Abstract
Background and objectives
Denmark experienced three waves of the new pandemic influenza A (H1N1)pdm09 from July 2009 to February 2011. The aim of the study was to describe the epidemiology and clinical characteristics of hospitalized patients in a defined population of North Denmark Region with a mixed urban and rural community of 579 000 inhabitants.
Methods
Review of medical records of all hospitalized patients with confirmed influenza A from July 2009 to February 2011.
Results
Two hundred and seventy‐three patients were admitted to hospital. The age‐related population incidences of hospitalization were as follows: 0–14 years: 111/100 000, 15–64 years: 39/100 000, and ≥65 years: 17/100 000. During the first wave (July 2009–August 2009), three patients were admitted – none received treatment in intensive care units (ICUs), during the second wave (October 2009–January 2010), 158 patients were admitted – nine received treatment in ICUs, and during the third wave (December 2010–February 2011), 112 patients were admitted – 25 received treatment in ICUs. Fourteen patients (5%) died within 30 days of diagnosis (median 55 years; range 14–76 years) and additional seven patients (2·6%) died within 365 days (median 25 years; range 1–86 years).
Conclusions
Patients hospitalized with pandemic influenza A (H1N1)pdm09 were predominantly children and younger adults, and only a few patients were >65 years. The third wave was the most severe taking the number and percentage of patients admitted to ICUs and 30‐day mortality into consideration. We observed that the incidence of hospitalizations as well as clinical severity among younger adults did not decline from the second to the third wave.
Keywords: Epidemiology, hospitalization, ICU, influenza, outcome, pandemic
Background and objectives
A new influenza A (H1N1) virus emerged in Mexico in April 2009, leading to an outbreak of respiratory disease through human‐to‐human transmission, which subsequently emerged as the first pandemic of the 21st century. Denmark experienced three waves of this new virus from July 2009 to February 2011, although the first clinical case was diagnosed on the first of May 2009. A national surveillance of pandemic influenza A (H1N1)pdm09 admissions to intensive care units (ICUs) during the first two waves has been conducted.1 However, the present study is the first detailed description of all three waves in North Denmark Region. The aim of the study was to describe the epidemiology and clinical characteristics of hospitalized patients in a defined population of North Denmark Region with at mixed urban and rural community. We present patterns of morbidity and mortality, clinical features, underlying medical conditions, treatment and concomitant and secondary bacterial co‐infections. Finally, we compare our data with observations from previous pandemics.2, 3, 4
Methods
Patients and study design
North Denmark Region has a mixed urban and rural community of 579 000 inhabitants, approximately 10% of inhabitants in Denmark. The region has no remote areas and only small differences in population density throughout the region. The age distribution did not change during the study period. The region is served by seven hospitals with a total capacity of 535 beds at Departments of Internal Medicine, 51 beds at Departments of Paediatrics, and 42 beds at ICUs. The distance between the seven hospitals is a maximum of 50 kilometers, and all hospitals were capable of receiving patients with influenza A (H1N1)pdm09. Nonetheless, far the greatest part and especially all patients requiring intensive vasopressor or respiratory support (IVRS) were transferred to the referral university hospital in Aalborg. All clinical samples for influenza A (H1N1)pdm09, from all parts of the North Denmark Region, were examined at Department of Clinical Microbiology, Aalborg Hospital, Aarhus University Hospital. Samples positive for influenza A (H1N1)pdm09 were linked to the patients medical records via the unique patient identifier, the Danish Civil Registry System. Only inpatients were included in the study, and patients were only enrolled once despite several positive samples within a follow‐up period of 365 days.
