Abstract
Objective
Screening is a key strategy to address the rising burden of chronic kidney disease (CKD) in low-income and middle-income countries. However, there are few reports regarding the implementation of screening programmes in resource-limited settings. The objectives of this study are to (1) to share programmatic experiences implementing CKD screening in a rural, resource-limited setting and (2) to assess the burden of renal disease in a community-based diabetes programme in rural Guatemala.
Design
Cross-sectional assessment of glomerular filtration rate (GFR) and urine albumin.
Setting
Central Highlands of Guatemala.
Participants
We enrolled 144 adults with type 2 diabetes in a community-based CKD screening activity carried out by the sponsoring institution.
Outcome measures
Prevalence of renal disease and risk of CKD progression using Kidney Disease: Improving Global Outcomes definitions and classifications.
Results
We found that 57% of the sample met GFR and/or albuminuria criteria suggestive of CKD. Over half of the sample had moderate or greater increased risk for CKD progression, including nearly 20% who were classified as high or very high risk. Hypertension was common in the sample (42%), and glycaemic control was suboptimal (mean haemoglobin A1c 9.4%±2.5% at programme enrolment and 8.6%±2.3% at time of CKD screening).
Conclusions
The high burden of renal disease in our patient sample suggests an imperative to better understand the burden and risk factors of CKD in Guatemala. The implementation details we share reveal the tension between evidence-based CKD screening versus screening that can feasibly be delivered in resource-limited global settings.
Keywords: chronic renal failure, general diabetes, international health services
Strengths and limitations of this study.
This study is one of the first to describe the implementation of a chronic kidney disease (CKD) screening programme in a rural area of a low-income or middle-income country.
The primary strength of this study relates to the practical barriers that were overcome to implement a guideline-directed CKD screening programme in this setting.
We investigated CKD in a small diabetes sample of 144 people, limiting the generalisability of our results.
Our results could have overestimated the overall prevalence of CKD among people with diabetes in rural Guatemala, given that we sampled from a single institution’s diabetes cohort rather than using a population-based sampling strategy.
Our sample was predominantly composed of women, which reflects known challenges in enrolling men in chronic disease programmes in Latin America.
Introduction
Chronic kidney disease (CKD) is a critical global health problem.1–3 The worldwide CKD prevalence rate is 11%–13%.4 From 2005 to 2015, deaths due to CKD rose from 0.9 to 1.2 million per year, primarily owing to increases in CKD caused by diabetes and hypertension.5 Data on CKD are limited in low-income and middle-income countries (LMICs), but age-adjusted prevalence and mortality rates may be greater than in high-income countries.6 7 The causes of CKD in LMICs are heterogeneous and incompletely understood, and most individuals are undiagnosed.2 8 9 A growing proportion of those with CKD in LMICs develop end-stage renal disease (ESRD), yet most do not have access to life-saving renal replacement therapy (RRT).10 11
The region of interest in this study is Latin America. Here, marked disparities exist with regard to the nephrology workforce and RRT rates.12 Latin America has the highest CKD death rate in the world,5 and diabetes is the leading cause of ESRD.12 Recent high-quality evidence from Mexico suggests that diabetes is a potent risk factor for CKD and death from renal disease in this region.13
CKD screening and management in resource-limited settings
Scaling up screening is an important strategy to address the burden of CKD in LMICs.14–16 International clinical guidelines recommend CKD screening for individuals with risk factors such as diabetes, using laboratory assessments of glomerular filtration rate (GFR) and urine albumin excretion.17–20 In the case of diabetes, interventions shown to slow disease progression for individuals who screen positive for CKD include glycaemic control, blood pressure management and renoprotection with ACE inhibitors or angiotensin receptor blockers.21
However, there are many barriers to implementing CKD screening in resource-limited settings. Screening for CKD may be cost-effective in high-income countries in high-risk patients such as those with diabetes,22 but the cost-effectiveness in LMICs is uncertain. This is in large part because international CKD screening guidelines require access to specialised laboratory testing,21 which is frequently unavailable at the primary care level in LMICs.23 Furthermore, many national health systems in LMICs are not equipped or funded to deliver integrated care for individuals with CKD once they are detected by screening.23 24 Finally, there are few published reports documenting the practical details of implementing CKD screening programmes in LMICs. Scaling up CKD care requires that all implementers more readily share their experiences in designing and evaluating CKD screening programmes.
Study objectives
This study describes the implementation and outcomes of a small, community-based CKD screening programme for patients with type 2 diabetes in rural Guatemala. The objectives are (1) to share our programmatic experiences implementing CKD screening in a rural, resource-limited setting and (2) to assess the burden of renal disease in a community-based diabetes programme in rural Guatemala.
