Abstract
The central nervous system has the remarkable ability to convert changes in the environment in the form of sensory experience into long-term alterations in synaptic connections and dendritic arborization, in part through changes in gene expression. Surprisingly, the molecular mechanisms that translate neuronal activity into changes in neuronal connectivity and morphology remain elusive. Rem2, a member of the Rad/Rem/Rem2/Gem/Kir (RGK) subfamily of small Ras-like GTPases, is a positive regulator of synapse formation and negative regulator of dendritic arborization. Here we identify that one output of Rem2 signaling is the regulation of gene expression. Specifically, we demonstrate that Rem2 signaling modulates the expression of genes required for a variety of cellular processes from neurite extension to synapse formation and synaptic function. Our results highlight Rem2 as a unique molecule that transduces changes in neuronal activity detected at the cell membrane to morphologically relevant changes in gene expression in the nucleus.
Keywords: Rem2, Excitatory synapse, activity-regulated, Lrrtm4, Glypican-5 (Gpc5)
Introduction
During central nervous system (CNS) development, processes such as synapse formation and dendritic and axonal outgrowth establish connectivity between neurons in order to construct functional neuronal circuits. This developmental program utilizes “hard-wired” genetic programing, but is further refined by sensory input [1–3]. Experience-driven structural changes require, in part, the precise regulation of activity-dependent signaling pathways [4–6]. These pathways activate downstream transcription factors that ultimately result in changes in gene expression [4–7]. Thus, not surprisingly, a large proportion of the studies elucidating the molecular mechanisms of synapse formation and dendritic arbor elaboration have focused on either transcription factors or cell surface receptors [8–13]. Therefore, much remains to be determined regarding the identity and function of cytosolic signaling molecules that transduce changes in neuronal activity into changes in gene expression and ultimately, neuronal structure and function.
Rem2 is a positive regulator of excitatory synapse formation in cultured rodent hippocampal neurons and an activity-dependent, negative regulator of dendritic complexity in both cultured rodent neurons and in Xenopus laevis optic tectum [14–18]. Rem2 is a member of the Rad/Rem/Gem/Kir (RGK) family of non-canonical Ras-like GTPases and is primarily expressed in the nervous system [19]. Unlike canonical Ras-like GTPases, Rem2 has amino acid substitutions at conserved residues in its GTP binding domain, which results in a low rate of GTP hydrolysis [20, 21]. The nucleoside diphosphate kinase, Nm23, has been identified as a GTPase activating protein (GAP) for Rad [22, 23]. However, neither GAPs nor Guanine exchange factors (GEFs) have been identified for Rem2 or the other RGK proteins thus far. These data and other lines of evidence [14] suggest that Rem2 does not transduce signals like a classical GTPase, regulated by its nucleotide binding state, but is instead activated by other means, for example by post-translational modification [20]. In addition, in both cultured rat neurons and in Xenopus optic tectum, Rem2 mRNA expression is upregulated in response to neuronal depolarization [15]. The increase in Rem2 mRNA expression is dependent on calcium entry specifically through voltage-gated calcium channels [15]. Therefore, Rem2 is well poised to be a cytosolic signal transducer that translates changes in neuronal activity into changes in dendritic branching and neuronal connectivity.
To identify the mechanism(s) by which Rem2 regulates synapse formation and neuronal morphology, we took an unbiased approach to investigate Rem2-dependent changes in gene expression. We employed RNA-sequencing (RNA-seq) of mRNA isolated from cultured mouse neurons in which Rem2 had been knocked out. Using this approach, we identified 24 genes whose expression was altered by Rem2 knockout. Importantly, we observed that Rem2 knockout caused a decrease in expression of at least two genes that regulate synapse formation: Leucine Rich Repeat Transmembrane Neuronal 4 (Lrrtm4) and Glypican-5 (Gpc5). Gpc5 is a GPI-linked heparan sulfate proteoglycan that can act as a ligand for Lrrtm4 to promote excitatory synapse formation [24].
