeletal muscle atrophy, functional decline and an associated risk of liver transplant waiting list mortality. However, there is limited research investigating the impact of exercise on patient outcomes pre and post liver transplantation. The waitlist period for patients listed for liver transplantation provides a unique opportunity to provide and assess interventions such as prehabilitation.
Methods and analysis
This study is a phase I observational study evaluating the feasibility of conducting a randomised control trial (RCT) investigating the use of a home-based exercise programme (HBEP) in the management of patients awaiting liver transplantation. Twenty eligible patients will be randomly selected from the Queen Elizabeth University Hospital Birmingham liver transplant waiting list. Participants will be provided with an individually tailored 12-week HBEP, including step targets and resistance exercises. Activity trackers and patient diaries will be provided to support data collection. For the initial 6 weeks, telephone support will be given to discuss compliance with the study intervention, achievement of weekly targets, and to address any queries or concerns regarding the intervention. During weeks 6–12, participants will continue the intervention without telephone support to evaluate longer term adherence to the study intervention. On completing the intervention, all participants will be invited to engage in a focus group to discuss their experiences and the feasibility of an RCT.
Ethics and dissemination
The protocol is approved by the National Research Ethics Service Committee North West - Greater Manchester East and Health Research Authority (REC reference: 17/NW/0120). Recruitment into the study started in April 2017 and ended in July 2017. Follow-up of participants is ongoing and due to finish by the end of 2017. The findings of this study will be disseminated through peer-reviewed publications and international presentations. In addition, the protocol will be placed on the British Liver Trust website for public access.
Trial registration number
NCT02949505; Pre-results.
Keywords: prehabilitation, functional capacity, end-stage liver disease, liver transplantation
Strengths and limitations of this study.
This is the first study to investigate a home-based exercise programme in patients with end-stage liver disease and listed for liver transplantation.
This is an extensive clinical evaluation of functional capacity and quality of life in a high-risk group of patients in whom there is a pressing need for optimisation prior to transplantation.
This is a pilot study of small sample size, but with the aim and design focusing on the feasibility of a randomised control trial of prehabilitation in patients awaiting liver transplantation.
Introduction
Liver disease is the third most common cause of premature mortality in the UK.1 Currently, a liver transplant is the only cure for end-stage liver disease.2 The existing shortage of donor organs highlights the importance of being able to accurately identify those individuals who will benefit the most from transplantation.
Frailty is defined as the biological syndrome of decreased reserve and resistance to stressors, which cause vulnerability to adverse outcomes.3 Liver failure accelerates this process, resulting in skeletal muscle atrophy (sarcopaenia), reduced functional capacity and an associated increased risk of liver transplant waiting list mortality.4
Evidence suggests that a subgroup of patients with end-stage liver disease who have low functional capacity, defined as an anaerobic threshold of less than 9 mL/kg/min, have lower survival rates post-transplantation5 and predict a longer hospital stay.6 Despite these findings, current management of end-stage liver disease tends to focus on preventing and treating complications (ie, variceal haemorrhage, ascites), rather than prospective strategies to improve functional capacity.
Research in non-end-stage liver disease populations has demonstrated the potential role of preoperative exercise programmes, ‘known as prehabilitation’, in optimising patients’ functional capacity prior to abdominal surgery and reducing postoperative complications.7 8 Furthermore, exercise training has been shown to improve functional capacity and quality of life in a wide variety of chronic diseases.9–11 The time period for patients while active on the liver transplant waiting list provides a unique opportunity to provide physical interventions, such as prehabilitation. This could potentially have a significant effect on short-term, medium-term and long-term outcomes at a relatively low cost.12
Recently, studies have demonstrated significant improvement in functional capacity following delivery of an exercise programme in patients with all causes of liver disease.13–17 Furthermore, significant improvements in muscle mass14 15 and EuroQol Group EQ-visual analogue of self-perceived health status15 were shown. Although all studies suggest exercise is a safe intervention in this patient population, three of the five studies excluded patients with end-stage cirrhosis.14–16 In view of this, as well as small participant numbers in each study, the safety of this intervention cannot be certain. Moreover, all studies were undertaken with weekly, directly supervised exercise sessions only. The seven UK National Health Service (NHS) liver transplant centres cover a vast geographical area; therefore, twice-weekly visits by patients to their nearest transplant centres are unlikely to be feasible. Interventions that can be conducted local to the patient’s homes or indeed in the patient’s own homes need to be evaluated in a randomised control trial (RCT).