Case definition
Nasopharyngeal swabs or combined nasopharyngeal swabs and lower airway specimens were tested by real‐time PCR for influenza A. Cases were children as well as adults, with a confirmed diagnosis of influenza A from 15 July 2009 (the first case) to 22 February 2011 (the last case). All samples were subtyped at the National Reference Laboratory at Statens Serum Institut, and all cases confirmed to be of pandemic (H1N1)pdm09 subtype. During the second wave, 33 of 158 (21%) strains were genotyped for the H275Y mutation, none were positive, and during the third wave, 90 of 112 strains (80%) were genotyped for the H275Y mutation, 2 (2·2%) were positive. This is in accordance with national data.5
Data collection and analysis
Data collection included patients' age and sex, date of admission, length of hospitalization including duration at ICUs, patients' pre‐existing medical conditions, 30‐day and 365‐day mortality as well as antiviral treatment modalities, IVRS and dialysis, radiology and concomitant and secondary bacterial co‐infections. Criteria for admission to ICUs were life‐threatening organ failures, most often respiratory failure with severe hypoxemia and requirement for mechanical ventilation. The patients were classified with a pre‐existing medical condition if they fell into one or more of three categories. The first category was patients with immunodeficiencies: hematological patients, organ‐transplanted, cancer patients receiving systemic chemotherapy, other patients receiving systemic immunosuppressive treatment and HIV‐positive patients with a CD4‐count below 350 × 10*9/l. The second category was immunocompetent adult patients at high risk of a severe and complicated outcome: >65 years, suffering from chronic illnesses, diabetes, neuromuscular disease, morbid obesity, hemoglobinopathy, and pregnancy. The third category was immunocompetent children at high risk of a severe and complicated outcome: prematurity, hemoglobinopathy, and chronic illnesses, as mentioned above or with specific difficulties of clearing airway when infected. Thirty‐day mortality and 365‐day mortality were defined as whether the patient died within 30 or 365 days of diagnosis, respectively. Antiviral treatment regimes consisted of either oseltamivir administered orally or via a nasogastric tube 150–300 mg daily, zanamivir administered intravenously 600 mg daily, or a combination. Dosages were adjusted for impaired renal function as recommended by the manufacturer. As a treatment modality we recorded days of mechanical ventilation and dialysis when admitted to ICUs. Data of radiology, that is, chest X‐ray and/or CT‐scan were also recorded. At the discretion of the attending physician, additional cultures from blood and lower respiratory tract for bacterial co‐infection were performed.
Statistical analysis
Data were entered using the Epidata software (The EpiData Association, Odense, Denmark) and analyzed using Stata® software, version 10 (College Station, TX, USA).
Results
Patients with confirmed influenza A (H1N1)pdm09
From July 15, 2009 to February 22, 2011, a total of 273 patients with influenza A (H1N1)pdm09 were hospitalized in North Denmark Region (Figure 1). The median age of patients with a positive influenza test was 23 years (range 0–86 years, IQR 45 years), and there were 129 women (47%) and 144 men (53%). During the study period, 111 (41%) children (0–14 years) and 162 (59%) adults (>15 years) were admitted (Figure 2). During the first wave (July 2009–August 2009), one child and two adults were hospitalized, during the second wave (October 2009–January 2010), 84 children and 74 adults were hospitalized, and during the third wave (December 2010–February 2011), 26 children and 86 adults were hospitalized. A 21‐year woman was hospitalized in both second and third wave.
Figure 1.
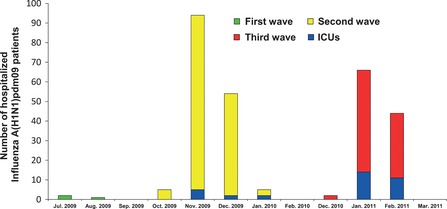
Monthly distribution of hospitalized Influenza A (H1N1)pdm09 patients including ICU patients during 2009–2011 in North Denmark Region. Note the discontinuous time line on the x‐axis.
Figure 2.

Age distribution of cumulative incidences (per 100 000 inhabitants) of hospitalization with Influenza A (H1N1)pdm09 for all three waves during 2009–2011 in North Denmark Region.