In a separate set of experiments, we identified Rem2 and activity-dependent changes in gene expression by depolarizing Rem2 knockout or control neurons with high extracellular potassium followed by RNA-Seq. Using this approach, we identified 217 genes whose expression was altered by Rem2 knockout in an activity-dependent manner. We found that in the context of neuronal depolarization, the expression of 94 genes is normally promoted by Rem2 signaling while expression of 123 genes is normally repressed by Rem2 signaling. Our results suggest that Rem2 is an important component of a signaling network that translates changes in neuronal activity to changes in gene expression and ultimately, synaptic connectivity.
Results and Discussion
Rem2 knockout results in altered gene expression
We sought to identify genes whose expression is modulated by Rem2 using next generation RNA-sequencing of samples isolated from cortical neurons in the presence or absence of Rem2. To perform these studies, we took advantage of a Cre-dependent conditional allele of Rem2, Rem2flx/flx, that was generated in our laboratory [25]. In brief, exons 2 and 3 of the Rem2 allele, which encode the GTP-binding domain, were flanked by LoxP sites. Therefore, when Cre recombinase is expressed, exons 2 and 3 are excised, resulting in a Rem2 null allele [25].
Dissociated cortex from E16 Rem2flx/flx mice was cultured at high density and infected at 1 DIV with either a control virus (AAV-GFP) or a virus expressing Cre-recombinase (AAV-Cre-GFP) to knockout Rem2. By DIV 5, GFP expression was observed in approximately 85% of the neurons in each condition. All neurons were treated with the voltage-gated sodium channel blocker, tetrodotoxin (TTX), overnight to suppress activity-dependent transcriptional programs in the culture. On DIV 6, total RNA from cells cultured in each condition (Control and Rem2 KO) was harvested and reverse transcribed. For each biological replicate, quantitative PCR (q-PCR) was performed to verify that Rem2 exons 2 and 3 were deleted (Fig. 1A). Next, we enriched mature mRNA from total RNA pool by performing a polyA selection and prepared RNA-seq libraries using standard protocols for high throughput sequencing. We confined our analysis to differential expression of protein coding genes only.
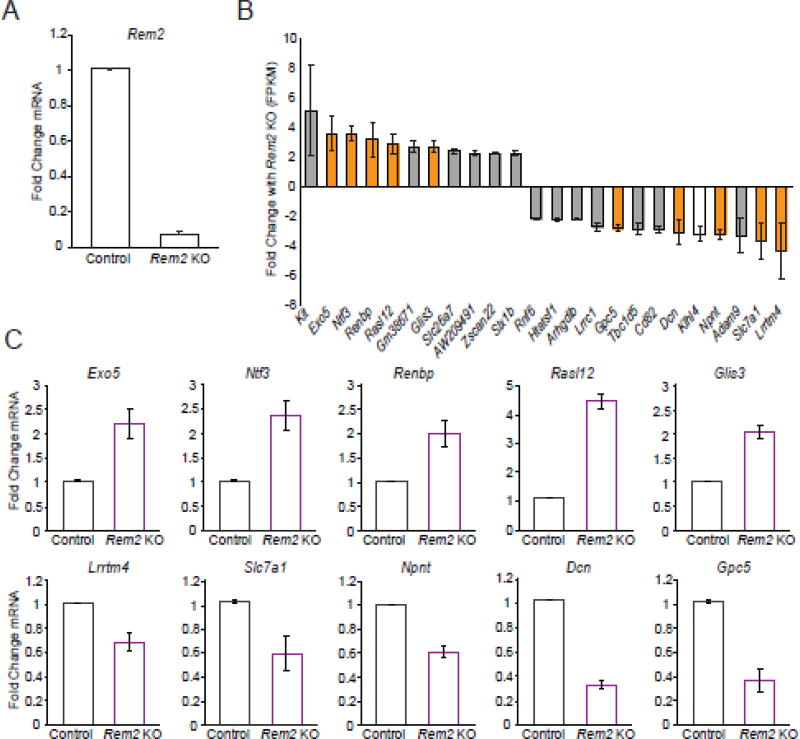
Figure 1. Knockout of Rem2 results in changes in gene expression.
