Abstract
Objective
To investigate evolving patterns in antithrombotic treatment in UK patients with newly diagnosed non-valvular atrial fibrillation (AF).
Design
Prospective, multicentre, international registry.
Setting
186 primary care practices in the UK.
Participants
3482 participants prospectively enrolled in four sequential cohorts (cohort 2 (C2) n=830, diagnosed September 2011 to April 2013; cohort 3 (C3) n=902, diagnosed April 2013 to June 2014; cohort 4 (C4) n=850, diagnosed July 2014 to June 2015; cohort 5 (C5) n=900, diagnosed June 2015 to July 2016). Participants had newly diagnosed non-valvular AF and at least one risk factor for stroke, were aged ≥18, and provided informed consent.
Main outcome measures
Antithrombotic treatment initiated at diagnosis, overall and according to stroke and bleeding risks. Stroke risk was retrospectively calculated using CHA2DS2-VASc (cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)–vascular disease, age 65–74 and sex category (female)) and bleeding risk using HAS-BLED (hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, elderly (>65), drugs/alcohol concomitantly (1 point each)).
Results
42.7% were women and the mean age was 74.5 years. The median CHA2DS2-VASc score was 3 in all cohorts and the median HAS-BLED score was 2 in all cohorts. There was a statistically significant increase in the use of anticoagulant therapy from C2 to C5 (C2 54.7%, C3 60.3%, C4 73.1%, C5 73.9%; P value for trend <0.0001). The increase in the use of anticoagulant was mainly in patients with CHA2DS2-VASc ≥2. The use of vitamin K antagonists (VKAs)±antiplatelet (AP) drugs decreased from C2 to C5 (C2 53.3%, C3 52.1%, C4 50.3%, C5 30.6%), while the use of non-vitamin K antagonist oral anticoagulants (NOACs)±AP increased (C2 1.3%, C3 8.0%, C4 22.7%, C5 43.3%). The use of AP only decreased (C2 36.4%, C3 25.5%, C4 11.9%, C5 10.5%), as did the combination therapy of VKA+AP (C2 13.6%, C3 11.0%, C4 9.6%, C5 5.8%).
Conclusion
There has been a progressive increase in the proportion of patients newly diagnosed with AF receiving guideline-recommended therapy in the UK, potentially driven by the availability of NOACs.
Trial registration number
NCT01090362; Pre-results.
Keywords: atrial fibrillation, antithrombotic therapy, anticoagulation, newly diagnosed, stroke prophylaxis
Strengths and limitations of the study.
This study describes real-world clinical practice in the UK for treatment initiated at atrial fibrillation (AF) diagnosis in patients with AF and at least one risk factor for stroke.
Eligible patients were enrolled prospectively and consecutively without exclusions according to comorbidities or treatment.
Patients were recruited in primary care in the UK, encompassing patients diagnosed in a comprehensive range of national care settings.
This study does not include patients without capacity to consent.
Introduction
Atrial fibrillation (AF) is a potent risk factor for stroke and mortality; people with AF have a fivefold increased risk of stroke and a twofold increased risk of death.1 2 AF-related strokes are more serious and are more likely to be fatal or lead to long-term disability than strokes in people without this arrhythmia.3 Stroke prevention is therefore a principal goal in the treatment of AF4 and is a major public health priority.5 Fortunately, there are effective therapies, with anticoagulation shown to mitigate up to two-thirds of this stroke risk.
Since 2010, changes in treatment guidelines from the European Society of Cardiology and the National Institute for Clinical Excellence (NICE) have widened the criteria for patients with AF that should be considered for antithrombotic therapy and now advocate anticoagulants (ACs) as the only appropriate antithrombotic therapy in patients with AF.4 5 ACs include vitamin K antagonists (VKAs; typically warfarin) and, recently, non-VKA oral anticoagulants (NOACs), comprising factor Xa inhibitors and direct thrombin inhibitors. Whereas the only anticoagulant previously recommended was warfarin, the updated AF guidelines from NICE include recommendations for NOACs for patients with non-valvular AF.
In 2014, NICE updated its guidelines on the management of AF, recommending the CHA2DS2-VASc (cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)–vascular disease, age 65–74 and sex category (female)) stroke risk tool for assessing stroke risk in patients with AF and further recommending anticoagulation therapy for patients at high risk (CHA2DS2-VASc ≥2), a consideration of anticoagulant therapy for patients at moderate risk (CHA2DS2-VASc=1) and no anticoagulant or antiplatelet (AP) treatment for patients at low risk (defined as CHA2DS2-VASc=0 for men and CHA2DS2-VASc=1 for women).5 In addition, the emergence of NOACs in the UK since 2012 has provided a wider range of AC options, particularly for patients for whom warfarin may not be appropriate. The change in guidelines coupled with the emergence of NOACs has the potential to transform clinical practice; however, the impact on the use of ACs in patients with AF in the UK is unclear.
More than 46 000 new cases of AF are diagnosed in the UK every year. Many studies have reported a long-standing problem of undertreatment with ACs of patients at high risk of stroke6 7; UK studies in the last decade also report suboptimal treatment,8–11 though there is limited evidence of AF management since the introduction of NOACs. Little is known about the contemporary real-world management of patients newly diagnosed with AF who are perceived to be at risk of stroke by their physicians. The Global Anticoagulant Registry in the FIELD–Atrial Fibrillation (GARFIELD-AF) aims to determine real-life treatment patterns and clinical outcomes of patients with newly diagnosed non-valvular AF and at least one investigator-determined risk factor for stroke.12 13 This paper investigates the evolving patterns of antithrombotic treatment of UK patients enrolled in the GARFIELD-AF registry from September 2011 to July 2016.
Methods
Study design
GARFIELD-AF is an ongoing, prospective, non-interventional, international registry of adults (≥18 years) diagnosed with AF. Patients were recruited into five independent cohorts; the first cohort also included a validation cohort of retrospective patients.
Participants
Inclusion criteria for the prospective cohort comprised a new diagnosis of non-valvular AF of up to 6 weeks prior to entry into the registry and an investigator-determined risk factor for stroke. Eligible patients were recruited consecutively at participating sites in order to prevent selection bias. The retrospective cohort comprised patients diagnosed 6–24 months before enrolment. Patients are followed up for a minimum of 2 years. Patients with transient AF, secondary to a reversible cause, and patients for whom follow-up was not possible were excluded from the registry. Full methods of the GARFIELD-AF registry have been previously reported.12 13
This paper reports baseline characteristics and treatment patterns in UK participants enrolled into cohorts 2 to 5; participants enrolled into cohort 1 were excluded as it consisted predominantly of a retrospective validation cohort.
Setting
Enrolment of UK patients into cohorts 2 to 5 was undertaken between September 2011 and July 2016 at 186 general practices (GPs) across the UK (161 in England, 8 in Wales, 8 in Northern Ireland and 9 in Scotland). The necessary regulatory approvals were obtained prior to recruitment, and all patients provided written informed consent prior to enrolment into the registry. The standard national diagnostic criteria for AF apply for GARFIELD-AF, and for the UK this was by electrocardiographic confirmation.