Before an RCT can be conducted, a feasibility study is required to determine if a larger trial is possible, and if so outline the optimal design features. Therefore, the aim of this study is to conduct a single-centre feasibility trial of a novel home-based exercise programme (HBEP) in patients with end-stage liver disease awaiting liver transplantation.
Methods and analysis
Study design overview
The proposed feasibility trial is a single-arm, single-centre study of an HBEP for patients listed for liver transplantation.
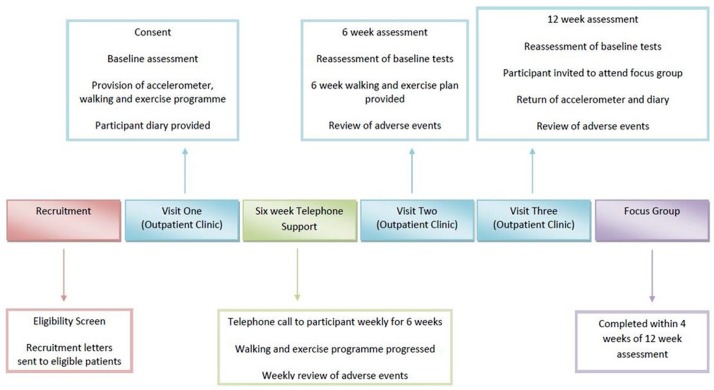
Patients recruited to the study at the Queen Elizabeth University Hospital Birmingham (QEUHB) UK liver transplant unit will be treated with a 12-week HBEP (figure 1). Functional capacity, health-related quality of life, anxiety and depression, anthropometry, and adverse events will be assessed at baseline, 6 weeks and 12 weeks after the study intervention is commenced.
Figure 1.
Study design overview.
On completion of the HBEP intervention, participants will be invited to attend a process evaluation focus group. The purpose of the focus group is to identify attitudes, motivators and barriers to the study intervention, as well as to reflect on the usefulness/acceptability of the study materials and equipment. Data will be collected and used to address the research questions outlined in the box.
Box. Process evaluation focus group research questions.
What motivated the participants to adhere to the study intervention?
Did the participants identify any barriers to completing the study intervention?
How useful did the participants find the accelerometers?
How useful did the participants find the weekly telephone support?
Ethical and regulatory approval
The National Research Ethics Service, Health Research Authority and the University Hospital Birmingham (UHB)Research and Dissemination group will be informed of any protocol modifications within 7 days. All participants will provide informed written consent.
Sample and selection
Twenty eligible patients will be selected from the QEUHB liver transplantation waiting list using a stratified random sampling method, completed by MJA. Subgroups will include four patients from each of the following disease types: alcoholic liver disease, non-alcoholic fatty liver disease, autoimmune liver disease, genetic liver disease and viral hepatitis. This is to ensure that various forms of liver disease will be represented in the study. Patients will be eligible to be included in the study if they meet the following criteria:
Inclusion criteria
Meet the UK liver transplant criteria for listing.18
Accepted on the liver transplant waiting list for a primary transplant.
Adults ≥18 years.
Exclusion criteria
Significant cardiovascular instability including a recent myocardial infarction (MI), recent cerebrovascular accident (CVA) and/or a recent unstable cardiac arrhythmia.
Unstable encephalopathy—open to interpretation by the chief investigator.
Neither patient or next-of-kin non-English-speaking.
Inpatient at the time of screening.
Refusal or lacks capacity to give informed consent.
Once deemed eligible, patients will be sent a letter of invitation to be involved in the study, along with a participant information sheet. Patients will be contacted by telephone 5–7 days after the letters are sent by the chief investigator or a nominated member of the research team. If participants are willing to take part, an appointment will be arranged within 6 weeks, when patients will be able to provide informed written consent.
Methods
Patients on the QEUHB liver transplant waiting list routinely attend outpatient clinics on six-weekly basis. All study visits will be incorporated into their routine clinical follow-up. On attendance to clinic (baseline study visit), participants will complete baseline assessments of functional capacity, anthropometry and questionnaires to assess quality of life and anxiety and depression. The study intervention will be completed for 6 weeks with weekly telephone support, including a telephone questionnaire (see online supplementary appendix 1). On return to clinic at week 6 (study visit 2), functional capacity, quality of life and anxiety/depression scores will be reassessed. For the remaining 6 weeks of the study, participants will continue with the HBEP, but without telephone support. This is to assess the carryover effect of information provided and assess the ability of the participants to continue the HBEP independently while on the waiting list. On return to clinic at 12 weeks (study visit 3), all participants will be reassessed in terms of functional capacity, quality of life and anxiety scores. If a participant is unable to participate in exercise due to illness for a week or number of weeks, then intermittent participation will be permitted. Periods of illness and intermittent participation will be recorded on the case report form (CRF) and accounted for in the data analysis.