Methods
Setting
This study was carried out in Guatemala by the non-governmental healthcare organisation Wuqu’ Kawoq (www.wuqukawoq.org). Since 2007, the organisation has operated a free, comprehensive type 2 diabetes programme serving approximately 250 rural patients in the Central Highlands of Guatemala. We previously have published implementation details and a clinical protocol of this programme.25 The programme is community based with the majority of patients entering on a first-come, first-served basis based on programme capacity.
Guatemala is a Latin American country with a population of approximately 16 million. Approximately 40% of Guatemalans are indigenous Maya,26 a predominantly rural ethnolinguistic group with poverty rates approaching 80% or greater,27 poor general health indicators28 and limited access to healthcare.29 30 The rural health system in Guatemala is fragmented and highly privatised, and people with chronic diseases face profound barriers when seeking healthcare.31 Individuals with diabetes typically have experienced years of uncontrolled disease prior to presentation to our institution’s clinic.32
While the epidemiology and risk factors for CKD have not been well studied in Guatemala, crude renal failure mortality rates are among the highest in the Americas.33 In addition to rising rates of traditional CKD risk factors like diabetes, some studies show that the newly described disease entity ‘CKD of non-traditional causes’ may also be affecting rural Guatemalan populations.34 35 Guatemala also has one of the world’s highest rates of child malnutrition,36 which is a risk factor for reduced renal function.37 With 54 total nephrologists, Guatemala has only 3.3 nephrologists per million population, below the Latin America average.12 Nevertheless, the number of people on RRT has increased rapidly, and Guatemala now has one of the highest per capita peritoneal dialysis rates in the world.38
Implementation of a community-based CKD screening and management programme in Guatemala
Our organisation previously had screened pragmatically for CKD in patients with diabetes using measurement of serum creatinine and manual assessment of urine dipstick protein.18 39 In 2014, after an increase in morbidity and mortality secondary to diabetes nephropathy including more than a dozen cases of ESRD, we redesigned our screening protocol to include assessment of both serum creatinine and urine albumin/creatinine excretion as recommended in the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.21
Implementing a KDIGO-aligned CKD screening programme posed significant logistical and financial challenges in our rural Guatemalan context. Reliable assays were only available at urban laboratory facilities, so we funded individual patient transportation or paid for couriers to transport specimens collected in rural villages. Assay costs were approximately US$8 per test for urine creatinine and urine albumin, and US$2.50 per test for serum creatinine. Collection and transportation fees approximately doubled these per-assay costs. These expenses were significant given that our overall budget for comprehensive diabetes care is approximately US$250 per patient per year, a sum we finance through donor fundraising.
Patients identified with CKD, including moderately increased albuminuria (albumin-to-creatinine ratio (ACR) of 30–300 mg/g), received guideline-directed medical care to prevent CKD progression,21 including management of hyperglycaemia with oral antidiabetic medications and insulin, treatment of albuminuria with ACE inhibitors, efforts to control hypertension with ACE inhibitors and other classes of antihypertensive agents, and integration of a behavioural intervention to improve exercise and diet. As we have described elsewhere,25 in our clinical protocols, we target haemoglobin A1c (HbA1c) <8.0% and blood pressure <140/90 mm Hg; these targets are less strict than those recommended in some guidelines but rational given our rural setting with minimal access to emergency services in the event of medication-related adverse effects. In line with KDIGO guidance,21 we monitored creatinine and potassium after initiating or escalating ACE inhibitor therapy, which added further costs to the programme. Of note, angiotensin receptor blockers are cost-prohibitive in our setting.
We referred patients with GFR below 30 mL/min/1.73 m2 and who wished to be considered for dialysis to the National Center for Chronic Renal Disease, a public health facility in Guatemala City that provides nephrology care, including haemodialysis and continuous ambulatory peritoneal dialysis (CAPD). Our organisation provides patient navigation and primary care services for rural patients with diabetes who carry out home-based CAPD.40
Study design and sample
We used data collected from a retrospective chart review of CKD screening among individuals with diabetes treated at our institution to carry out a cross-sectional assessment of GFR and urine albumin. Inclusion criteria were age ≥18 years, enrolment in our diabetes programme, and at least one measurement of HbA1c between 3 January 2014 (when the CKD screening programme was implemented) and 5 January 2015 (when data extraction began). Exclusion criteria were incomplete albuminuria data, diagnosis of type 1 diabetes, diagnosis of gestational diabetes or current use of dialysis. A total of 157 patients met the study’s inclusion criteria. Thirteen patients were subsequently excluded: two patients with type 1 diabetes, one patient with gestational diabetes, three patients receiving dialysis (one of whom also had type 1 diabetes) and eight patients with missing urine albumin data. A final list of 144 patients was identified that comprise the sample in this paper.