Data sources
Data collected at baseline comprised demographics, body mass index, type of AF, care setting of diagnosis, treatment strategy initiated at diagnosis, reason for treatment decision and medical history. Data were collected through review of medical records by trained site staff using an electronic case report form.
Stroke risk was calculated retrospectively using CHA2DS2-VASc score-based variables: heart failure, hypertension, age ≥75 years and 65–74 years, diabetes mellitus, prior stroke or transient ischaemic attack (TIA), left ventricular ejection fraction <40%, prior thromboembolism, vascular disease and female gender. HAS-BLED scores were calculated retrospectively using the variables hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, elderly (>65) and drugs/alcohol concomitantly.
Data for the analysis in this report were extracted from the study database on 28 July 2016.
Definitions
ACs include VKAs and NOACs. NOACs include oral direct factor Xa inhibitors and oral direct thrombin inhibitors.
Vascular disease was defined as peripheral artery disease and/or coronary artery disease (CAD) with a history of acute coronary syndromes. Hypertension was defined as a documented history of hypertension or blood pressure >140/90 mm Hg. Chronic kidney disease (CKD) was classified according to the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines14: moderate to severe includes stages III to V; none or mild includes all other patients.
Statistical analysis
Patient characteristics and medical history are described by cohort. Continuous variables are expressed as number of patients and mean±SD and or median and IQR. Categorical variables are expressed as frequencies and percentages. Treatment patterns were analysed by cohort, and by cohort and CHA2DS2-VASc or HAS-BLED. Trends were assessed using an extension of the Wilcoxon rank-sum test.
Logistic regression models were used to assess the risk factors associated with the prescribing of NOACs (vs VKA). The following risk factors were included in the model: gender, age group, race, smoking, congestive heart failure, hypertension, diabetes, CAD, vascular disease, dementia, moderate to severe CKD, non-steroidal anti-inflammatory drug (NSAID) usage, history of bleeding, previous stroke/TIA/systemic embolism (SE) and cohort. ORs with 95% CIs were estimated to describe the associations of the risk factors and prescribing of NOACs versus VKA, as well as AP and no treatment (No ACs) versus ACs.
Multiple Imputation by Chained Equations was used to fill in missing values, creating five complete datasets.15 16 Logistic regression was performed using the imputed datasets. First-degree interaction between comorbidities and time (cohort) was tested using likelihood ratio tests. Only significant interactions were included in the final model.
Statistical analysis was performed using both SAS software V.9.4 (SAS Institute, Cary, NC, USA) and Stata Statistical Software V.14 (StataCorp, College Station, TX, USA).
Results
Patient distribution and characteristics
In the UK, 3482 patients were enrolled into cohorts 2 to 5 between September 2011 and July 2016: cohort 2 (C2) consisted of 830 patients diagnosed with AF between September 2011 and April 2013, cohort 3 (C3) consisted of 902 patients diagnosed between April 2013 and June 2014, cohort 4 (C4) consisted of 850 patients diagnosed between July 2014 and June 2015, and cohort 5 (C5) consisted of 900 patients diagnosed between June 2015 and July 2016. Overall, 42.7% of patients were women, mean age (SD) at diagnosis was 74.5 years (9.5) and 89.7% had a CHA2DS2-VASc score of ≥2 (table 1).
Table 1.
Baseline characteristics of patients in cohorts 2 to 5
| Variable | Cohort 2 (n=830) (2011–2013) |
Cohort 3 (n=902) (2013–2014) |
Cohort 4 (n=850) (2014–2015) |
Cohort 5 (n=900) (2015–2016) |
Total C2 to C5 (n=3482) |
| Women, n/N (%) | 376/850 (45.3) | 391/902 (43.3) | 343/850 (40.4) | 378/900 (42.0) | 1488/3482 (42.7) |
| Age at diagnosis, years, mean (SD) | 75.2 (9.7) | 73.8 (9.7) | 74.2 (9.6) | 74.8 (9.0) | 74.5 (9.5) |
| Age at diagnosis, years, median (IQR) | 77.0 (70.0 to 82.0) | 75.0 (68.0 to 81.0) | 75.0 (69.0 to 81.0) | 75.0 (69.0 to 81.0) | 75.0 (69.0 to 81.0) |
| Age group, n/N (%) | |||||
| <65 | 110/830 (13.3) | 133/902 (14.7) | 116/850 (13.6) | 96/900 (10.7) | 455/3482 (13.1) |
| 65–74 | 222/830 (26.7) | 315/902 (34.9) | 293/850 (34.5) | 322/900 (35.8) | 1152/3482 (33.1) |
| ≥75 | 498/830 (60.0) | 454/902 (50.3) | 441/850 (51.9) | 482/900 (53.6) | 1875/3482 (53.8) |
| Caucasian race, n/N (%) | 804/816 (98.5)a | 867/884 (98.1)b | 832/837 (99.4)c | 853/860 (99.2)d | 3356/3397 (98.8)e |
| Medical history, n/N (%) | |||||
| Congestive heart failure | 70/830 (8.4) | 69/902 (7.6) | 56/850 (6.6) | 57/900 (6.3) | 252/3482 (7.2) |
| Coronary artery disease | 166/830 (20.0) | 165/902 (18.3) | 164/850 (19.3) | 174/900 (19.3) | 669/3482 (19.2) |
| Acute coronary syndrome | 87/830 (10.5) | 74/896 (8.3)f | 90/847 (10.6)g | 89/897 (9.9)h | 340/3470 (9.8)i |
| Vascular disease | 109/830 (13.1) | 112/895 (12.5)j | 125/848 (14.7)k | 125/898 (13.9)l | 471/3471 (13.6)m |
| Systemic embolism | 9/830 (1.1) | 4/893 (0.4) | 3/842 (0.4) | 6/893 (0.7) | 22/3458 (0.6) |
| Stroke/TIA | 101/830 (12.2) | 105/902 (11.6) | 116/850 (13.6) | 106/900 (11.8) | 428/3482 (12.3) |
| History of bleeding | 28/830 (3.4) | 26/899 (2.9) | 23/845 (2.7) | 27/895 (3.0) | 104/2574 (3.0) |
| Hypertension | 607/830 (73.1) | 637/899 (70.9) | 566/847 (66.8) | 607/897 (67.7) | 2417/3473 (69.6) |
| Diabetes mellitus | 136/830 (16.4) | 156/902 (17.3) | 168/850 (19.8) | 154/900 (17.1) | 614/3482 (17.6) |
| Moderate to severe CKD* | 244/830 (29.4) | 241/902 (26.7) | 199/850 (23.4) | 196/900 (21.8) | 880/3482 (25.3) |
| Risk scores | |||||
| CHA2DS2-VASc, median (IQR) | 3.0 (2.0 to 4.0)n | 3.0 (2.0 to 4.0)o | 3.0 (2.0 to 4.0)p | 3.0 (2.0 to 4.0)q | 3.0 (2.0 to 4.0)r |
| CHA2DS2-VASc, 0–1, n/N (%) | 73/795 (9.2) | 93/844 (11.0) | 90/801 (11.2) | 81/835 (9.7) | 337/3275 (10.3) |
| HAS-BLED, median (IQR) | 2.0 (1.0 to 2.0)s | 2.0 (1.0 to 2.0)t | 2.0 (1.0 to 2.0)u | 2.0 (1.0 to 2.0)v | 2.0 (1.0 to 2.0)w |
| HAS-BLED, 0–2, n/N (%) | 437/574 (76.1) | 510/641 (79.6) | 535/638 (83.9) | 524/615 (85.2) | 2006/2468 (81.3) |
Patients missing: a14, b18, c13, d40, e85, f6, g3, h3, i12, j7, k2, l1, m11, n35, o58, p49, q65, r207, s256, t261, u212, v285, w1014.