bmjopen-2017-019298supp001.pdf (333.2KB, pdf)
Intervention: HBEP
Participants will be provided with a 6-week HBEP including daily step targets and functional resistance-based exercises (figure 2). Participants will be provided with an accelerometer (COOSA Heart Rate Monitor) to aid tracking of their daily steps and activity levels. In addition, participants will be asked to record their activity in a diary to aid self-reporting at the weekly telephone contact.
Figure 2.
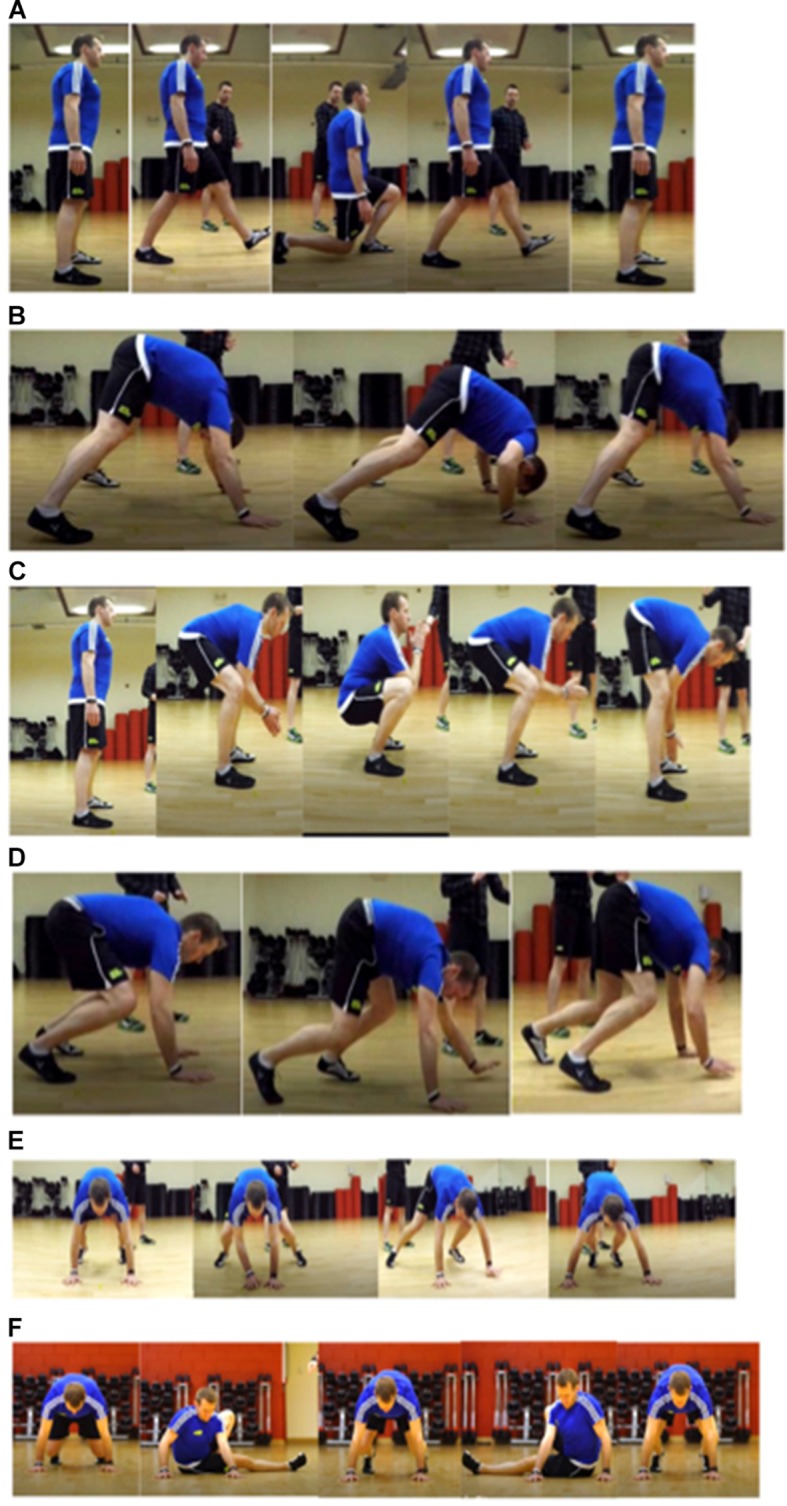
Functional resistance exercises: (A) lunge, (B) rock press, (C) frog squat, (D) bear crawl, (E) side bear crawl and (F) kick sit.