A) Fold change in Rem2 mRNA by qPCR after 5 days of Cre-mediated Rem2 exon 2 and 3 deletion. mRNA levels were first normalized to Actb and then to the control condition within each replicate then averaged across biological replicates. Data is presented as mean ± SEM. Rem2 KO condition is significantly decreased from control by student’s t-test (P<0.05) B) RNA-seq results: Genes up or downregulated greater than 2-fold with Rem2 knockout. Orange bars indicate genes whose expression change with Rem2 knockout was validated by qPCR while the white bar shows the gene whose expression profile that did not recapitulate the RNA-seq results. The gray bars show genes that were not chosen for qPCR validation. Data is presented as the mean fold change across biological replicates in FPKM (Fragments per kilobase of transcript per million mapped reads) from control ± SEM. C) Validation of gene expression changes (of the genes shown in orange in B) using qPCR. Fold change in mRNA expression for each indicated gene was averaged across 3 biological replicates. First, mRNA levels were normalized to Actb and then to the control condition. Data is presented as mean ± SEM. All Rem2 KO conditions are significantly increased (top row) or decreased (bottom row) from controls by student’s t-test (P<0.05).
To begin, we determined the effect of knocking out Rem2 on gene expression. To that end, we compared the gene expression profile in the control neurons to the expression profile of Rem2 KO neurons. When analyzing the RNA-sequencing data, a gene was considered to be a “hit” if the FPKM (fragments per kilobase of transcript per million mapped reads) was greater than 0.5, its expression changed ≥ 2-fold in the Rem2 knockout condition compared to the control condition, and the expression changes were observed in both biological replicates. We found that knockout of Rem2 led to increased expression (using our 2-fold cutoff) of 11 protein-coding genes, suggesting that Rem2 signaling normally functions to inhibit the expression of these genes (Fig. 1B). We also found decreased expression of 13 protein-coding genes, suggesting that Rem2 normally functions to promote expression of these genes (Fig. 1B). From these data, we conclude that Rem2 signaling both positively and negatively regulates gene expression.
To confirm that the results from our RNA-sequencing analysis were representative of the changes in gene expression observed between conditions in our biological replicates, we performed qPCR using probe sets designed to quantify expression of 11 genes identified by our RNA-seq experiment. We chose 6 genes whose expression decreased and 5 genes whose expression increased with Rem2 KO. For the qPCR validation, we probed RNA from each RNA-seq biological replicate and RNA from a third, independent biological replicate that was not subjected to deep sequencing. We found that 10 of the 11 genes identified as having significant expression changes by RNA-seq were validated by qPCR in all three biological replicates tested (Fig. 1C). Expression of the 11th gene, Klhl4, shows a small but not significant decrease in the Rem2 KO compared to control by qPCR from the first biological replicate, so its expression in the subsequent biological replicates was not determined (Fig. S1). Taken together, these data suggest that our RNA-seq experiment has a false discovery rate of <10%.
Knockdown of Rem2 target genes Lrrtm4 and Gpc5 decrease excitatory synapse density
Rem2 is a positive regulator of excitatory synapse formation [16, 18]. Therefore, we reasoned that at least one function of a subset of genes affected by Rem2 deletion could be to regulate synapse formation. Further support of this hypothesis comes from the fact that it has been previously reported that Lrrtm4, whose expression is decreased upon Rem2 deletion (Fig. 1B–C), promotes excitatory synapse formation [24, 26]. In addition, Gpc5 acts as a ligand for Lrrtm4 to mediate synapse formation [24]. However, a loss of function analysis of Gpc5 in neurons has never been performed.
We obtained siRNA Smartpools (Dharmacon) targeting Gpc5 and Lrrtm4 and assayed the effect of RNAi-mediated knockdown on excitatory synapse density in cultured neurons. To perform these experiments, we utilized our previously established assay to quantify excitatory synapse formation [14–18]. Hippocampal neurons were sparsely transfected with GFP and the siRNA Smartpools using the calcium phosphate method such that the vast majority of synaptic connections made onto the transfected neurons are from non-transfected, “wildtype” neurons. Thus, the sparseness of our transfection is a key element of the experimental design as it allows us to test a role for the targeted gene in the postsynaptic neuron only. This approach differs from the high percentage of transduced Rem2flx/flx cultured neurons achieved with Cre-expressing AAV infection which was necessary for our RNA-seq experiments. As a result, we also included a well-validated RNAi reagent that specifically targets Rem2 [14–18] in our sparse transfection paradigm as a positive control for decreased excitatory synapse density. We have previously shown that the decreased synapse density observed with transfection of this Rem2 shRNA can be rescued by co-transfection of a vector encoding an RNAi-resistant Rem2 cDNA [14–17].