Laboratory assessment of kidney function
At contracting commercial laboratories, serum creatinine was assessed with the Microlab 300 analyser (ELITech, Paris, France). Urine ACR was carried out using the Cobas 6000/c501 analyser (Roche, Basel, Switzerland). Specimens were collected, packaged and shipped to in accordance with the guidelines of Mayo Medical Laboratories.41
Data analysis and definitions
Participant data were extracted from our electronic medical record to a spreadsheet and then imported to Stata V.13 for statistical analyses. Two independent data coders reviewed the spreadsheet for errors. We first calculated baseline characteristics using descriptive statistics. We compared the underlying characteristics of patients in the sample versus those with missing urine albumin data using the two-sample rank-sum test and Fisher’s exact test.
Second, we assessed CKD staging using GFR and albuminuria categories as recommended by KDIGO.21 We calculated GFR using the 2009 CKD-Epidemiology Collaboration (CKD-EPI) equation.42 Due to inherent limitations of our organisation’s screening programme, ACR and serum creatinine testing often did not occur on the same day. Mapping of risk stratification of disease progression was performed using KDIGO nomenclature and summarised visually with a waffle chart. Finally, we evaluated the use and indication of ACE inhibitors between albuminuria category and hypertension diagnosis. KDIGO has released detailed guidelines on the management of hypertension and albuminuria in people with diabetes,21 43 including a weaker recommendation for treatment of moderately increased albuminuria versus severely increased albuminuria. For the purposes of this analysis, we assumed that an ACE inhibitor was indicated if a patient had a diagnosis of hypertension and/or ACR greater than 30 mg/g.
Consent
A waiver of consent was obtained as the research presented no more than minimal risk of harm to subjects and involved no procedures for which written consent is normally required outside of the research context.
Results
Patient characteristics
Baseline demographic and clinical information is presented in table 1. The patient sample was predominantly women (83%), mostly Mayan speaking (61%) and had low levels of educational attainment (median 2 years). At the date of creatinine measurement, mean age was 54.6±11.8 years, and patients reported living with diabetes for a median of 6 (IQR 3–10) years. Median enrolment in the clinical diabetes programme was 1.5 (IQR 0.2–2.8) years, and mean HbA1c at enrolment was 9.4%±2.5%. At most recent HbA1c measurement, the mean was 8.6%±2.3%, and 47% of patients had met the predefined programmatic target of ≤8.0%. Forty-two per cent of the sample carried a diagnosis of hypertension, and 67% of patients had blood pressure <140/90 mm Hg at the most recent reading. A substantial proportion of patients had body mass index in the overweight (72%) and obese range (29%) range. Regarding medications, 90% were prescribed metformin, 53% a sulfonylurea, 22% insulin, 33% an ACE inhibitor and 15% at least one additional antihypertensive agent that was not an ACE inhibitor. In comparing characteristics of the sample to the eight patients excluded due to missing urine ACR, there were no significant differences in most variables (age, gender, initial HbA1c, most recent HbA1c, diagnosis of hypertension), but GFR was lower among those with missing albuminuria values.
Table 1.
Demographic and clinical profile
| Characteristic (n=144) | Value* |
| Age (years) | 54.6±11.8 |
| Female (%) | 83 |
| Language preference (n=134) | |
| Mayan Kaqchikel or K’iche’ (%) | 61 |
| Spanish (%) | 39 |
| Years of schooling (years, n=132) | 2 (0–4) |
| Years with diabetes (years, n=139) | 6 (3–10) |
| Years in clinical diabetes programme | 1.5 (0.2–2.8) |
| HbA1c | |
| At enrolment (%) | 9.4±2.5 |
| Current (%) | 8.6±2.3 |
| ≤8.0% (%) | 47 |
| Blood pressure | |
| Diagnosis of hypertension (%) | 42 |
| Systolic blood pressure (mm Hg) | 128±21 |
| Diastolic blood pressure (mm Hg) | 77±11 |
| <140/90 mm Hg (%) | 67% |
| Body mass index (n=132) | |
| Mean (kg/m2) | 27.6±4.7 |
| ≥25 (%) | 72 |
| ≥30 (%) | 29 |
| Medication prescriptions | |
| Metformin (%) | 90 |
| Sulfonylurea (%) | 53 |
| Insulin (%) | 22 |
| ACE inhibitors (%) | 33 |
| Other antihypertensive agent (beta-blocker, calcium-channel blocker or thiazide diuretic) | 15% |
*Normally distributed values are described as mean±SD and non-normally distributed values as median (IQR).
HbA1c, haemoglobin A1c.