Daily step programme
During the first week, participants will be asked to monitor their daily step count via their accelerometer. Following weekly telephone contact, participants will be advised to increase their daily step count by 200–500 steps each day every week depending on the level of function and achievement of step target of the previous week.
Functional resistance exercise sessions
During the initial assessment patients will be taught functional resistance exercises to complete at home (figure 2). Information provided will be followed up with an exercise worksheet as well as a video to aid patient understanding and adherence. Exercises will be regressed if the participant is unable to complete any of the techniques demonstrated in figure 2. For example, a step or bed will be used for hand positioning in the rock press and bear crawl exercises. The public and patient involvement group advised to keep exercise sessions short to aid compliance. Therefore, sessions will be 20–25 min for each individual, but the difficulty of the session will be split into five levels as described in table 1. Participants will be advised to complete the level most suitable for them depending on their baseline functional capacity scores. Participants will be asked to achieve a work rate of 12–13 on the Borg Rate of Perceived Exertion (RPE) score (6–20 scale).19 An RPE score of 12–13 has been shown to correlate with anaerobic threshold in healthy individuals20 and will therefore guide the participants to work to a training level that will elicit change in functional capacity. Participants will be advised to stop exercising if they reached above 15 on the RPE score or if they feel a change in symptoms, including dizziness, light-headedness and chest pain. Participants will be advised to progress to each level depending on their RPE scores and results of the telephone health call questionnaire. At the 6-week assessment, participants will be advised to progress to a different level of exercise and to continue to increase their step count by 200–500 steps per day, per week depending on the results of their functional capacity scores. Additional exercises, as shown in levels 4 and 5 in table 1, will be taught if needed.
Table 1.
Levels of difficulty for each exercise session
| Level | Exercises | Work to rest timings | Number of circuits | Total session time (min) |
| 1 | Frog squat Rock press Lunge Bear crawl |
20 s of each exercise 40 s rest |
5 | 20 |
| 2 | Frog squat Rock press Lunge Bear crawl |
30 s of each exercise 30 s rest |
5 | 20 |
| 3 | Frog squat Rock press Lunge Bear crawl |
40 s of each exercise 20 s rest |
5 | 20 |
| 4 | Frog squat Rock press Lunge Bear crawl Side bear crawl |
40 s of each exercise 20 s rest |
4 | 20 |
| 5 | Frog squat Rock press Lunge Bear crawl Side bear crawl Kick sit |
40 s of each exercise 20 s rest |
4 | 24 |
Telephone health call
During the first 6 weeks of the study intervention, participants will receive one 20 min telephone call weekly from the chief investigator or a nominated member of the research team. The purpose of the telephone call is to provide support and guidance with the study intervention and address the following areas:
Compliance to the study intervention.
Achievement of weekly preagreed step count and functional resistance exercise level.
Step target for the following week.
Queries or concerns regarding the intervention.
Incidence of any adverse events.
After 6 weeks of the HBEP study intervention, participants will continue with the intervention without telephone support. This aims to assess longer term adherence to the study intervention without weekly telephone support.
Process evaluation focus group
Within 6 weeks of completing the 12-week study intervention, all participants will be invited to attend one of two focus groups. The chief investigator, along with a member of the research team, will conduct two focus groups aiming to (1) explore the thoughts/experiences of the participants regarding the study process, and (2) explore acceptability of the exercise programme and support provided. All participants will be invited to capture the range of participant experiences.
Outcome measures
Primary outcome
The primary outcome of the study is feasibility whereby the decision to proceed to an RCT will be made on the following criteria:
No serious adverse events (defined as grade 3/4) directly related to the HBEP.
>66% of the active transplant waiting list for primary grafts must meet the eligibility criteria to achieve timely recruitment and representation of the cohort.
>90% recruitment to target number of participants (n=20) during the allotted study time period to achieve timely recruitment and assess willingness of patients to participate.
>66% compliance with the step count (including ranges) while active on the transplant waiting list.
>66% compliance with resistance exercises while active on the transplant waiting list.
Of those who undergo initial assessment, >66% complete 6 weeks of HBEP.
Feedback will be documented from those participants who are approached but who refuse to consent or withdraw from the study, on the understanding that this feedback will be optional.