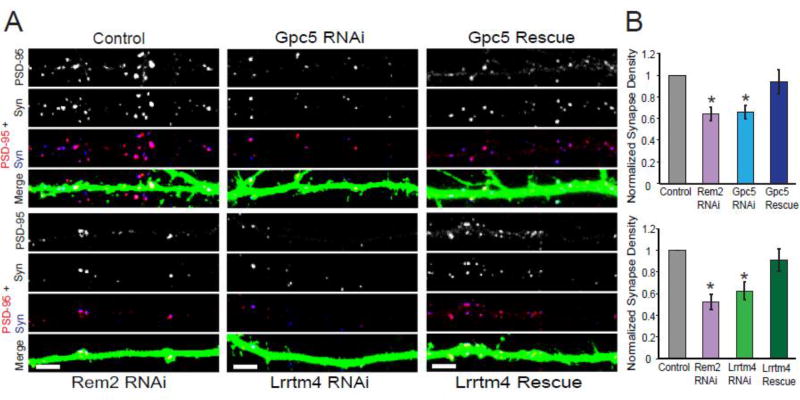
Neurons were fixed at 14 DIV and stained for the excitatory synaptic markers PSD-95 and Synapsin. The transfected neurons were then imaged and analyzed for changes in excitatory synapse density by quantifying the co-localization of PSD-95 and Synapsin on a transfected neuron. We found, as previously reported [24, 26], that knockdown of Lrrtm4 expression led to a decrease in excitatory synapse density, as did knockdown of Rem2 (Fig. S2A, B). This result serves to verify the biological significance of our RNA-seq findings and further, places Rem2 signaling upstream of and positively regulating Lrrtm4 expression.
Interestingly, we also observed that knockdown of Gpc5 expression was sufficient to decrease excitatory synapse density (Fig. S2A, B), consistent with the hypothesis that Rem2 promotes the expression of genes that regulate synapse formation. In addition, these data support the model that Gpc5 is a ligand for Lrrtm4 in regulating synapse formation, as knockdown of Lrrtm4 and Gpc5 yields similar phenotypes. Lastly, these data add new insight into a possibly novel signaling mechanism of Gpc5 and Lrrtm4 as knockdown of either gene in the postsynaptic neuron causes a decrease in synapse density, suggesting that Gpc5 could interact with Lrrtm4 in cis to promote synapse formation.
We sought to confirm that the decreased synapse density phenotype we observed with the knockdown of Lrrtm4 and Gpc5 was due to the knockdown of the targeted gene and not an off-target effect of the siRNA pools. To accomplish this, we designed an shRNA targeting Gpc5 and utilized a previously published shRNA targeting Lrrtm4 [26]. We also engineered Lrrtm4 and Gpc5 cDNA constructs containing silent mutations in the nucleotide sequence targeted by the respective shRNAs, rendering the cDNAs RNAi resistant. We confirmed that the shRNAs knocked down Lrrtm4 or Gpc5 protein expression and that the RNAi resistant cDNAs were indeed resistant to shRNA targeting in HEK 293T cells by Western blotting of cellular lysates (Fig. S2C).
Next, we transfected cultured hippocampal neurons with shRNAs targeting Lrrtm4 or Gpc5 alone or with the appropriate RNAi resistant cDNA; the cultures were subsequently processed for immunostaining for synaptic markers at 14 DIV. We found that decreased expression of either Lrrtm4 or Gpc5 resulted in a decrease in excitatory synapse density which could be rescued to control synapse density upon co-transfection of the corresponding RNAi resistant cDNA (Fig. 2). These results demonstrate that the loss of synapse density was due to knockdown of either Lrrtm4 or Gpc5 specifically, and not to an off-target effect of the RNAi. Taken together, our data indicate that the function of both Lrrtm4 and Gpc5 is required in postsynaptic neurons to regulate the formation of excitatory synapses. Glypican proteins can be either membrane associated or cleaved from the cell surface by phospholipases [27], suggesting multiple possible signaling configurations for Gpc5 and Lrrtm4. Taken together with previous studies demonstrating that Gpc5 can signal through Lrrtm4 to mediate synapse formation [24], our data suggests that Gpc5 may signal in an autocrine manner to regulate this process.