*Includes NKF KDOQI stages III–V.
CHA2DS2-VASc, cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)–vascular disease, age 65–74 and sex category (female); CKD, chronic kidney disease; HAS-BLED, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, elderly (>65), drugs/alcohol concomitantly (one point each); NKF KDOQI, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative; TIA, transient ischaemic attack.
Participants were diagnosed in a broad range of care settings representative of those in the UK: more than half of the patients (2124/3482; 61.0%) were diagnosed in primary care. The remainder were diagnosed in internal (general) medicine (21.9%), cardiology (15.2%), geriatrics (1.8%) and neurology (0.1%). Of the 3482 participants, 1370 (39.3%) had new or unclassified AF, 640 (18.4%) had paroxysmal AF, 272 (7.8%) had persistent AF and 1200 (34.5%) had permanent AF. There were some variations in baseline characteristics across the four cohorts (table 1), though the median CHA2DS2-VASc and HAS-BLED scores were similar.
Antithrombotic therapy use by cohort
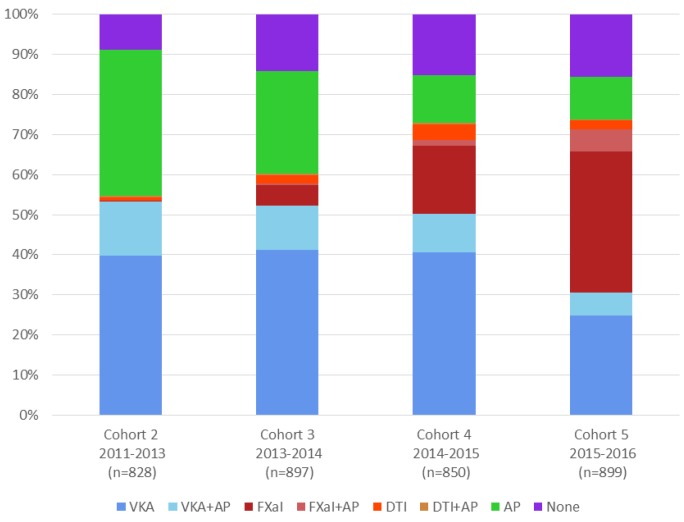
Figure 1 shows the treatment patterns at diagnosis in each of the four cohorts. The proportion of patients prescribed AC therapy at diagnosis, with or without an AP, increased consistently from C2 to C5 (54.7%, 60.3%, 73.1% and 73.9%; for trend <0.0001), whereas the use of AP only decreased (36.4%, 25.5%, 11.9% and 10.5%). At the same time, there was an increase in the proportion of patients receiving NOACs with or without AP from C2 to C5 (1.3%, 8.1%, 22.7%, 43.3%); the proportion of patients not receiving any antithrombotic therapy increased from C2 to C4 (8.9%, 14.4%, 15.1%) then stayed similar in C5 (15.7%). Co-prescription of AC and AP was variable (C2 14.0%, C3 11.8%, C4 11.4%, C5 11.7%). Table 2 shows selected baseline characteristics for all patients (C2 to C5 combined) according to treatment group. Patients receiving no treatment generally had a lower incidence of comorbidities, apart from history of bleeding; however, patients aged ≥75 years were more likely not to receive treatment.
Figure 1.
Antithrombotic treatment at diagnosis by cohort. AP, antiplatelet; DTI, direct thrombin inhibitor; FXaI, factor Xa inhibitor; VKA, vitamin K antagonist.
Table 2.
Baseline characteristics of patients in cohorts 2 to 5 by antithrombotic treatment type
| Variable | None (n=470) |
AP alone (n=725) |
VKA alone (n=1267) |
NOAC alone (n=587) |
AC+AP (n=425) |
AC±AP (n=2279) |
| Women, n (%) | 201 (42.8) | 291 (40.1) | 565 (44.6) | 262 (44.6) | 167 (39.3) | 994 (43.6) |
| Age, mean (SD) | 73.3 (10.5) | 75.3 (9.7) | 74.2 (9.4) | 75.0 (9.4) | 74.7 (8.2) | 74.5 (9.2) |
| Age 65–74, n (%) | 153 (32.6) | 217 (29.9) | 430 (33.9) | 198 (33.7) | 150 (35.3) | 778 (34.1) |
| Age ≥75, n (%) | 227 (48.3) | 417 (57.5) | 676 (53.4) | 319 (54.3) | 234 (55.1) | 1229 (53.9) |
| Medical history, n (%) | ||||||
| Heart failure (any) | 22 (4.7) | 46 (6.3) | 97 (7.7) | 36 (6.1) | 49 (11.5) | 182 (8.0) |
| Hypertension (any) | 325 (78.1) | 531 (77.7) | 961 (79.2) | 451 (80.0) | 331 (80.3) | 1743 (79.6) |
| Diabetes mellitus | 51 (10.9) | 105 (14.5) | 249 (19.7) | 94 (16.0) | 112 (26.4) | 455 (20.0) |
| Stroke | 12 (2.6) | 55 (7.6) | 78 (6.2) | 46 (7.8) | 52 (12.2) | 176 (7.7) |
| Systemic embolism | – | 5 (0.7) | 12 (1.0) | 1 (0.2) | 4 (1.0) | 17 (0.8) |
| CAD (any) | 37 (7.9) | 187 (25.8) | 168 (13.3) | 90 (15.3) | 182 (42.8) | 440 (19.3) |
| Vascular disease | 23 (4.9) | 120 (16.6) | 125 (9.9) | 64 (10.9) | 137 (32.5) | 326 (14.4) |
| History of bleeding | 34 (7.3) | 35 (4.9) | 14 (1.1) | 15 (2.6) | 6 (1.4) | 35 (1.5) |
| Moderate to severe CKD* | 94 (20.0) | 208 (28.7) | 331 (26.1) | 128 (21.8) | 117 (27.5) | 576 (25.3) |
| Risk scores | ||||||
| CHA2DS2-VASc, mean (SD) | 2.8 (1.4) | 3.3 (1.5) | 3.3 (1.4) | 3.3 (1.4) | 3.8 (1.5) | 3.4 (1.4) |
| CHA2DS2-VASc, median (IQR) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) | 4.0 (3.0 to 5.0) | 3.0 (2.0 to 4.0) |
| CHA2DS2-VASc, 0–1, n (%) | 75 (18.1) | 73 (10.8) | 107 (8.9) | 57 (10.1) | 24 (5.9) | 188 (8.6) |
| HAS-BLED, mean (SD) | 1.4 (0.9) | 2.4 (0.8) | 1.4 (0.8) | 1.4 (0.8) | 2.4 (0.8) | 1.6 (0.9) |
| HAS-BLED, median (IQR) | 1.0 (1.0 to 2.0) | 2.0 (2.0 to 3.0) | 1.0 (1.0 to 2.0) | 1.0 (1.0 to 2.0) | 2.0 (2.0 to 3.0) | 2.0 (1.0 to 2.0) |
| HAS-BLED, 0–2, n (%) | 249 (88.7) | 306 (61.3) | 855 (90.2) | 398 (91.9) | 193 (63.9) | 1446 (85.8) |
*Includes NKF KDOQI stages III–V.