Candidate primary outcomes
The following candidate outcomes will be assessed at baseline (pre-HBEP), and after 6 and 12 weeks of the HBEP. Feasibility will be determined according to the acceptability and usefulness of these outcome measures, as well as time and resources needed to collect data.
Anthropometry
At each study visit body mass index, hand grip strength (kg) (Cranlea Human Performance Digital Hand Grip Dynamometer), mid-arm circumference (cm) and triceps skinfold (mm) (Holtain Tanner/Whitehouse Skinfold Caliper) will be assessed. These assessments are currently completed as part of standard care by the QEUHB liver dietetics team and will be used in the study to ensure control of variables and inform the researchers of any change in nutrition.
Incremental Shuttle Walk Test
The Incremental Shuttle Walk Test (ISWT) is a standardised, externally paced, incremental field-walking test that evaluates maximal exercise capacity. The patient is progressively stressed to a symptom-limited maximal performance by walking at different speeds around a 10 m course, which is dictated by an audio signal. It is a reliable21 and valid measure that has been used in a wide range of chronic diseases,22–24 as well as a predictor of mortality postabdominal surgery.25
Short Performance Battery Test
The Short Performance Battery Test is a physical functional tool that can identify disability and predict mortality through assessment of gait speed, balance and repeated chair stands. It is a valid tool used within a population of patients with liver cirrhosis. A score of less than 9 has been associated with a 45% increase in waiting list mortality in patients listed for liver transplantation, independent of the Model for End-Stage Liver Disease (MELD) score.4
EurolQol-5D (EQ-5D) (V.2.1)
The EQ-5D (V.2.1) is a reliable and validated tool used in a wide range of health conditions and treatments. It provides information on health status, which will be used to help evaluate the clinical and economic value of the study intervention.26
Hospital Anxiety and Depression Score
The Hospital Anxiety and Depression Score is a reliable and valid tool for assessing anxiety and depression in medical patients.27 It will be used to identify if there is a need to include psychological support in future larger research projects. Participants will be advised on the participant information sheet that the purpose of the study is not to address any anxiety or depression concerns, and if they feel this is a concern they should contact their general practitioners.
Telephone questionnaire
This will be completed weekly throughout the first 6 weeks of the study intervention. The telephone questionnaire provides a standardised framework for assessing the participant’s weekly progress and identifying any areas of concern. Furthermore, the answers will provide guided goal setting for the following week.
Other outcomes
Disease severity
To understand the relationship between the severity of liver disease and functional capacity at baseline, and possibly inform the need for stratification in the future RCT, the Child-Turcotte-Pugh, MELD and the UK Model for End Stage Liver Disease will be reported. These scores will be used to compare the study sample selected with the entire waiting list to ensure there is a representative balance of disease severity in the study. In addition, these scores will be calculated at 6 and 12 weeks to inform future hypothesis development for the future RCT.
Number and reason for dropouts
All registered dropouts will be recorded according to their reason, including1 (1) withdrawal of consent, (2) liver transplantation, (3) acute decompensation leading to incapacity to follow the study intervention or (4) death. This will provide valuable information when planning recruitment for the RCT.
Data analysis plan
All quantitative data will be entered into a purposefully designed secure access database and exported to SPSS (V.24) for statistical analysis. Feasibility decision rules and primary candidate outcomes will be analysed and presented using descriptive statistics.
Adverse events reported by telephone or in person will be descriptively reported in terms of frequency (%). To determine compliance with the intervention, the number of days when participants achieved their step count and completed the functional resistance exercises will be reported as categorical variables on a week-by-week basis (weeks 1–5).
Two focus groups will be conducted with three thematic components: (1) barriers to the intervention, (2) facilitators of adherence and (3) level of support received, although, where appropriate, sufficient scope will be given to explore novel themes. Two members of the research team will conduct the focus group. Each session will be digitally recorded, transcribed verbatim and uploaded into NVivo V.10 software to aid organisation and analysis of data. NVivo will be used to store data transcripts, and as a means by which codes could be highlighted and collated based on the themes described above, as well as to explore any new emerging themes.
Adverse events and analysis
An adverse event (AE) would be unlikely in this study due to the extensive investigations the patients have undergone prior to being listed for liver transplantation. However, the reporting period for AE will start at initial screening and continue until the end of the second focus group. Serious adverse events (SAEs) will be reported until 30 days post each participant’s liver transplant. All SAEs and adverse reactions will be evaluated and recorded using the National Cancer Institute’s common terminology criteria for AEs (V.4.0, 2010) and reported to the principal investigator. All SAEs will be reported to the sponsor’s research and development department via the SAE form in the CRF. Only those events classified as probable or definitely related will be reported to the Research Ethics Committee.