Figure 2. Lrrtm4 and Gpc5 are required for proper excitatory synapse formation.
A) Immunostaining for PSD-95 (red) and Synapsin I (blue) in the dendrites of transfected neurons. A synapse is defined as the overlap of red and blue puncta (shown in white) on a GFP positive dendrite. Scale bar = 5µm. B) Quantification of PSD-95/Synapsin puncta for neurons transfected with GFP and either: a pSuper empty vector (Control), shRNA targeting Rem2 (Rem2 RNAi), shRNA targeting Gpc5 (Gpc5 RNAi), shRNA targeting Gpc5 and an RNAi resistant Gpc5 cDNA (Gpc5 Rescue), shRNA targeting Lrrtm4 (Lrrtm4 RNAi) or a shRNA targeting Lrrtm4 and an RNAi resistant Lrrtm4 cDNA (Lrrtm4 Rescue). Data is presented as mean of normalized PSD-95/Synapsin puncta density ± SEM. Asterisks indicate p<0.05 compared to control by two-way ANOVA with Tukey post-hoc test. Normalized values are the average of two biological replicates and n ≥ 30 neurons for each condition.
Overexpression of Lrrtm4 or Gpc5 restores the Rem2 RNAi-dependent decrease in synapse density
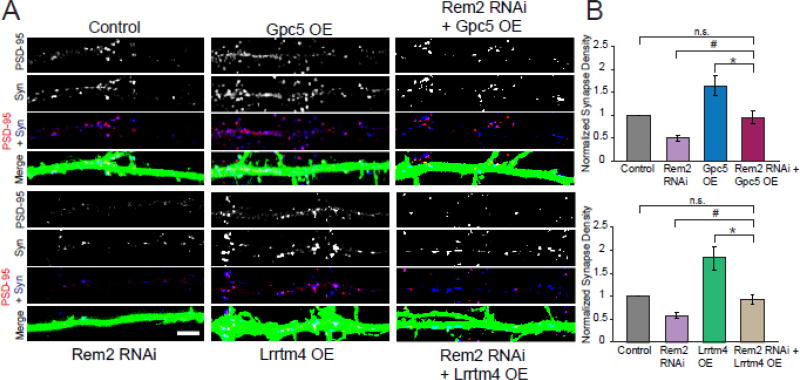
In order to determine if Lrrtm4 and Gpc5 function downstream of Rem2 in a signaling pathway that promotes excitatory synapse formation, we performed a series of epistasis experiments in cultured hippocampal neurons. We asked if overexpression of either Lrrtm4 or Gpc5 could restore decreased synapse density that occurs upon Rem2 knockdown by RNAi. Cultured hippocampal neurons were transfected at 4DIV with either a shRNA targeting Rem2 or cDNAs that express either Lrrtm4 or Gpc5. We also co-transfected Rem2 shRNA and Lrrtm4 cDNA, or Rem2 shRNA and Gpc5 cDNA. At 14 DIV we fixed and stained the neurons as described above to assay excitatory synapse density. We found that overexpression of either Lrrtm4 or Gpc5 alone significantly increased excitatory synapse density, suggesting that both Lrrtm4 and Gpc5 are sufficient to promote excitatory synapse density [24] (Fig. 3). This result again suggests that Gpc5 acts in an autocrine manner to promote synapse formation, as overexpression of Gpc5 increases synapse density in that same neuron.
Figure 3. Overexpression of Gpc5 or Lrrtm4 restores the Rem2 RNAi-dependent decrease in synapse density.