Incidences of hospitalization and ICU admission
The age distribution in North Denmark Region in the study period was as follows: <1 year: 5943 inhabitants, 1–4 years: 25 426 inhabitants, 5–14 years: 68 351 inhabitants, 15–24 years: 74 317 inhabitants, 25–44 years: 137 949 inhabitants, 45–64 years: 161 050 inhabitants and ≥65 years: 106 788 inhabitants. The age‐related population incidences of hospitalization for the whole study period were as follows: <1 year: 286/100 000, 1–4 years: 181/100 000, 4–14 years: 70/100 000, 15–24 years: 40/100 000, 25–44 years: 35/100 000, 45–64 years: 40/100 000 and ≥65 years: 17/100 000. When separating data for the three waves, incidences are as shown in Figure 2. At the peak of wave two, the weekly incidence of hospitalization was 5·4/100 000 in our region. During the pandemic 34 patients, one child and 33 adults were admitted to an ICU. During the first wave, none received treatment in ICUs, during the second wave, nine patients (1·6/100 000) received treatment in ICUs, and during the third wave, 25 patients (4·3/100 000) received treatment in ICUs. The proportion of patients admitted to ICUs was 12·5% (34/273) on average, although the proportion increased successively during the study period, (data not shown).
Length of stay
The median length of hospitalization for all 273 patients was 2 days (range 1–147 days), although the average days of hospitalization was shorter for children (4 days) compared with adults (11 days). Thirty‐four patients with a median age of 54 years (range 1–76, IQR 18 years) were admitted to ICUs. The median length of stay in ICUs was 22 days (range 1–50 days). Because the capacity in ICUs in the region is 7/100 000 and taking length of stay into consideration, critically ill influenza patients occupied approximately 8% and 20% of ICU beds during the second and third wave, respectively.
Pre‐existing medical conditions
We found that 117 of 273 patients (43%) had one or more risk factors that fell into the three categories, 19 children (17%) and 98 adults (60%). Seven children were immunodeficient. Three of those had an underlying hematological disease, one had a hepatoblastoma, one had kidney transplantation, one was treated for insufficiency of the hypophysis, and another child had regularly treatment intravenously with corticosteroids for ciliary dysfunction. Twelve children were immunocompetent, but suffered from different syndromes and malformations subsequently leading to difficulties with clearing airways from mucus. Thirty‐five adults were immunodeficient. They suffered from malignant hematological disease, solid cancers, transplantation of solid organs and in immunosuppressive treatment, immunosuppressive treatment for other reasons, and one had HIV infection. Sixty‐three adults were immunocompetent, but suffered from different chronic diseases.
30‐day and 365‐day mortality
Fourteen patients (5%) died within 30 days of diagnosis (median 55 years; range 14–76 years). One was a child (14 years) with myelodysplasia treated with chemotherapy. Thirteen were adults of whom two had no pre‐existing medical conditions, three were immunodeficient – two with chronic lymphatic leukemia and one with T‐prolymphocytic leukemia. Eight adults suffered from a variety of chronic diseases having one or more risk factors – among these morbid obesity (four patients), chronic obstructive lung disease (three patients), diabetes (one patient). Seven additional patients (2·6%) died within 365 days of follow‐up (median 25 years; range 1–86 years).
Antiviral treatment
In total, 132 of 273 patients (48%) received treatment with oseltamivir given as tablets, either orally or via a nasogastric tube. The average period of treatment was 6 days (range 1–29 days, median 5 days). Nevertheless, it was mainly adults receiving this treatment, because only 18 of 111 children (16%) were treated, in contrast to 114 of 162 adults (70%). Two children were treated for <5 days, 15 children for 5 days, and one child for >5 days (10 days). Six adults were treated for <5 days, 80 for 5 days, and 28 for >5 days (6–29 days). Zanamivir was only administered during the second and third wave and only for adults, who were admitted to ICUs and who had been treated with oseltamivir before shifting toward a regimen of either zanamivir as monotherapy or in combination with oseltamivir. In total, 14 of 273 patients (5%) or 14 of 162 adults (9%) received treatment with zanamivir given intravenously. The average period of treatment was 6 days (range 4–13 days, median 5 days). One adult was treated for <5 days, eight for 5 days, and 5 for >5 days (range 6–13 days). By resistance genotyping, only two cases of H275Y mutation were observed, and these were not related to therapeutic failure. The 14 cases receiving zanamivir treatment had clinical therapeutic failure with oseltamivir. Four patients were treated with a combination of oseltamivir and zanamivir for 1–6 days. We found that despite antiviral treatment, 35 patients had more than one positive test for influenza A (H1N1)pdm09 during hospitalization with a median interval between positive tests of 7 days (range 1–45 days). These patients tended to be most sick, as 23 of the 35 patients (66%) were admitted to ICUs and may therefore have been tested more repeatedly.