AC, anticoagulant; AP, antiplatelet; CAD, coronary artery disease; CHA2DS2-VASc, cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)–vascular disease, age 65–74 and sex category (female); CKD, chronic kidney disease; HAS-BLED, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, elderly (>65), drugs/alcohol concomitantly (1 point each); NKF KDOQI, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist.
Overall, 19.1% (666/3482) of patients were prescribed NOACs. Table 3 shows the baseline characteristics of patients on NOACs by cohort. There were no clear patterns of NOAC use by patient characteristics; however, patients diagnosed in cardiology in the earlier cohorts were more likely to be given NOACs than those in the later cohorts, while among patients diagnosed in primary care the later cohorts were more likely to receive NOACs than earlier cohorts. Of the patients prescribed either NOACs or VKA, those with dementia were significantly more likely to receive NOACs than VKA compared with patients without a history of the condition (table 4). Also, patients were more likely to receive NOACs over VKA as the cohorts progressed, from C2 to C5; however, no interaction between cohort and covariates was statistically significant.
Table 3.
Baseline characteristics of patients on NOACs by cohort
| Variable | Cohort 2 (n=11) |
Cohort 3 (n=73) |
Cohort 4 (n=193) |
Cohort 5 (n=389) |
Total C2 to C5 (n=666) |
| Female, n (%) | 4 (36.4) | 42 (57.5) | 80 (41.5) | 165 (42.4) | 291 (43.7) |
| Age at diagnosis, years, mean (SD) | 75.9 (10.3) | 74.8 (9.2) | 74.7 (10.1) | 74.7 (9.0) | 74.7 (9.4) |
| Age at diagnosis, years, median (IQR) | 75.0 (69.0 to 86.0) | 74.0 (69.0 to 81.0) | 76.0 (68.0 to 82.0) | 75.0 (69.0 to 81.0) | 75.0 (69.0 to 82.0) |
| Age group, n (%) | |||||
| <65 | 2 (18.2) | 8 (11.0) | 30 (15.5) | 43 (11.1) | 83 (12.5) |
| 65–74 | 3 (27.3) | 29 (39.7) | 59 (30.6) | 138 (35.5) | 229 (34.4) |
| ≥75 | 6 (54.5) | 36 (49.3) | 104 (53.9) | 208 (53.5) | 354 (53.2) |
| Care setting at diagnosis, n (%) | |||||
| Internal medicine | 2 (18.2) | 18 (24.7) | 53 (27.5) | 108 (27.8) | 181 (27.2) |
| Cardiology | 4 (36.4) | 11 (15.1) | 21 (10.9) | 59 (15.2) | 95 (14.3) |
| Neurology | – | – | 1 (0.5) | 1 (0.3) | 2 (0.3) |
| Geriatrics | – | 2 (2.7) | 2 (1.0) | 7 (1.8) | 11 (1.7) |
| Primary care/general practice | 5 (45.5) | 42 (57.5) | 116 (60.1) | 214 (55.0) | 377 (56.6) |
| Medical history, n (%) | |||||
| Congestive heart failure | 2 (18.2) | 4 (5.5) | 14 (7.3) | 23 (5.9) | 43 (6.5) |
| History of hypertension | 10 (90.9) | 48 (65.8) | 139 (72.8) | 276 (71.1) | 473 (71.3) |
| Diabetes mellitus | 2 (18.2) | 9 (12.3) | 35 (18.1) | 69 (17.7) | 115 (17.3) |
| Stroke | – | 7 (9.6) | 16 (8.3) | 32 (8.2) | 55 (8.3) |
| Systemic embolism | – | – | 1 (0.5) | 2 (0.5) | 3 (0.5) |
| Coronary artery disease | 1 (9.1) | 11 (15.1) | 43 (22.3) | 73 (18.8) | 128 (19.2) |
| Vascular disease | 1 (9.1) | 7 (9.7)a | 37 (19.3)b | 50 (12.9) | 95 (14.3)c |
| History of bleeding | – | 3 (4.1) | 2 (1.0) | 11 (2.8) | 16 (2.4) |
| Moderate to severe CKD* | – | 26 (35.6) | 47 (24.4) | 70 (18.0) | 143 (21.5) |
| Risk scores | |||||
| CHA2DS2-VASc, mean (SD) | 3.3 (1.7) | 3.3 (1.4)d | 3.4 (1.5)e | 3.3 (1.4)f | 3.3 (1.5)g |
| CHA2DS2-VASc, median (IQR) | 4.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) |
| CHA2DS2-VASc, 0–1, n (%) | 2 (18.2) | 7 (9.9) | 19 (10.4) | 37 (9.9) | 65 (10.2) |
| HAS-BLED, mean (SD) | 1.2 (0.8)h | 1.7 (0.8)i | 1.5 (0.8)j | 1.4 (0.8)k | 1.5 (0.8)l |
| HAS-BLED, median (IQR) | 1.0 (1.0 to 2.0) | 2.0 (1.0 to 2.0) | 1.0 (1.0 to 2.0) | 1.0 (1.0 to 2.0) | 1.0 (1.0 to 2.0) |
| HAS-BLED, 0–2, n (%) | 6 (100) | 52 (86.7) | 129 (89.0) | 255 (92.4) | 442 (90.8) |
Patients missing: a1, b1, c2, d2, e10, f16, g28, h5, i13, j48, k113, l179.
*Includes NKF KDOQI stages III–V.
CHA2DS2-VASc, cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)–vascular disease, age 65–74 and sex category (female); CKD, chronic kidney disease; HAS-BLED, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, elderly (>65), drugs/alcohol concomitantly (1 point each); NKF KDOQI, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative; NOAC, non-vitamin K antagonist oral anticoagulant.
Table 4.