A) Immunostaining for PSD-95 (red) and Synapsin I (blue) in the dendrites of transfected neurons. A synapse is defined as the overlap of red and blue puncta (shown in white) on a GFP positive dendrite. Scale bar = 5µm. B) Quantification of PSD-95/Synapsin puncta for neurons transfected with GFP and either: (Top) a pSuper empty vector (Control), shRNA targeting Rem2 (Rem2 RNAi), Gpc5 cDNA (Gpc5 OE), or shRNA targeting Rem2 and Gpc5 cDNA (Rem2 RNAi + Gpc5 OE) and (Bottom) a pSuper empty vector (Control), shRNA targeting Rem2 (Rem2 RNAi), Lrrtm4 cDNA (Lrrtm4 OE), or shRNA targeting Rem2 and Lrrtm4 cDNA (Rem2 RNAi + Lrrtm4 OE). Data is presented as mean of normalized PSD-95/Synapsin puncta density ± SEM. * indicates p < 0.005 and # indicates P < 0.085 between conditions indicated by bars using two-way ANOVA with Tukey post-hoc test. In both top and bottom graphs, control versus Rem2 RNAi was different at P < 0.005 but this was not indicated on the graph for clarity. Normalized values are the average of ≥ 2 biological replicates and n ≥ 32 neurons for each condition.
Importantly, overexpression of either Lrrtm4 or Gpc5 was able to compensate for the decreased synapse density observed upon Rem2 knockdown and restore synapse density to control levels (Fig. 3). One interpretation of this experiment is that Lrrtm4 and Gpc5 function downstream of Rem2 in a linear signaling pathway: Rem2 promotes the expression of Gpc5 and Lrrtm4 who signal together, in the same cell, to promote synapse formation. Thus, in the absence of Rem2, increased expression of either the receptor Lrrtm4 or its ligand Gpc5 can promote excitatory synapse formation. Alternatively, it could be that Rem2, Lrrtm4, and Gpc5 function in parallel pathways to regulate synapse formation. Given our RNA-seq data indicating that Rem2 signaling promotes the expression of Lrrtm4 and Gpc5 (Fig. 1), we believe that the linear pathway model is the most parsimonious explanation for our results.
Rem2 regulates activity-dependent gene expression
Treatment of cultured rodent neurons with high extracellular potassium has been shown to mimic the cellular response to neuronal activity. Increased activity stimulates the transcription of immediate early genes (IEGs) such as Fos, Jun and Egr1 which encode transcription factors [28, 29]. This process occurs in a transcription-independent manner [30]. Once active, IEGs promote the transcription of late response genes in an activity-dependent and cell-type specific manner [4, 6, 28, 31–33]. Importantly, these transcriptional responses mimic gene induction events observed in intact rodent brain in response to physiological stimuli such as visual experience, seizures and cocaine exposure [34–37].
Therefore, in parallel to the above experiments, we performed RNA-seq using RNA isolated from control and Rem2 KO neurons that were depolarized with high extracellular potassium (55mM KCl) for 4 hours to determine the effect of Rem2 deletion on the subset of genes that are specifically upregulated in response to neuronal depolarization. Our previous work demonstrated that a 4 hour exposure to high extracellular potassium produced a robust increase in Rem2 mRNA expression while still displaying a significant induction of the IEG Fos [15]. For each biological replicate, we performed qPCR to confirm that the expression of the activity-regulated genes Rem2 and Fos were upregulated following a 4 hour treatment of high extracellular potassium (KCl, Fig. 4A).
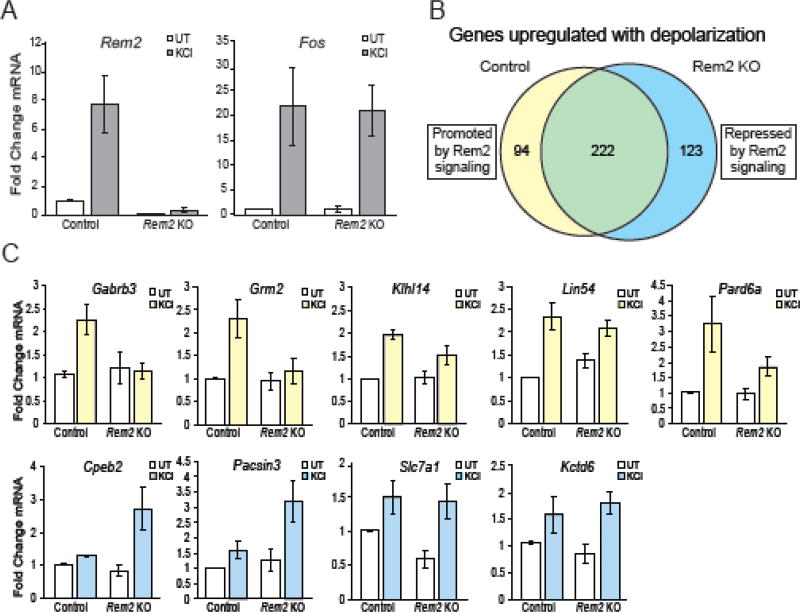
Figure 4. Rem2 knockout causes changes in activity-dependent gene expression.