IVRS and dialysis
Twenty‐six of 34 patients (76%) admitted to ICUs received intensive vasopressor support, mainly norepinephrine, but also epinephrine and dopamine. Thirty of 34 patients (88%) admitted to ICUs received intensive respiratory support with mechanical ventilation for a median of 17 days (range 1–50 days). Another two patients (6%) received continuous positive airway pressure (CPAP), and two patients (6%) received bi‐level positive airway pressure (Bi‐PAP) for a few days. Seven of 34 patients (21%) admitted to ICUs received dialysis for a median of 19 days (range 3–50 days).
Radiology
Thirty‐one children (28%) had a chest X‐ray, which revealed infiltrates in 20 cases (65%).
One child with a positive chest X‐ray had an additional CT‐scan also revealing infiltrates. Seven of 20 children (35%) with positive radiology had a positive bacterial culture of lower respiratory tract specimens. One‐hundred and nineteen (73%) adults had a chest X‐ray, which revealed infiltrates in 65 cases (55%). Twenty adults had an additional CT‐scan, revealing infiltrates in 19 cases (95%). Twenty‐one adult patients (32%) with positive radiology had a positive bacterial culture of lower respiratory tract specimens.
Concomitant and secondary bacterial coinfection
Thirty‐five patients (13%) had bacterial coinfection, 33 patients (12%) from lower respiratory tract specimens, and nine patients (3%) from blood cultures. Overall, Streptococcus pneumoniae was the most frequent causative bacteria at both sites. In respiratory tract specimen, there was growth of Streptococcus pneumoniae in ten cases (30%), Haemophilus influenzae in seven cases, Staphylococcus aureus in three cases, Pseudomonas aeruginosa in three cases, Escherichia coli in three cases, and other species in seven cases. In blood cultures, there was growth of Streptococcus pneumoniae in three cases (33%), Escherichia coli in three cases, and Pseudomonas aeruginosa in two cases.
The patients with concomitant and secondary bacterial coinfection tended to have a more severe clinical outcome, because 30 of 35 patients (86%) had radiological changes, 19 of 35 patients (54%) required therapy in ICU, and seven and eight patients (20 and 23%) died within 30 and 365 days, respectively.
Discussion
It was a major finding of our study that the severity of hospitalized patients and requirement for ICU admission increased in the third (post‐pandemic) wave as compared with the second (pandemic) wave, which is opposite reports from 1918 to 19.2, 3, 6 It is not easy to predict the behavior of emerging influenza pandemics, because prior pandemics have evolved in different ways. The 1918–19 pandemic occurred in three waves: a mild first wave in spring and summer 1918, a severe and lethal second wave in autumn 1918, followed by a third wave the following season with less severity.2, 3 Due to lack of viral assessment of pathogenecity during the waves of 1918–19, it is unclear whether the reported incidences were related to change in virulence, host immune response or other epidemiological factors. Experiences with the two other pandemics of the past century, 1957 and 1968, revealed patterns of varying severity,4 so it is of crucial importance to observe detailed patterns of hospitalization and mortality of any new pandemic strain. However, our results could be supported by recent studies from the US and Spain of a higher mortality of hospitalized H1N1pdm09 patients in the post‐pandemic season.7, 8 Nevertheless, in other countries with a very high mortality, the results may be different.9 Furthermore, although incidence of hospitalization of children remained high during the second and third wave, rates among older persons significantly increased during the post‐pandemic (third) wave. This trend resembles those reported during successive waves in previous pandemics.4 Because children are important drivers of influenza virus transmission and likely are very susceptible to any new pandemic virus, the changing demography between 1918 and 19 (high proportion of children in society) to contemporary decades of developed countries (high proportion of older persons in society) part of the difference between pandemics could relate to changing transmission levels in an aging population.