Use of NOACs in relation to baseline characteristics for patients on an AC at baseline
| Variable | Cohorts 2 to 5 OR (95% CI) |
| Gender | |
| Female | 1 |
| Male | 0.90 (0.72 to 1.12) |
| Age (years) | |
| 65 | 1 |
| 65–80 | 0.66 (0.47 to 0.92) |
| 80–85 | 0.71 (0.48 to 1.07) |
| >85 | 1.02 (0.66 to 1.59) |
| Medical history* | |
| Congestive heart failure | 0.88 (0.58 to 1.34) |
| Hypertension (history or >140/90 mm Hg) | 1.23 (0.93 to 1.62) |
| Diabetes | 0.78 (0.59 to 1.02) |
| Coronary artery disease | 1.14 (0.80 to 1.65) |
| Vascular disease | 1.14 (0.76 to 1.71) |
| Dementia | 3.58 (1.15 to 11.15) |
| Moderate to severe CKD† | 0.85 (0.65 to 1.10) |
| NSAID usage | 0.57 (0.44 to 0.74) |
| Bleeding | 1.90 (0.86 to 4.19) |
| Previous stroke/TIA/SE | 1.29 (0.96 to 1.75) |
| Smoking | |
| Never | 1 |
| Ex-smoker | 1.03 (0.82 to 1.29) |
| Current smoker | 0.61 (0.38 to 0.97) |
| Cohort | |
| 2 | 1 |
| 3 | 6.14 (3.28 to 11.52) |
| 4 | 7.24 (9.43 to 31.53) |
| 5 | 55.21 (30.29 to 100.62) |
*Reference group is patients with no history of disease (for congestive heart failure, hypertension, diabetes, coronary artery disease, vascular disease, dementia, moderate to severe CKD, NSAID usage, bleeding, previous stroke/TIA/SE).
†Includes NKF KDOQI stages III–V; none or mild (reference group) includes all other patients.
An OR > 1 implies that NOACs are more frequent than VKAs, while an OR < 1 means that VKAs are more frequent than NOACs. No interaction between cohort and covariates was statistically significant.
AC, anticoagulant; CKD, chronic kidney disease; NKF KDOQI, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative; NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug; SE, systemic embolism; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
Table 5 shows the baseline characteristics of patients who received no AC therapy (34.3%, 1195/3482) by cohort. There were no clear changes over time in ‘no AC’ use when considering individual patient characteristics. Nevertheless, in the whole population, ‘no AC’ was less likely (relative to AC therapy) in patients aged 65–80 years, with diabetes, or a history of vascular disease and previous stroke/TIA/SE than in patients without these conditions or other age groups (table 6). ‘No AC’ was more likely if patients had a history of bleeding or with NSAID usage. Over time, UK physicians became increasingly less likely to choose ‘no AC’ with each successive cohort of patients enrolled between 2011 and 2016.
Table 5.
Baseline characteristics of patients not on AC by cohort
| Variable | Cohort 2 (n=375) |
Cohort 3 (n=356) |
Cohort 4 (n=229) |
Cohort 5 (n=235) |
Total C2 to C5 (n=1195) |
| Women, n (%) | 166 (44.3) | 140 (39.3) | 89 (38.9) | 97 (41.3) | 492 (41.2) |
| Age at diagnosis, years, mean (SD) | 75.2 (9.8) | 74.0 (9.9) | 73.8 (10.7) | 74.9 (9.9) | 74.5 (10.0) |
| Age at diagnosis, years, median (IQR) | 77.0 (69.0 to 82.0) | 75.0 (69.0 to 81.0) | 74.0 (68.0 to 81.0) | 75.0 (69.0 to 82.0) | 75.0 (69.0 to 82.0) |
| Age group, n (%) | |||||
| <65 | 51 (13.6) | 60 (16.9) | 38 (16.6) | 32 (13.6) | 181 (15.1) |
| 65–74 | 102 (27.2) | 114 (32.0) | 78 (34.1) | 76 (32.3) | 370 (31.0) |
| ≥75 | 222 (59.2) | 182 (51.1) | 113 (49.3) | 127 (54.0) | 644 (53.9) |
| Care setting at diagnosis, n (%) | |||||
| Internal medicine | 66 (17.6) | 73 (20.5) | 49 (21.4) | 37 (15.7) | 255 (18.8) |
| Cardiology | 54 (14.4) | 53 (14.9) | 30 (13.1) | 29 (12.3) | 166 (13.9) |
| Neurology | – | – | 1 (0.4) | 1 (0.4) | 2 (0.2) |
| Geriatrics | 7 (1.9) | 8 (2.2) | 3 (1.3) | 4 (1.7) | 22 (1.8) |
| Primary care/general practice | 248 (66.1) | 222 (62.4) | 146 (63.3) | 164 (69.8) | 780 (65.3) |
| Medical history, n (%) | |||||
| Congestive heart failure | 25 (6.7) | 18 (5.1) | 10 (4.4) | 15 (6.4) | 68 (5.7) |
| History of hypertension | 269 (71.7) | 245 (68.8) | 135 (59.2) | 141 (60.3) | 790 (66.2) |
| Diabetes mellitus | 46 (12.3) | 50 (14.0) | 29 (12.7) | 31 (13.2) | 156 (13.1) |
| Stroke | 23 (6.1) | 20 (5.6) | 7 (3.1) | 17 (7.2) | 67 (5.6) |
| Systemic embolism | 2 (0.5) | 2 (0.6) | – | 1 (0.4) | 5 (0.4) |
| Coronary artery disease | 80 (21.3) | 57 (16.0) | 44 (19.2) | 43 (18.3) | 224 (18.7) |
| Vascular disease | 46 (12.3) | 34 (9.6)a | 31 (13.5) | 32 (13.7)b | 143 (12.0)c |
| History of bleeding | 23 (6.1) | 19 (5.4) | 13 (5.7) | 14 (6.0) | 69 (5.8) |
| Moderate to severe CKD* | 108 (28.8) | 82 (23.0) | 47 (20.5) | 65 (27.7) | 302 (25.3) |
| Risk scores | |||||
| CHA2DS2-VASc, mean (SD) | 3.2 (1.5)d | 3.0 (1.4)e | 3.0 (1.5)f | 3.2 (1.5)g | 3.1 (1.5)h |
| CHA2DS2-VASc, median (IQR) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.0) |
| CHA2DS2-VASc, 0–1, n (%) | 41 (11.6) | 46 (13.8) | 34 (16.5) | 27 (13.4) | 148 (13.5) |
| HAS-BLED, mean (SD) | 2.2 (0.9)i | 2.1 (0.9)j | 1.7 (1.0)k | 1.9 (1.1)l | 2.0 (1.0)m |
| HAS-BLED, median (IQR) | 2.0 (2.0 to 3.0) | 2.0 (2.0 to 3.0) | 2.0 (1.0 to 2.0) | 2.0 (1.0 to 3.0) | 2.0 (1.0 to 3.0) |
| HAS-BLED, 0–2, n (%) | 164 (66.6) | 173 (71.1) | 122 (77.7) | 96 (71.6) | 555 (71.2) |
Patients missing: a1, b1, c2, d22, e24, f22, g34, h102, i129, j113, k72, l101, m415.
*Includes NKF KDOQI stages III–V.