Storage of data
All data for an individual participant will be collected by the principal investigator or their delegated nominees and recorded in the CRF. Participant identification on the CRF will be through their unique participant study number, which will be allocated at the time of consideration for the study. Data will be collected from the time the patient is considered for entry into the study through to 30 days after they receive their liver transplant. All clinical data will be stored as per NHS regulations and held on the UK National Transplant Database.
Data from the CRF will be entered into a secure password-protected database held on a University Hospitals Birmingham Trust computer. Due care will be taken to ensure data safety and integrity, and compliance with the Data Protection Act 1998. All essential documentation and trial records will be stored in conformance with the applicable regulatory requirements, and access to stored information will be restricted to authorised personnel. Coded research data will be stored for 5 years anonymously under the property of University Hospitals Birmingham in keeping with good clinical practice.
Case report form
CRFs will include baseline/follow-up functional capacity, anthropometry and questionnaire scores to capture changes in outcomes. Other CRFs incorporated in the electronic database will include medical history, eligibility screening, date of transplant, donor organ and operation data, length of Intensive Care Unit(ICU) stay, 30-day outcome post-transplant, safety monitoring, AE reporting, study treatment adherence and attendance to focus groups.
Sponsorship, indemnity and monitoring
The QEUHB will act as the sponsor through the duration of the study. As sponsor, the QEUHB will be responsible for the general conduct of the study and indemnify the study centre against any claims arising from any negligent act or omission by the hospital, in fulfilling the sponsor role in respect to the study.
Contact name of trial sponsor: Dr Chris Counsell.
Contact information of trial sponsor: chris.counsell@uhb.nhs.uk.
Discussion
This is the first feasibility trial to investigate an HBEP in patients listed for liver transplantation. To date 46 patients have been randomly screened for eligibility, of whom 32 are eligible and 26 have agreed to participate in the trial.
Safety
Few small studies have investigated exercise therapy in patients with chronic liver disease.13–16 Each study reported the safe use of exercise therapy with no AEs described. However, participant numbers were small (n≤24), and three of the four studies included patients with only mild liver disease, who are not as high risk as patients with end-stage liver disease. Furthermore, exercise was supervised by a health professional ensuring that participants exercised within safe training zones and were able to guide participants when to stop. To ensure safe delivery of exercise therapy in this study, education will be given to participants regarding RPE with clear colour-coded training zones. Furthermore, participants will have the contact numbers of the physiotherapists working on the study and will be advised to inform them if they experience any AE. This will also be automatically checked at the weekly telephone contact. To minimise the risk of AEs, the design of the exercise programme was based on well-documented training models delivered to other patients with chronic cardiovascular and respiratory disease in terms of number of sessions per week, length of exercise programme (6–12 weeks) and intensity.28 29
Although this study includes participants with end-stage liver disease, certain medical conditions will be excluded from the study, including cardiovascular instability and unstable encephalopathy, to minimise the risk of an SAE. Furthermore, unstable encephalopathy may affect the participant’s ability to consistently and adequately follow the exercise programme. This would affect the analysis of feasibility, as well as put unnecessary demand on the main carer to support the patient through the process.
Challenges in study design
There are currently no validated outcome measures to assess change in functional capacity in patients with end-stage liver disease. The ISWT will be used in this study because it is a recognised measure of maximal exercise capacity and has been shown to correlate well with volume oxygen (VO2) peak when compared with the gold standard cardiopulmonary exercise test.22 It has been previously used to measure change in functional capacity in other chronic disease types such as respiratory and cardiovascular diseases.23 24 Moreover, the ISWT has been shown to predict postsurgical morbidity in patients undergoing abdominal surgery.30
In view of a home-based set-up, it is important to promote adherence and compliance to the exercise programme. Although it is understood that patients listed for liver transplantation have a lower quality of life, compared with healthy individuals, it is not understood what the motivational influences of this patient population are. To promote adherence to the programme, a self-reported diary and an accelerometer will be given to each participant to provide daily visual feedback and empower responsibility for their daily and weekly goals. Additionally, following demonstration of the functional resistance exercises at their initial assessment, participants will be provided with written and pictorial instructions, as well as a DVD of all of the exercises with front and side views including verbal instruction from an exercise trainer. At the end of the study, each participant will be invited to attend a focus group to feedback on their experience of the study, with particular reference to the level of support they receive, the clarity of the programme and motivational influences.