Several other factors may modify the pattern of pandemic phases, including vaccination and immune protection after mild to moderate disease. It has been suggested that immune protection naturally acquired during the first 1918 influenza wave decreased morbidity and mortality during the successive pandemic wave.2 However, as demonstrated by our case of repeated severe illness in the same patient in the third wave despite illness during the second wave, protective immunity not always develops following infection. Likewise, immunological memory of past pandemics may confer protection in the population, and it is likely that the very low incidence in patients older than 65 years in our study relates to cross‐protective antibodies generated prior to the 1957 pandemic event.10 In Denmark, the uptake of vaccination in 2009–2010 (second wave) was modest and directed toward risk groups, in contrast to mass vaccinations in the population of other countries. Surveillance data from Denmark and North Denmark Region report an uptake in risk groups of 50% and 48%, respectively, in 2010,5 and a recent Danish study found that only 10 of 53 patients admitted to ICUs in 2009–2010 had been vaccinated, mostly due to chronic co‐morbidities.1 The role of vaccination is unclear at the individual level, but effectiveness at the public health level varying from 49% to 93% is reported in recent studies from the US, Canada, and Australia11, 12, 13; hence, the importance is acknowledged by WHO.
Antiviral therapy with oseltamivir was common in our patients as soon as the diagnosis was suspected on hospitalization, but in some cases, the disease progressed to respiratory failure very quickly, and the impact of antiviral therapy on outcome is unclear. Immediate initiation of oseltamivir treatment potentially reduces the risk of complications and requirement for ICU admission,14 and we observed no difference in time from diagnosis to initiation of antiviral therapy between the second and third wave. In the critically ill patients with pandemic influenza, it has been suggested to increase the dose of oseltamivir to 300 mg daily,15, 16 but this is not supported by clinical data and the pharmacokinetics of oseltamivir 75 mg twice daily in intensive care patients have been demonstrated to be comparable to patients with mild disease.17 The majority of our patients were treated with 150 mg daily. During the second and third wave, 14 adult patients with clinical non‐response to oseltamivir were switched to intravenous zanamivir, but due to the uncontrolled nature, we cannot assess whether zanamivir contributed to survival. It remains unclear whether zanamivir administered to critically ill influenza A (H1N1)pdm09 patients will have any role.18 We only rarely used combination antiviral therapy, because this is experimental and has so far not demonstrated any benefit.16 Corticosteroids were not routinely used, as this may add to the risk of a worse outcome 19, 20
A majority of hospitalized patients requiring therapy in ICUs were adults <65 years who on admission had respiratory failure due mainly to primary influenza pneumonia in agreement with other observations,8, 15, 21, 22, 23, 24 and in clear contrast to seasonal influenza. The number of patients that are admitted to an ICU may vary related to criteria used by the institution. Consequently, the mortality in ICU differs has been reported from 11% to 25%.15, 21, 22, 23, 24, 25 Many patients with severe disease have co‐morbidities, but it is well described that fatalities from pandemic influenza may occur in previously healthy subjects. Mortality in the ICU was reported to be similar between the pandemic and the post‐pandemic wave at a single institution in Spain.26 Mortality in hospitalized patients was reported to be 4%7 with no difference between waves, and in our cohort, the 30‐day crude mortality was 5%. Due to low number of deaths in our study, it is not possible to compare the three waves for mortality rates.
Viral shedding may be prolonged in severe cases of influenza A27, 28 with immunosuppression and mechanical ventilation as specific risk factors.27 Antiviral therapy obviously could influence the duration of viral shedding. There is possibility of confounding by indication due to the fact that patients with more severe disease (e.g., in ICU) received protracted care for longer duration and were sampled repeatedly compared to those with milder symptoms. To et al.29 reported a slower decline in viral shedding in patients with severe disease, and Meschl et al.28 associated extended shedding with complicated pneumonia. Another possibility for prolonged virus replication could be emerging viral resistance with reduced susceptibility to neuraminidase inhibitors, which, however, was not the case in our cohort. Only two cases of H275Y resistance mutations were observed in our cohort, and they were unrelated cases during the third wave.