AC, anticoagulant; CHA2DS2-VASc, cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)–vascular disease, age 65–74 and sex category (female); CKD, chronic kidney disease; HAS-BLED, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, elderly (>65), drugs/alcohol concomitantly (1 point each), NKF KDOQI, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative.
Table 6.
Use of antiplatelet and no treatment (no AC) versus anticoagulant in relation to baseline characteristics
| Variable | Cohorts 2 to 5 OR (95% CI) |
| Gender | |
| Female | 1 |
| Male | 1.09 (0.91 to 1.30) |
| Age (years) | |
| <65 | 1 |
| 65–80 | 0.70 (0.54 to 0.90) |
| 80–85 | 0.75 (0.55 to 1.02) |
| >85 | 0.98 (0.70 to 1.36) |
| Medical history* | |
| Congestive heart failure | 0.73 (0.52 to 1.03) |
| Hypertension (history or >140/90 mm Hg) | 0.89 (0.72 to 1.09) |
| Diabetes | 0.57 (0.45 to 0.72) |
| Coronary artery disease | 0.84 (0.64 to 1.11) |
| Vascular disease | 0.63 (0.46 to 0.87) |
| Dementia | 0.72 (0.28 to 1.84) |
| Moderate to severe CKD† | 0.92 (0.75 to 1.12) |
| NSAID usage | 5.85 (4.89 to 7.00) |
| Bleeding | 6.30 (3.90 to 10.18) |
| Previous stroke/TIA/SE | 0.47 (0.36 to 0.62) |
| Smoking | |
| Never | 1 |
| Ex-smoker | 0.96 (0.81 to 1.15) |
| Current smoker | 1.04 (0.73 to 1.48) |
| Cohort | |
| 2 | 1 |
| 3 | 0.84 (0.67 to 1.05) |
| 4 | 0.55 (0.43 to 0.70) |
| 5 | 0.52 (0.41 to 0.66) |
*Reference group is patients with no history of disease (for congestive heart failure, hypertension, diabetes, coronary artery disease, vascular disease, dementia, moderate to severe CKD, NSAID usage, bleeding, previous stroke/TIA/SE)
†Includes NKF KDOQI stages III–V; none or mild (reference group) includes all other patients.
Please note: An OR > 1 implies that No ACs are more frequent than ACs, while an OR < 1 means that ACs are more frequent than No ACs. Odds ratios were adjusted for all variables in the model.
AC, anticoagulant; CKD, chronic kidney disease; NKF KDOQI, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative; NSAID, non-steroidal anti-inflammatory drug; SE, systemic embolism; TIA, transient ischaemic attack.
Antithrombotic therapy use according to risk score
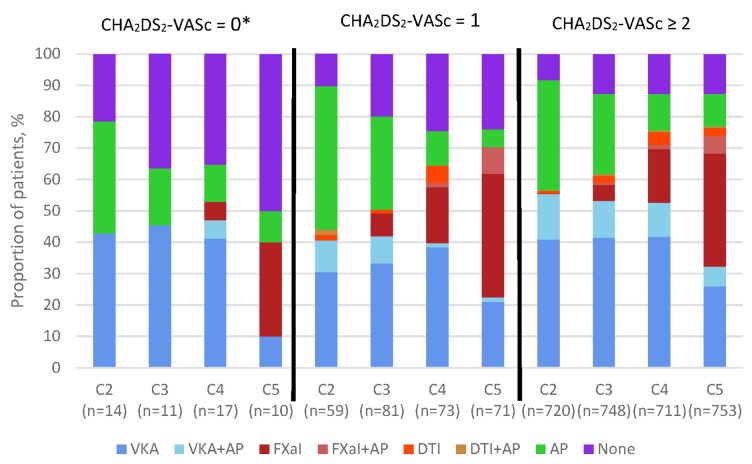
Figure 2 shows the use of antithrombotic therapy according to CHA2DS2-VASc score and cohort. Notably, the registry includes a few patients classified as low risk according to the CHA2DS2-VASc score (ie, 0 for men, 1 for women) because the determination of risk factors was left to the clinician’s judgement and not prespecified in the protocol. The use of AC±AP increased from C2 to C4 for patients at all levels of stroke risk (low, moderate and high risk), though the increase was highest in patients with a CHA2DS2-VASc of ≥2 (C2 56.7%; C4 75.6%). At the same time, there was a decline in the proportion of patients receiving AP only and an increase in the proportion of high-risk patients not receiving any antithrombotic therapy. The overall use of antithrombotic therapy decreased in patients with low risk of stroke from C2 to C4, driven by a decline in the use of AP only from 35.7% in C2 to 11.8% in C4. Also, the proportion of low-risk patients not receiving any antithrombotic therapy increased from 21.4% to 35.3%. There was a slightly different pattern from C4 to C5; there was a decrease in the use of AC±AP in patients at low risk (C4 53.0%, C5 40.0%) and C5 had the largest proportion of low-risk patients not receiving treatment (50.0%). C5 saw an increase in NOAC use across all stroke risk levels, along with a decrease in the use of VKA.
Figure 2.
Antithrombotic treatment at diagnosis by CHA2DS2-VASc and cohort, for patients with a score of 0, 1 and ≥2. *Includes women with no other risk factors. The total population represented by n excludes unknowns. Patients with missing CHA2DS2-VASc score: C2, 35; C3, 58; C4, 49; C5, 65. AP, antiplatelet; CHA2DS2-VASc, cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)–vascular disease, age 65–74 and sex category (female); DTI, direct thrombin inhibitor; FXaI, factor Xa inhibitor; VKA, vitamin K antagonist.
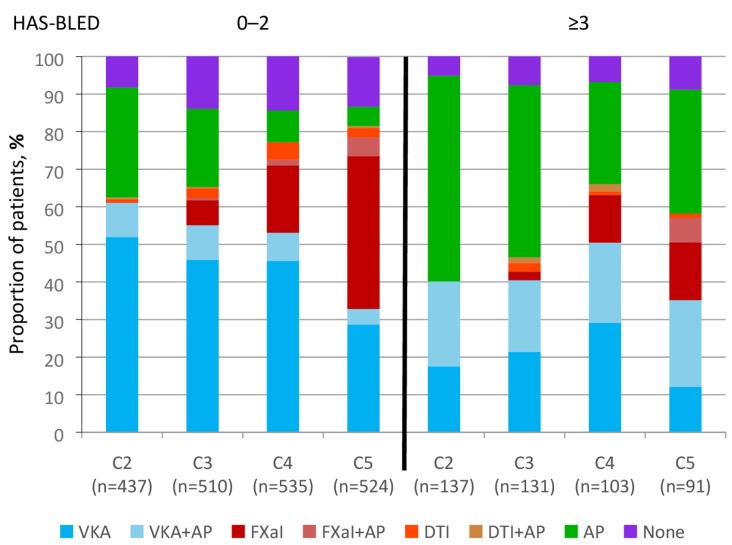
Figure 3 shows the use of antithrombotic therapy according to HAS-BLED score and cohort. There was an increase in AC use over the study period for patients with a HAS-BLED score of 0 to 2; notably, there was a steady increase in AC±AP use in patients with HAS-BLED ≥3, peaking at C4 (C2 40.1%, C3 46.7%, C4 66.0%, C5 58.2%) at the expense of AP use.