Due to the large geographical area the QEUHB liver unit covers, participants have to travel up to 300 miles per clinic appointment. It was, therefore, felt that limiting participant visits would facilitate recruitment and adherence to the study and reduce participant burden. Predominantly, patients on the liver transplant waiting list are reviewed on a six-weekly basis. Baseline assessments will be timed with their prearranged clinical appointment so that 6-week and 12-week follow-up will coincide with ongoing clinical appointments.
The HBEP was designed to use movements, which would challenge the cardiorespiratory system, but also encourage movement through multiple planes of motion to improve stability, flexibility and balance. Patients with end-stage liver disease vary in age, function and exercise experience. Exercises were chosen, along with appropriate progression and regressions, in order to adapt to individual needs. Additionally, five levels of intensity will be available based on increasing work time and reducing rest time. These will ensure participants exercise at a level consistent with their exercise capacity, but have room for progression over the 12-week period.
Future RCT considerations
NHS England aims to encourage and support healthier behaviours through the use of NHS accredited health apps.31 In the current study, participants will record their activity in a written diary and verbally report back at their weekly telephone support. In a larger RCT the use of accelerometers with live data collection would be considered. This would aim to empower patients to proactively monitor their activity and work towards patient-centred goals. Furthermore, the physiotherapist could monitor adherence and progression of the exercise programme on a daily basis. This would give better indication to tolerance to the exercise programme and would enable specific exercise intensity advice and avoid participant reporter bias. However, it is currently unknown if all patients have access to smart phones for live data to be recorded on an app. Likewise, virtual clinics could be used instead of telephone support. This would provide a more interactive experience for the patient. The physiotherapist could review exercise techniques and demonstrate alternatives as required.
This phase I trial is critical to understanding potential recruitment rates, withdrawal rates, patients undergoing transplantation or death in the study period and HBEP completion rates in order to accurately power the number of participants required for the future RCT.
Summary
To the best of our knowledge this is the first study to investigate an HBEP in patients listed for liver transplantation. The enrolment of participants to the study was completed in July 2017 and the final results are expected by May 2018.
Supplementary Material
Acknowledgments
Mr Brendan Turner (Virgin Active) made contribution and provided consent for use of photographs in figure 2.
Footnotes
Contributors: FRW: concept, design, recruitment, and first draft and review/editing of final manuscript. AV, TF, JF and MJA: concept, design, recruitment and review/editing of final manuscript. DK, SD, JT, JJ, TP, AH: review/editing of final manuscript. SD: provided consent for use of photographs in figure 2.
Funding: University Hospitals Birmingham Charities (grant number: 173858).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The National Research Ethics Service (NRES) Committee and Health Research Authority North West - Greater Manchester East (REC reference: 17/NW/0120) approved version 1.1 of the study protocol.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Williams R, Aspinall R, Bellis M, et al. . Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384:1953–97. 10.1016/S0140-6736(14)61838-9 [DOI] [PubMed] [Google Scholar]
- 2.Knaak J, McVey M, Bazerbachi F, et al. . Liver transplantation in patients with end-stage liver disease requiring intensive care unit admission and intubation. Liver Transpl 2015;21:761–7. 10.1002/lt.24115 [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M157. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 4.Lai JC, Dodge JL, Sen S, et al. . Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology 2016;63:574–80. 10.1002/hep.28316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentis JM, Manas DM, Trenell MI, et al. . Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transpl 2012;18:152–9. 10.1002/lt.22426 [DOI] [PubMed] [Google Scholar]
- 6.Epstein SK, Freeman RB, Khayat A, et al. . Aerobic capacity is associated with 100-day outcome after hepatic transplantation. Liver Transpl 2004;10:418–24. 10.1002/lt.20088 [DOI] [PubMed] [Google Scholar]
- 7.Dunne DF, Jack S, Jones RP, et al. . Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 2016;103:504–12. 10.1002/bjs.10096 [DOI] [PubMed] [Google Scholar]
- 8.Li C, Carli F, Lee L, et al. . Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 2013;27:1072–82. 10.1007/s00464-012-2560-5 [DOI] [PubMed] [Google Scholar]
- 9.Ochmann U, Kotschy-Lang N, Raab W, et al. . Long-term efficacy of pulmonary rehabilitation in patients with occupational respiratory diseases. Respiration 2012;84:396–405. 10.1159/000337271 [DOI] [PubMed] [Google Scholar]
- 10.Smart N, Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology 2011;16:626–32. 10.1111/j.1440-1797.2011.01471.x [DOI] [PubMed] [Google Scholar]
- 11.van der Meer S, Zwerink M, van Brussel M, et al. . Effect of outpatient exercise training programmes in patients with chronic heart failure: a systematic review. Eur J Prev Cardiol 2012;19:795–803. 10.1177/1741826711410516 [DOI] [PubMed] [Google Scholar]
- 12. Department of Health. An outcome strategy for COPD and Asthma: NHS Companion Document [Best Practice Guideline]. 2012. cited 4 Oct 2017.