Strengths of the present study are its complete coverage of a defined population with complete follow‐up after 365 days, the inclusion of all hospitalized cases irrespective of age, and the possibility to compare the clinical features and requirement for ICU admission between the three defined waves of a newly emerging pandemic influenza. We are able to report specific incidence rates for different age groups and to follow changes in epidemiology during the post‐pandemic third wave.
However, our study also has limitations that should be acknowledged. The population under observation is <1 million, and consequently, the number of patients included is limited, and the occurrence of rare events or complications cannot be estimated. Nevertheless, our regional data reveal the same pattern as national surveillance data, showing that at the peak of wave two, the incidence of hospitalized patients was 20% of the total community incidence.30 We have only incomplete information on vaccination status prior to admission and are thus unable to assess the role of vaccination. Criteria for hospitalization may have changed from the second to the third wave, especially for children, which may introduce confounding by indication and direct comparison of hospitalization rates between waves should be interpreted cautiously. However, criteria for admission to ICU remained the same throughout the study period, and direct comparisons are possible.
In conclusion, significant changes of hospitalizations and requirement for ICU admissions were observed in the post‐pandemic third wave of influenza A (H1N1)pdm09 in a defined population in North Denmark Region. The epidemiology behaved differently from the previous pandemics, which underlines that each new pandemic should be studied for individual characteristics.
Ørsted et al (2013) The first, second and third wave of pandemic influenza A (H1N1)pdm09 in North Denmark Region 2009–2011: a population‐based study of hospitalizations. Influenza and Other Respiratory Viruses 7(5), 776–782
References
- 1. Gubbels S, Perner A, Valentiner‐Branth P, Mølbak K. National surveillance of pandemic influenza A(H1N1) infection‐related admissions to intensive care units during the 2009–2010 winter peak in Denmark: two complementary approaches. Euro Surveill 2010; 15:19743. [DOI] [PubMed] [Google Scholar]
- 2. Barry JM, Viboud C, Simonsen L. Cross‐protection between successive waves of the 1918–1919 influenza pandemic: epidemiological evidence from US Army Camps and from Britain. J Infect Dis 2008; 198:1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control stategies. J Infect Dis 2008; 197:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis 1998; 178:53–60. [DOI] [PubMed] [Google Scholar]
- 5. Statens Serum Institut . EPI‐NYT. Available at http://www.ssi.dk (Accessed 8 June 2011).
- 6. Paulo AC, Correia‐Neves M, Dominguos T et al Influenza infectious dose may explain the high mortality of the second and third wave of 1918–19 influenza pandemic. PLoS ONE 2010; 5:e11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Truelove SA, Chitnis AS, Heffernan RT, Karon AE, Haupt TE, Davis JP. Comparison of patients hospitalized with pandemic 2009 influenza A (H1N1) virus infection during the first two pandemic waves in Wisconsin. J Infect Dis 2011; 203:828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Viasus D, Rodriguez‐Baño J, Oteo JA et al Changes in epidemiology, clinical features and severity of influenza A (H1N1) 2009 pneumonia in the first post‐pandemic influenza season. Clin Microbiol Infect 2012; 18:E55–E62. [DOI] [PubMed] [Google Scholar]
- 9. Ramakrishna K, Peter JV, Karthik G et al Influenza A (H1N1) 2009 pandemic: was there a difference in the two waves in patients requiring admission to the intensive‐care unit? Clin Microbiol Infect 2011; 17:1353–1358. [DOI] [PubMed] [Google Scholar]
- 10. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon N. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross‐sectional serological study. Lancet 2010; 375:1100–1108. [DOI] [PubMed] [Google Scholar]