Figure 3.
Antithrombotic treatment at diagnosis by HAS-BLED score and cohort, for patients with a score of 0–2 and ≥3. AP, antiplatelet; DTI, direct thrombin inhibitor; FXaI, factor Xa inhibitor; HAS-BLED, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, elderly (>65), drugs/alcohol concomitantly (1 point each); VKA, vitamin K antagonist.
Main reason anticoagulant was not used in patients with CHA2DS2-VASc ≥2
The main reasons why ACs were not used in patients with a CHA2DS2-VASc score of ≥2 are shown in table 7. The top two known reasons were patient refusal and physician’s choice. Patient refusal was variable, and in the most recent cohort (C5), it accounted for 11.2% of high-risk patients not receiving AC. There were also some variations in the reasons for physicians choosing not to give high-risk patients ACs across the cohorts; the main reason in C2 was fall risk, whereas the main reason in C5 was bleeding risk.
Table 7.
Main reason anticoagulant not used in patients with CHA2DS2-VASc ≥2
| Variable | Cohort 2 (n=307) n % |
Cohort 3 (n=279) n % |
Cohort 4 (n=171) n % |
Cohort 5 (n=170) n % |
| Main reason anticoagulant not used* | ||||
| Already taking antiplatelet drugs for other medical condition | 30 (9.8) | 11 (3.9) | 5 (2.9) | 9 (5.3) |
| Patient refusal | 44 (14.3) | 51 (18.3) | 24 (14.0) | 19 (11.2) |
| Previous bleeding event | 6 (2.0) | 5 (1.8) | 7 (4.1) | 5 (2.9) |
| Taking medication contraindicated or cautioned for use with VKA or AC | 1 (0.3) | 2 (0.7) | 1 (0.6) | 2 (1.2) |
| Other | 113 (36.8) | 100 (35.8) | 73 (42.7) | 79 (46.5) |
| Unknown | 70 (22.8) | 72 (25.8) | 46 (26.9) | 36 (21.2) |
| Physician’s choice† | 43 (14.0) | 38 (13.6) | 15 (8.8) | 20 (11.8) |
| Bleeding risk | 8 (18.6) | 10 (26.3) | 9 (60.0) | 13 (65.0) |
| Concern over patient compliance | 3 (7.0) | 1 (2.6) | – | – |
| Guideline recommendation | 8 (18.6) | 6 (15.8) | 1 (6.7) | 1 (5.0) |
| Fall risk | 13 (30.2) | 12 (31.6) | 2 (13.3) | 5 (25.0) |
| Low risk of stroke | 11 (25.6) | 9 (23.7) | 3 (20.0) | 1 (5.0) |
*Percentages are calculated with the column ‘n’ as denominator;
†Percentages in each category of the physician’s choice are calculated with the available (non-missing) data of the variable as denominator.
AC, anticoagulant; CHA2DS2-VASc, cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)–vascular disease, age 65–74 and sex category (female); VKA, vitamin K antagonist.
Discussion
These findings from the UK cohort of the GARFIELD-AF registry indicate a progressive improvement in the clinical management of AF, with newly diagnosed at-risk patients with AF more often receiving guideline-recommended therapy. The proportion of patients on AC increased (C2 54.5%, C3 60.1%, C4 72.9%, C5 73.9%) and the increase in the use of AC was mainly in patients with CHA2DS2-VASc ≥2. There was a notable increase in the use of NOACs±AP (C2 1.3%, C3 8.0%, C4 23.0%, C5 43.3%), with the main increase in NOAC prescribing being driven by the prescribing of factor Xa inhibitors; C5 saw a change in VKA prescribing, with NOACs being prescribed in place of VKA. The use of AP only decreased (C2 36.5%, C3 25.3%, C4 11.9%, C5 10.5%); however, the co-prescription of AC+AP did not change much (C2 14%, C3 11.8%, C4 11.4%, C5 11.7%). AC use decreased with bleeding risk, with people with HAS-BLED ≥3 less likely to be anticoagulated; nevertheless, use of AC in patients with HAS-BLED ≥3 increased notably from 40.1% in C2 to the peak of 66.0% in C4.
In addition, there was a decline in AP use in patients at low risk, with a corresponding increase in the proportion of patients in this category not receiving any antithrombotic therapy. However, an important proportion of low-risk patients received AC over the period, with 50% of low-risk patients receiving AC in the most recent cohort. For patients with a CHA2DS2-VASc score of 1, there was a notable increase in AC prescribing from C2 to C5 and a steep decline in the use of AP only.
Our findings are, to a large extent, consistent with changes in AF management guidelines. In the UK, NICE guidelines up until 2014 recommend that high-risk patients should be on warfarin, those at moderate risk should receive warfarin or aspirin, and low-risk patients should not be on warfarin (but could be prescribed aspirin).17 The current (2014) guidelines no longer recommend aspirin; patients should receive anticoagulation or not.5 The notable increase in AC use and corresponding decline in AP use fall within the guidelines; our data suggest patients that would have been given aspirin in earlier cohorts are now given AC, also that the increase in AC use is potentially driven by the availability of NOACs.
This is the first UK study to describe the reasons for not anticoagulating real-world patients in relation to stroke risk, and the findings corroborate our deduction that guidelines have influenced clinical practice. The data suggest that patient refusal (11.2% for high-risk patients in the most recent cohort) may be the main patient factor affecting rates of anticoagulation. There is little UK evidence on AC treatment rates in the post-VKA-only era; nevertheless, co-prescription of ACs and APs (15.1%) is higher than reported by Kassianos et al11 (11% initiated on ACs plus APs within 12 weeks of diagnosis of AF).
Strengths and limitations
This study describes real-world clinical practice in the UK for treatment initiated at AF diagnosis in patients with AF and at least one risk factor for stroke. Recruiting patients from primary care captures patients regardless of the care setting of diagnosis, therefore providing a pool of patients representative of UK patients diagnosed with AF. Study sites sought to recruit consecutive eligible patients, thereby reducing the risk of selection bias. In addition, the 6-week period between diagnosis and enrolment minimises the risk of excluding deceased patients.
The study is subject to the limitations inherent to observational studies, although efforts were made to standardise definitions and reduce missing data. Ethical approval for the study does not cover patients without the capacity to consent. The data on low-risk patients need to be interpreted with caution due to the low numbers in the UK sample. Comorbidities are likely confounders in treatment strategies; however, these were not comprehensively incorporated in this analysis.