- 13.Williams AM, Waits S, Englesbe MJ. The importance of prehabilitation in liver transplantation. Curr Transplant Rep 2015;2:312–5. 10.1007/s40472-015-0080-7 [DOI] [Google Scholar]
- 14.Román E, García-Galcerán C, Torrades T, et al. . Effects of an exercise programme on functional capacity, body composition and risk of falls in patients with cirrhosis: a randomized clinical trial. PLoS One 2016;11:e0151652 10.1371/journal.pone.0151652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zenith L, Meena N, Ramadi A, et al. . Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1920–6. 10.1016/j.cgh.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 16.Garcia AM, Veneroso CE, Soares DD, et al. . Effect of a physical exercise program on the functional capacity of liver transplant patients. Transplant Proc 2014;46:1807–8. 10.1016/j.transproceed.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 17.Debette-Gratien M, Tabouret T, Antonini MT, et al. . Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation 2015;99:145–50. 10.1097/TP.0000000000000245 [DOI] [PubMed] [Google Scholar]
- 18.Zalewska K. Liver Transplantation: Selection Criteria and Recipient Registration, 2017. [Google Scholar]
- 19.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81. 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 20.Scherr J, Wolfarth B, Christle JW, et al. . Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol 2013;113:147–55. 10.1007/s00421-012-2421-x [DOI] [PubMed] [Google Scholar]
- 21.Singh SJ, Morgan MD, Scott S, et al. . Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47:1019–24. 10.1136/thx.47.12.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luxton N, Alison JA, Wu J, et al. . Relationship between field walking tests and incremental cycle ergometry in COPD. Respirology 2008;13:856–62. 10.1111/j.1440-1843.2008.01355.x [DOI] [PubMed] [Google Scholar]
- 23.Fowler SJ, Singh SJ, Revill S. Reproducibility and validity of the incremental shuttle walking test in patients following coronary artery bypass surgery. Physiotherapy 2005;91:22–7. 10.1016/j.physio.2004.08.009 [DOI] [Google Scholar]
- 24.Lee AL, Cecins N, Holland AE, et al. . Field walking tests are reliable and responsive to exercise training in people with non-cystic fibrosis Bronchiectasis. J Cardiopulm Rehabil Prev 2015;35:439–45. 10.1097/HCR.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 25.Struthers R, Erasmus P, Holmes K, et al. . Assessing fitness for surgery: a comparison of questionnaire, incremental shuttle walk, and cardiopulmonary exercise testing in general surgical patients. Br J Anaesth 2008;101:774–80. 10.1093/bja/aen310 [DOI] [PubMed] [Google Scholar]
- 26.Van Reenan M, Janssen B. EQ-5D-5L User Guide: Basic Information on How to Use the EQ-5D-5L Instrument, 2015. [Google Scholar]
- 27.Rothenbacher D, Jaensch A, Mons U, et al. . Prognostic value of one-year course of symptoms of anxiety and depression in patients with coronary heart disease: Role of physical activity and unmet medical need. Eur J Prev Cardiol 2015;22:1129–38. 10.1177/2047487314545317 [DOI] [PubMed] [Google Scholar]
- 28.Bolton CE, Bevan-Smith EF, Blakey JD, et al. . British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013;68(Suppl 2):ii1–30. 10.1136/thoraxjnl-2013-203808 [DOI] [PubMed] [Google Scholar]
- 29. Rehabilitation AoCPiC. Standards for Physical Activity and Exercise in the Cardiovascular Population, 2015. cited 4 Oct 2017. [Google Scholar]
- 30.Murray P, Whiting P, Hutchinson SP, et al. . Preoperative shuttle walking testing and outcome after oesophagogastrectomy. Br J Anaesth 2007;99:809–11. 10.1093/bja/aem305 [DOI] [PubMed] [Google Scholar]
- 31. Care Quality Commission PHEaNI. The NHS Five Years Forward View. NHS England, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-019298supp001.pdf (333.2KB, pdf)