- 11. Griffin MR, Monto AS, Belongia EA et al Effectiveness of non‐adjuvanted pandemic influenza A vaccines for preventing pandemic influenza acute respiratory illness visits in 4 U.S. communities. PLoS ONE 2011; 6:e23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skowronski DM, Janjua NZ, Serres GD et al Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case‐control evaluation based on sentinel surveillance system in Canada, autumn 2009. BMJ 2011; 342:c7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng AC, Kotsimbos T, Kelly HA et al Effectiveness of H1N1/09 monovalent and trivalent influenza vaccines against hospitalization with laboratory‐confirmed H1N1/09 influenza in Australia: a test‐negative case control study. Vaccine 2011; 29:7320–7325. [DOI] [PubMed] [Google Scholar]
- 14. Hiba V, Chowers M, Levi‐Vinograd I, Rubinovitch B, Leibovici L, Paul M. Benefit of early treatment with oseltamivir in hospitalized patients with documented 2009 influenza A (H1N1): retrospective cohort study. J Antimicrob Chemother 2011; 66:1150–1155. [DOI] [PubMed] [Google Scholar]
- 15. Rello J, Rodriguez A, Ibanez P et al Intensive care adult patients with severe respiratory failure caused by influenza A (H1N1)v in Spain. Crit Care 2009; 13:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen E, Keld DB, Ellermann‐Eriksen S et al Failure of combination oral oseltamivir and inhaled zanamivir antiviral treatment in ventilator‐ and ECMO‐treated critically ill patients with pandemic influenza A (H1N1)v. Scand J Infect Dis 2011; 43:495–503. [DOI] [PubMed] [Google Scholar]
- 17. Ariano RE, Sitar DS, Zelenitsky SA et al Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. CMAJ 2010; 182:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fraaij PL, Vries E, Beersma MF et al Evaluation of the antiviral response to zanamivir administered intravenously for treatment of critically ill patients with pandemic influenza A(H1N1) Infection. J Infect Dis 2011; 204:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han K, Ma H, An X et al Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin Infect Dis 2011; 53:326–333. [DOI] [PubMed] [Google Scholar]
- 20. Martin‐Loeches I, Lisboa T, Rhodes A et al Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med 2011; 37:272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The ANZIC Influenza Investigators . Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 2009; 361:1925–1934. [DOI] [PubMed] [Google Scholar]
- 22. Louie JK, Acosta M, Winter K et al Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 2009; 302:1896–1902. [DOI] [PubMed] [Google Scholar]
- 23. Kumar A, Zarychanski R, Pinto R et al Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302:1872–1879. [DOI] [PubMed] [Google Scholar]
- 24. Fuhrman C, Bonmarin I, Bitar D et al Adult intensive‐care patients with 2009 pandemic influenza A(H1N1). Epidemiol Infect 2011; 139:1202–1209. [DOI] [PubMed] [Google Scholar]
- 25. Brandsaeter BJ, Pillgram M, Berild D, Kjekshus H, Kran AB, Bergersen BM. Hospitalised patients with suspected 2009 H1N1 in a hospital in Norway, July‐December 2009. BMC Infect Dis 2011; 11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pakalou G, Souto J, Balcells J et al First influenza season after the 2009 pandemic influenza: characteristics of intensive care unit admissions in adults and children in Vall d′Hebron Hospital. Clin Microbiol Infect 2012; 18:374–380. [DOI] [PubMed] [Google Scholar]
- 27. Gianella M, Alonso M, Viedma DG et al Prolonged viral shedding in pandemic influenza A(H1N1): clinical significance and viral load analysis in hospitalized patients. Clin Microbiol Infect 2011; 17:1160–1165. [DOI] [PubMed] [Google Scholar]
- 28. Meschi S, Selleri M, Lalle E et al Duration of viral shedding in hospitalized patients infected with pandemic H1N1. BMC Infect Dis 2011; 11:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. To KKW, Hung IFN, Li IWS et al Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis 2010; 50:850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harder KM, Andersen PH, Bæhr I et al Electronic real‐time surveillance for influenza‐like illness: experience from the 2009 influenza A(H1N1) pandemic in Denmark. Euro Surveill 2011; 16:pii=19767. [PubMed] [Google Scholar]