CKD indicators
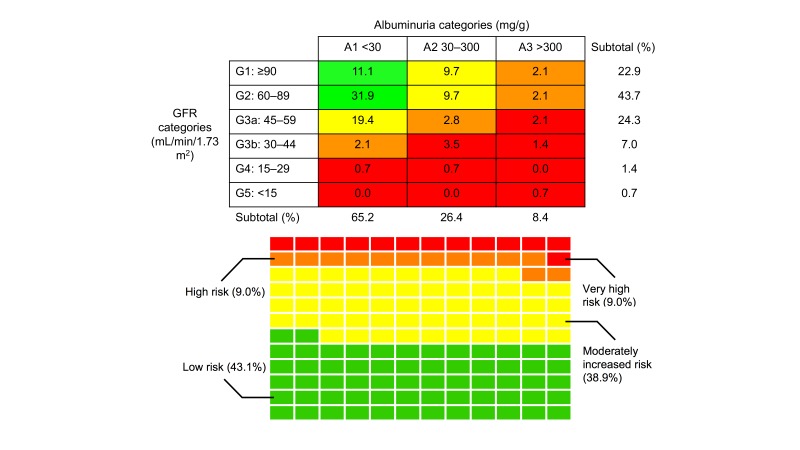
Risk of CKD progression according to the KDIGO system in our sample of patients with diabetes is summarised in figure 1. A majority (77.1%) of the sample had at least mildly decreased GFR (<90 mL/min/1.73 m2), and 33.4% had GFR <60 mL/min/1.73 m2 (G3a or worse). Most (65.2%) of the sample had ACR values in the normal range of <30 mg/g. Moderately increased albuminuria (30–300 mg/g) was observed in 26.4% and severely increased albuminuria (>300 mg/g) in 8.4% of patients. Overall, 57% had GFR <60 mL/min/1.73 m2 or ACR of >30 mg/g.
Figure 1.
Risk of chronic kidney disease (CKD) progression. (Top panel) Risk map for CKD progression. Cells coded by Kidney Disease: Improving Global Outcomes (KDIGO) risk level as follows: green, low risk; yellow, moderately increased risk; orange, high risk; red, very high risk. Glomerular filtration rate (GFR) was calculated using CKD-Epidemiology Collaboration equation. KDIGO designations are as follows: A1, normal to mildly increased albuminuria; A2, moderately increased albuminuria; A3, severely increased albuminuria; G1, normal or high GFR; G2, mildly decreased GFR; G3a, mildly to moderately decreased; G3b, moderately to severely decreased GFR; G4, severely decreased GFR; G5, kidney failure. (Bottom panel) Waffle chart of KDIGO CKD risk progression categories. Each rectangle denotes a single patient in the sample (n=144). Cells are coded by KDIGO risk as in the top panel.
Using the KDIGO classification for risk of CKD progression, 43.1% of patients were classified as low risk, 38.9% as moderately increased risk, 9.0% as high risk and 9.0% as very high risk. The median time interval between ACR and creatinine screening assessments was 56 (IQR 17–115) days.
Use of ACE inhibitors
Table 2 displays the proportion of patients prescribed an ACE inhibitor by albuminuria category and hypertension diagnosis prior to implementation of the KDIGO-aligned CKD screening programme. Sixty-six per cent (40/61) of patients with hypertension had an ACE inhibitor prescription. Among those patients with a diagnosis of hypertension, 53% (8/15) of participants with moderately increased microalbuminuria and 100% (9/9) with severely increased microalbuminuria had been prescribed an ACE inhibitor at the time of albuminuria assessment. Among patients who did not have a diagnosis of hypertension, ACE inhibitors had been prescribed in 17% (4/23) of cases with moderately increased microalbuminuria and 0% (0/3) of cases with severely increased microalbuminuria. There were three patients in the sample who had been prescribed an ACE inhibitor but who did not have hypertension or elevated albuminuria.
Table 2.
Proportion of sample prescribed ACE inhibitor by albuminuria category and hypertension diagnosis
| Albuminuria categories (mg/g) | Patients without hypertension | Patients with hypertension |
| <30 (A1) | 5% (3/57) | 62% (23/37)* |
| 30–300 (A2) | 17% (4/23)* | 53% (8/15)* |
| >300 (A3) | 0% (0/3)* | 100% (9/9)* |
| Total | 8% (7/83) | 66% (40/61) |
Kidney Disease: Improving Global Outcomes designations are as follows: A1, normal to mildly increased albuminuria; A2, moderately increased albuminuria; A3, severely increased albuminuria.
*ACE inhibitor was assumed to be indicated for the treatment of hypertension and/or elevated microalbuminuria.
Discussion
This was a retrospective chart review of a community-based CKD screening programme for adults with type 2 diabetes in rural Guatemala. Our programme’s implementation details and results raise key implications for CKD screening efforts in rural, resource-limited global settings.