Comparison with global GARFIELD-AF data
Evolving antithrombotic treatment patterns up to C4 for the global GARFIELD-AF population have previously been published18; our comparison is in relation to UK patients enrolled during the corresponding recruitment period (C2 to C4). Globally, a total of 34 170 patients were enrolled into C2 to C4 in 34 countries. UK patients were older than patients in the global study: mean age of 74.7 years compared with 69.9 years in the global study.18 UK patients had less heart failure (7.6% vs 19.8%), higher prevalence of CKD (26.5% vs 10.3%), but similar rates of coronary artery disease and acute coronary syndromes. UK patients had a higher proportion of those with CHA2DS2-VASc score of 0–1 (10.5% vs 14.7%) and a lower proportion with HAS-BLED of 0–2 (81.3% vs 88.7%).
Despite starting from a lower baseline, the use of AC in the UK in the most recent cohort is comparable to that in the global study (UK 54.7% to 73.1%, global 62.1% to 71.1%).18 Nevertheless, the uptake of NOACs is higher in the global study, with NOACs being prescribed in place of VKA, whereas VKA prescribing in the UK hardly changed up until C4 (NOAC use in C4: global 37.2%, UK 22.7%). In C5, however, UK data illustrate a decline in VKA prescribing matched by an increase in NOAC use. As in the UK population, overtreatment of patients at low risk of stroke was observed in the global population, and over 50% of low-risk patients in C4 received AC. This may be due to clinicians’ perception of stroke risk as all participants were deemed by the recruiting clinician to have an investigator-determined risk factor for stroke. Co-prescription of AC+AP was also an issue in the global population, with 6.8% affected in C4; however, the UK seems to have responded better to the renunciation of AP only as a treatment option: in C4, 11.7% of high-risk UK patients were given AP only compared with 16.0% in the global population.
Implications for practice
These data indicate progressive concordance with evidence-based guidelines and clinical practice in the UK for patients newly diagnosed with AF. More UK patients are receiving guideline-recommended therapy; this is significant, given the increasing prevalence of AF in the UK. Although the proportion of high-risk patients taking an AC in the most recent cohort is unprecedented, nearly a quarter of high-risk patients still do not receive AC therapy, indicating that there is further scope for improvement. It is important to elucidate the reasons why some high-risk patients do not receive anticoagulation; in particular, the reasons and circumstances for patient refusal need to be explored (and documented). An important proportion of low-risk patients are still receiving AC despite the proven capability of the CHA2DS2-VASc score to identify patients at truly low risk. Further attention to patients in this category will be beneficial. Also, patients are being co-prescribed ACs and aspirin (11.7% of high-risk patients in most recent cohort), a combination that is rarely indicated since it increases bleeding risk by over 50%; it might be worth exploring the rationale for this in future research.
The clinical management of patients with AF is evolving and treatment outcomes will become clearer with time. GARFIELD-AF provides real-world data on evolving treatment patterns, and further data will provide insight into corresponding treatment outcomes.
bmjopen-2017-018905supp001.pdf (98KB, pdf)
Supplementary Material
Acknowledgments
We thank the physicians, nurses and patients involved in the GARFIELD-AF registry. SAS programming support was provided by Madhusudana Rao (Thrombosis Research Institute, London, UK). Editorial support was provided by Emily Chu (Thrombosis Research Institute, London, UK). FDRH acknowledges part-funding from the NIHRSchool for Primary Care Research, NIHR CLARHC Oxford, NIHR Oxford BRC, and NIHROxford DEC.
Footnotes
Contributors: PNA contributed to the acquisition, analysis and interpretation of data for the study, and drafted the manuscript. HG contributed to the analysis and interpretation of the data and revised the work critically for intellectual content. FDRH contributed to the interpretation of the data and revised the work critically for intellectual content. DAF contributed to the acquisition, analysis and interpretation of the data and revised the work critically for intellectual content. FDRH and DAF contributed to the initial methods of GARFIELD-AF in the UK. DAF is also the Principal Investigator and guarantor for the UK study. All authors approved the final version of the manuscript and are accountable for all aspects of the work.
Funding: The GARFIELD-AF registry is sponsored by the Thrombosis Research Institute, London, UK. Funding of the registry is provided through an educational research grant from Bayer AG (Berlin, Germany).
Competing interests: FDRH personal fees and other from BMS/Pfizer, personal fees and other from BI, personal fees and other from Bayer, outside the submitted work.
Patient consent: Obtained.
Ethics approval: The UK has received ethical approval from the South East London Research Ethics Committee 5 (REC 5) on 29 September 2010; REC reference 10/H0805/48.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
Collaborators: A full list of the UK GARFIELD-AF Investigators is given in the online supplementary appendix 1.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. 10.1161/01.STR.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 1987;147:1561–4. [PubMed] [Google Scholar]
- 3.Jørgensen HS, Nakayama H, Reith J, et al. . Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke 1996;27:1765–9. 10.1161/01.STR.27.10.1765 [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Kirchhof P, Lip GY, et al. . Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- 5. National Instisute for Health and Clinical Excellence (NICE). Nice Clinical Guideline 180; Atrial Fibrillation: the management of atrial fibrillation. 2014. https://www.nice.org.uk/guidance/cg180
- 6.Ogilvie IM, Newton N, Welner SA, et al. . Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638–45. 10.1016/j.amjmed.2009.11.025 [DOI] [PubMed] [Google Scholar]
- 7.Baczek VL, Chen WT, Kluger J, et al. . Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta-analysis. BMC Fam Pract 2012;13:5 10.1186/1471-2296-13-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammed MA, Marshall T, Nirantharakumar K, et al. . Patterns of warfarin use in subgroups of patients with atrial fibrillation: a cross-sectional analysis of 430 general practices in the United Kingdom. PLoS One 2013;8:e61979 10.1371/journal.pone.0061979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt TA, Hunter TD, Gunnarsson C, et al. . Risk of stroke and oral anticoagulant use in atrial fibrillation: a cross-sectional survey. Br J Gen Pract 2012;62:710–7. 10.3399/bjgp12X656856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan C, Healicon R, Robson I, et al. . The use of anticoagulants in the management of atrial fibrillation among general practices in England. Heart 2013;99:1166–72. 10.1136/heartjnl-2012-303472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kassianos G, Arden C, Hogan S, et al. . Current management of atrial fibrillation: an observational study in NHS primary care. BMJ Open 2013;3:e003004 10.1136/bmjopen-2013-003004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakkar AK, Mueller I, Bassand J-P, et al. . International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J 2012;163:13–19. 10.1016/j.ahj.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 13.Apenteng PN, Murray ET, Holder R, et al. . An international longitudinal registry of patients with atrial fibrillation at risk of stroke (GARFIELD): the UK protocol. BMC Cardiovasc Disord 2013;13:31 10.1186/1471-2261-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1. [PubMed] [Google Scholar]
- 15.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 16.Raghunathan TE, Lepkowski JM, Van Hoewyk J, et al. . A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology 2001;27:85–96. [Google Scholar]
- 17. National Instisute for Health and Clinical Excellence (NICE). Clinical Guideline CG36—Atrial Fibrillation: the management of atrial fibrillation. 2006. http://www.nice.org.uk/CG36.
- 18.Camm AJ, Accetta G, Ambrosio G, et al. . Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart 2017;103:307–14. 10.1136/heartjnl-2016-309832 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018905supp001.pdf (98KB, pdf)