First, we report a very high burden of renal disease among our sample of adults with type 2 diabetes in rural Guatemala. Nearly 20% of individuals were classified as high or very high risk for CKD progression, and 57% of the sample met GFR and/or albuminuria criteria suggestive of CKD. For comparison, the prevalence of CKD among people with diabetes in the USA is approximately 26%,44 and in high-risk cohorts in other LMICs has ranged from 19% to 49%.9 Surprisingly, most participants with reduced GFR had normoalbuminuria (65.2%), a finding at odds with the prevalence of normoalbuminuric CKD described in high-quality longitudinal and cross-sectional studies.45 Additionally, the proportion of participants with hypertension (42%) was lower than in other cohorts of people with type 2 diabetes.46 We are uncertain if these findings reflect a unique feature of the diabetes epidemic among Maya indigenous populations or are an artefact of sampling and data collection. In other settings, emerging evidence suggests that the profile of people with CKD differs across LMICs.47 Given the limited data on the epidemiology of CKD in Guatemala,12 35 48 our findings show that it is imperative to better study the burden and risks factors of CKD in this setting through representative population-based surveys.
Second, while our study was not designed or powered to analyse associations of CKD with glycaemic control or blood pressure, it was notable that the mean HbA1c was quite high (9.4%±2.5% at programme enrolment and 8.6%±2.3% at time of CKD screening) and that a sizeable proportion of the sample (42%) had a diagnosis of hypertension. However, one-third of those with hypertension had not been prescribed an ACE inhibitor, one-third of the sample did not meet blood pressure goal of <140/90 mm Hg, and only a small number of patients (15% of sample) were taking a separate antihypertensive agent. These findings reiterate the fundamental importance of quality management of diabetes and hypertension in addressing CKD in our context and other resource-limited settings.
Finally, we describe the practical barriers in implementing our CKD screening programme, including cost, geographical constraints and lack of access to laboratory facilities. Three additional implementation issues that arose as we designed and implemented our screening programme are worth describing in more detail: (1) use of urine dipsticks, (2) point-of-care (POC) creatinine testing and (3) monitoring adverse effects of ACE inhibitor treatment. Regarding urine dipsticks, some clinical groups suggest using dipstick proteinuria as a CKD screening test in resource-limited settings.18 39 However, dipsticks are insensitive to low levels of urine albumin49 and perform poorly when interpreted visually,50 51 as typically must be done in rural clinics. Low-cost dipsticks to detect albuminuria are in development,52 53 but currently available models are insensitive and require use of an analyser.54 Regarding POC serum creatinine assessment, our institution has had success using POC tests for HbA1c,25 and reliable POC serum creatinine assays are available.55 However, cost and durability represent major limitations to the widespread implementation of POC serum creatinine assays in global CKD screening programmes. Regarding ACE inhibitors, reports from LMICs often do not describe protocols, training or costs associated with monitoring adverse effects of these drugs.
These implementation and screening challenges reveal the tension between evidence-based CKD screening and screening that can feasibly be delivered in rural, under-resourced areas. There is a dearth of guidelines specifically tailored for CKD screening in resource-limited settings. Important exceptions include the International Diabetes Federation,18 a CKD chapter in Partners In Health’s field manual for non-communicable diseases in in Rwanda39 and CKD screening reports from a handful of other LMICs.56–58 We urge other global health workers to share their experiences implementing CKD screening programmes and adapting international CKD guidelines to local contexts.
In our clinical programming, our pragmatic response to the work described in this paper has been to prioritise hypertension and diabetes control as the most practical CKD response in our context. We have de-emphasised the more costly screening with ACR and reinvested in quality improvement initiatives relating to management of hypertension and diabetes. This view of addressing CKD by prioritising treatment of comorbid non-communicable diseases echoes similar proposals from WHO.59 We continue to contemplate the role of low-cost urine dipstick for field screening of proteinuria, while acknowledging that a CKD screening approach without ACR will fail to detect normotensive patients with diabetes with moderately increased albuminuria who would benefit from ACE inhibitors (13% of our sample).60 Given the extent of CKD in our sample, we recognise that aggressive treatment of hypertension with ACE inhibitors entails a high risk of side effects. To minimise harm, we have decided to aggressively monitor side effects in line with KDIGO standards,21 though this practice involves increased costs.
Our research has several weaknesses and limitations. We investigated CKD in a small community-based diabetes cohort rather than a large, representative sample, so our results cannot be generalised within Guatemala or to other countries. Due to challenges assessing CKD, other reports from LMICs also have used convenience sampling.9 57Compared with the overall rural diabetes population in Guatemala, our reported CKD prevalence could have been overestimated, as our programme occasionally accepts referral patients with known diabetes complications. At the same time, CKD prevalence could have been underestimated as we excluded patients with ESRD from our analysis. Our sample was predominantly composed of women (83%), reflecting known difficulties enrolling men in chronic disease programmes in Latin America.61
A final set of weaknesses relate to our laboratory assessment of CKD. Due to the programmatic, retrospective nature of this research, we did not often measure serum creatinine and urine ACR on the same date. Like other groups,9 57 we calculated CKD prevalence rates and the risk of progression based on a single, cross-sectional assessment; not conducting confirmatory testing after 3 months, as indicated in KDIGO guidelines,21 may have overestimated the true CKD prevalence rate and risk profile of the sample.62 Additionally, our contracting commercial laboratory could not confirm the specific assay used to assess serum creatinine or traceability to IDMS. Finally, we used the 2009 CKD-EPI equation to calculate GFR, but this equation has not been validated in Guatemala. Our decision to use this equation is supported by KDIGO guidelines,21 which recommend using CKD-EPI unless an alternative estimation has been found to be superior for a specific target population.
Supplementary Material
Acknowledgments
We thank Sandy Mux, Carol Teleguario, Waleska Lopez, German Obispo and Luisa Ixjotop for assisting in study activities and for delivering high-quality diabetes care in rural Guatemala.
Footnotes
Contributors: DF and PR designed the study, secured funding and carried out fieldwork. DF, PG, KD and JH extracted and cleaned the data. DF, PG and PR analysed the data. DF and PG wrote the initial draft with input from PR. All authors were involved in the manuscript revisions and approved the final version.
Funding: This work was supported from operating funds of Wuqu’ Kawoq and a grant from the Center for Primary Care at Harvard Medical School.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: This study was approved by the Institutional Review Board of Wuqu’ Kawoq/Maya Health Alliance (WK-2017-002) and conforms to the principles embodied in the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: De-identified replication data are available through Wuqu’ Kawoq’s Dataverse site at doi:10.7910/DVN/NSE8BB.
References
- 1.Jha V, Arici M, Collins AJ, et al. . Conference Participants. Understanding kidney care needs and implementation strategies in low- and middle-income countries: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int 2016;90:1164–74. 10.1016/j.kint.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 2.Stanifer JW, Muiru A, Jafar TH, et al. . Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant 2016;31:868–74. 10.1093/ndt/gfv466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuen BL, Chadban SJ, Demaio AR, et al. . Chronic kidney disease and the global NCDs agenda. BMJ Glob Health 2017;2:e000380 10.1136/bmjgh-2017-000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill NR, Fatoba ST, Oke JL, et al. . Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 2016;11:e0158765 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1459–544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills KT, Xu Y, Zhang W, et al. . A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 2015;88:950–7. 10.1038/ki.2015.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Institute for Health Metrics and Evaluation (IHME). GBD Compare data visualization. Seattle, WA: IHME, University of Washington, 2016. (accessed 15 Jul 2017). [Google Scholar]
- 8.Jha V, Garcia-Garcia G, Iseki K, et al. . Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 9.Ene-Iordache B, Perico N, Bikbov B, et al. . Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health 2016;4:e307–e319. 10.1016/S2214-109X(16)00071-1 [DOI] [PubMed] [Google Scholar]
- 10.Liyanage T, Ninomiya T, Jha V, et al. . Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015;385:1975–82. 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 11.Ashuntantang G, Osafo C, Olowu WA, et al. . Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob Health 2017;5:e408–e417. 10.1016/S2214-109X(17)30057-8 [DOI] [PubMed] [Google Scholar]
- 12.Cusumano AM, Rosa-Diez GJ, Gonzalez-Bedat MC. Latin American dialysis and transplant registry: experience and contributions to end-stage renal disease epidemiology. World J Nephrol 2016;5:389–97. 10.5527/wjn.v5.i5.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alegre-Díaz J, Herrington W, López-Cervantes M, et al. . Diabetes and cause-specific mortality in Mexico city. N Engl J Med 2016;375:1961–71. 10.1056/NEJMoa1605368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perico N, Bravo RF, De Leon FR, et al. . Screening for chronic kidney disease in emerging countries: feasibility and hurdles. Nephrol Dial Transplant 2009;24:1355–8. 10.1093/ndt/gfp039 [DOI] [PubMed] [Google Scholar]
- 15.George C, Mogueo A, Okpechi I, et al. . Chronic kidney disease in low-income to middle-income countries: the case for increased screening. BMJ Glob Health 2017;2:e000256 10.1136/bmjgh-2016-000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cusumano AM, González Bedat MC. Chronic kidney disease in Latin America: time to improve screening and detection. Clin J Am Soc Nephrol 2008;3:594–600. 10.2215/CJN.03420807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Diabetes Association. Standards of medical care in diabetes-2017: summary of revisions. Diabetes Care 2017;40(Suppl 1):S1–135. 10.2337/dc17-S003 [DOI] [PubMed] [Google Scholar]
- 18. International Diabetes Federation. Global guideline for type 2 diabetes. Brussels, Belgium: International Diabetes Federation, 2012. [Google Scholar]
- 19.Inker LA, Astor BC, Fox CH, et al. . KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–35. 10.1053/j.ajkd.2014.01.416 [DOI] [PubMed] [Google Scholar]
- 20. Asociación Latinoamericana de Diabetes. Guías ALAD sobre el diagnóstico, control y tratamiento de la diabetes mellitus tipo 2 con medicina basada en evidencia edición 2013. Revista de la ALAD 2013:1–142. [Google Scholar]
- 21. Kidney Disease: Improving Global Outcomes. KDIGO 2012 clinical practice guideline for the evaluation andmanagement of chronic kidney disease. Kidney Int 2013;3:i–150. [DOI] [PubMed] [Google Scholar]
- 22.Komenda P, Ferguson TW, Macdonald K, et al. . Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis 2014;63:789–97. 10.1053/j.ajkd.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 23.Bello AK, Levin A, Tonelli M, et al. . Assessment of global kidney health care status. JAMA 2017;317:1864–81. 10.1001/jama.2017.4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samb B, Desai N, Nishtar S, et al. . Prevention and management of chronic disease: a litmus test for health-systems strengthening in low-income and middle-income countries. Lancet 2010;376:1785–97. 10.1016/S0140-6736(10)61353-0 [DOI] [PubMed] [Google Scholar]
- 25.Flood D, Mux S, Martinez B, et al. . Implementation and outcomes of a comprehensive type 2 diabetes program in rural Guatemala. PLoS One 2016;11:e0161152 10.1371/journal.pone.0161152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Instituto Nacional de Estadística. Encuesta nacional de condiciones de vida 2014: tomo I. Guatemala, Central America: Instituto Nacional de Estadística, 2016. [Google Scholar]
- 27.Sanchez SM, Scott K, Humberto Lopez J. Guatemala - closing gaps to generate more inclusive growth: systematic country diagnostic. Washington, DC: The World Bank, 2015. [Google Scholar]
- 28. PAHO. Health in the Americas, 2012. Guatemala: PAHO, 2012. Country Volume. [Google Scholar]
- 29.Cerón A, Ruano AL, Sánchez S, et al. . Abuse and discrimination towards indigenous people in public health care facilities: experiences from rural Guatemala. Int J Equity Health 2016;15:77 10.1186/s12939-016-0367-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hautecoeur M, Zunzunegui MV, Vissandjee B. [Barriers to accessing health care services for the indigenous population in Rabinal, Guatemala]. Salud Publica Mex 2007;49:86–93. [DOI] [PubMed] [Google Scholar]
- 31.Chary A, Rohloff P, Privatization and the new medical pluralism: shifting healthcare landscapes in Maya Guatemala. Lanham, Maryland: Lexington Press, 2015. [Google Scholar]
- 32.Chary A, Greiner M, Bowers C, et al. . Determining adult type 2 diabetes-related health care needs in an indigenous population from rural Guatemala: a mixed-methods preliminary study. BMC Health Serv Res 2012;12:476 10.1186/1472-6963-12-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. PAHO and WHO. Renal failure and chronic kidney disease (CKD) mortality visualization. 2014. http://www.paho.org/hq/index.php?option=com_content&view=article&id=9402&Itemid=41166&lang=en (accessed 4 Feb 2017).
- 34.Laux TS, Barnoya J, Cipriano E, et al. . Prevalence of chronic kidney disease of non-traditional causes in patients on hemodialysis in southwest Guatemala. Rev Panam Salud Publica 2016;39:186–93. [PubMed] [Google Scholar]
- 35.Laux TS, Barnoya J, Guerrero DR, et al. . Dialysis enrollment patterns in Guatemala: evidence of the chronic kidney disease of non-traditional causes epidemic in Mesoamerica. BMC Nephrol 2015;16:54 10.1186/s12882-015-0049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black RE, Victora CG, Walker SP, et al. . Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- 37.Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes--a global concern. Nat Rev Nephrol 2015;11:135–49. 10.1038/nrneph.2014.251 [DOI] [PubMed] [Google Scholar]
- 38.Jain AK, Blake P, Cordy P, et al. . Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012;23:533–44. 10.1681/ASN.2011060607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Partners In Health. "The PIH guide to chronic care integration for endemic non-communicable diseases: Rwanda edition": Partners in Health, 2011. [Google Scholar]
- 40.Flood DC, Chary AN, Austad K, et al. . A patient navigation system to minimize barriers for peritoneal dialysis in rural, low-resource settings: case study from Guatemala. Kidney Int Rep 2017;2:762–5. 10.1016/j.ekir.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mayo Medical Laboratories, Mayo Clinic. Collect, package, & ship. 2017. http://www.mayomedicallaboratories.com/specimen/ (accessed 24 Mar 2017).
- 42.Levey AS, Stevens LA, Schmid CH, et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012;2. [Google Scholar]
- 44.Afkarian M, Zelnick LR, Hall YN, et al. . Clinical manifestations of kidney disease among us adults with diabetes, 1988-2014. JAMA 2016;316:602–10. 10.1001/jama.2016.10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pugliese G, Solini A, Bonora E, et al. . chronic kidney disease in type 2 diabetes: lessons from the renal insufficiency and cardiovascular events (RIACE) Italian multicentre study. Nutr Metab Cardiovasc Dis 2014;24:815–22. 10.1016/j.numecd.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 46.Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes 2013;6:327–38. 10.2147/DMSO.S51325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anand S, Zheng Y, Montez-Rath ME, et al. . Do attributes of persons with chronic kidney disease differ in low-income and middle-income countries compared with high-income countries? Evidence from population-based data in six countries. BMJ Glob Health 2017;2:e000453 10.1136/bmjgh-2017-000453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Unidad Nacional de Atención al Enfermo Renal Crónico (UNAERC). Pacientes activos por programa y consulta del mes según bioestadísticas. 2015. http://unaerc.gob.gt/estadisticas/pacientes-activos-por-programa-y-consulta-del-mes-segun-bioestadisticas/ (accessed 24 Oct 2017).
- 49.Park JI, Baek H, Kim BR, et al. . Comparison of urine dipstick and albumin:creatinine ratio for chronic kidney disease screening: a population-based study. PLoS One 2017;12:e0171106 10.1371/journal.pone.0171106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rumley A. Urine dipstick testing: comparison of results obtained by visual reading and with the Bayer CLINITEK 50. Ann Clin Biochem 2000;37(Pt 2):220–1. 10.1258/0004563001899041 [DOI] [PubMed] [Google Scholar]
- 51.Waugh JJ, Bell SC, Kilby MD, et al. . Optimal bedside urinalysis for the detection of proteinuria in hypertensive pregnancy: a study of diagnostic accuracy. BJOG 2005;112:412–7. 10.1111/j.1471-0528.2004.00455.x [DOI] [PubMed] [Google Scholar]
- 52.Tugirimana PL, Delanghe JR. Development of an affordable dye-stained microalbuminuria screening test. Nephrol Dial Transplant 2009;24:1485–90. 10.1093/ndt/gfn705 [DOI] [PubMed] [Google Scholar]
- 53. Grand Challenges Canada. Urine dipsticks as a screening tool for chronic renal disease (CRD). http://www.grandchallenges.ca/grantee-stars/0518-01-10/ (accessed 28 Jul 2017).
- 54.McTaggart MP, Newall RG, Hirst JA, et al. . Diagnostic accuracy of point-of-care tests for detecting albuminuria: a systematic review and meta-analysis. Ann Intern Med 2014;160:550–7. 10.7326/M13-2331 [DOI] [PubMed] [Google Scholar]
- 55.Gbinigie O, Price CP, Heneghan C, et al. . Creatinine point-of-care testing for detection and monitoring of chronic kidney disease: primary care diagnostic technology update. Br J Gen Pract 2015;65:608–9. 10.3399/bjgp15X687613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma SK, Ghimire A, Carminati S, et al. . Management of chronic kidney disease and its risk factors in eastern Nepal. Lancet Glob Health 2014;2:e506–e507. 10.1016/S2214-109X(14)70281-5 [DOI] [PubMed] [Google Scholar]
- 57.Cravedi P, Sharma SK, Bravo RF, et al. . Preventing renal and cardiovascular risk by renal function assessment: insights from a cross-sectional study in low-income countries and the USA. BMJ Open 2012;2:bmjopen-2012-001357 10.1136/bmjopen-2012-001357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sumaili EK, Cohen EP. Screening for chronic kidney disease in sub-Saharan Africa. Lancet 2010;376:418 10.1016/S0140-6736(10)61222-6 [DOI] [PubMed] [Google Scholar]
- 59. WHO. Action plan for the prevention and control of non-communicable diseases, 2013–2020. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 60.Ravid M, Lang R, Rachmani R, et al. . Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. A 7-year follow-up study. Arch Intern Med 1996;156:286–9. 10.1001/archinte.1996.00440030080010 [DOI] [PubMed] [Google Scholar]
- 61.Fort MP, Castro M, Peña L, et al. . Opportunities for involving men and families in chronic disease management: a qualitative study from Chiapas, Mexico. BMC Public Health 2015;15:1019 10.1186/s12889-015-2361-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bottomley MJ, Kalachik A, Mevada C, et al. . Single estimated glomerular filtration rate and albuminuria measurement substantially overestimates prevalence of chronic kidney disease. Nephron Clin Pract 2011;117:348–52. 10.1159/000321515 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.