A) Fold change in Rem2 or Fos mRNA expression by qPCR averaged across biological replicates. mRNA levels were first normalized to Actb and then to the control untreated condition within each biological replicate. Data is presented as mean ± SEM. B) Venn diagram showing the number of genes upregulated ≥ 2-fold following KCl stimulation. The 316 genes represented by the yellow circle were upregulated by KCl treatment in control neurons. Of the 316 genes upregulated in control neurons, 94 of those genes were only upregulated ≥ 2-fold in the control condition. The blue circle represents the 345 genes upregulated ≥ 2-fold by KCl stimulation in the Rem2 KO condition. 123 of the 345 genes were upregulated ≥ 2-fold in the Rem2 KO condition only. C) Validation of expression profiles of genes identified in panel B. Fold change in mRNA expression by qPCR for each indicated gene was averaged across biological replicates. Hollow bars represent untreated conditions while yellow bars represent KCl treated conditions for genes promoted by Rem2 signaling and blue bars represent KCl treated conditions for genes repressed by Rem2 signaling. mRNA levels were first normalized to Actb and then to the control untreated (UT) condition within each biological replicate. Data is presented as mean ± SEM. Data shown for the untreated control and Rem2 KO conditions for Rem2 and Slc7a1 are the same data as shown in Figure 1A and 1C respectively. The data is included here again for clarity.
Using this experimental paradigm, we identified 316 genes whose expression increased greater than 2-fold following KCl stimulation in the control condition (i.e. infection with AAV-GFP virus; Fig. 4B, yellow circle). Included among this gene set are known activity-regulated genes Fos, Jun and Npas4 (Table. S2). Additionally, we found increased expression of 345 genes upon KCl stimulation in the absence of Rem2 (Fig. 4B; blue circle). Comparison of these two data sets revealed that 222 of the genes were upregulated both in the presence and absence of Rem2, indicating that Rem2 signaling has no effect on their expression in the context of KCl stimulation (Fig. 4B; green, Table. S2). Thus, expression of 94/316 genes were upregulated upon KCl stimulation in a Rem2-dependent manner, indicating that Rem2 signaling is required to increase expression of these genes in the context of neuronal depolarization (Fig. 4B; yellow, Table. S3). Conversely, expression of 123/345 genes were up-regulated in response to KCl stimulation only in neurons in which Rem2 was deleted, suggesting that Rem2 signaling normally functions to inhibit expression of these genes in response to neuronal depolarization (Fig. 4B; blue, Table. S4).
We validated the expression profiles for 12 differentially regulated genes, 6 from the 94 genes promoted by Rem2 signaling and 6 from the 123 genes repressed by Rem2 signaling. For the qPCR validation, we probed RNA from each RNA-seq biological replicate and RNA from a third, independent biological replicate that was not subjected to deep sequencing. We found that 5/6 genes whose expression is promoted by Rem2 signaling and 4/6 genes whose expression is repressed by Rem2 signaling matched the expression profile identified in our RNA-seq experiments (Fig. 4C). The qPCR expression profiles for the genes that did not recapitulate results from the RNA-seq are shown in Figure S3. Surprisingly we observed that, with the exception of the gene Slc7a1, the set of genes regulated by Rem2 signaling at basal levels of activity versus those regulated with KCl stimulation were non-overlapping. Specifically, our data demonstrate that Slc7a1 mRNA expression decreases with Rem2 knockout (Fig. 1), but is upregulated upon Rem2 deletion with KCl stimulation (Fig. 4C). Thus, without stimulation, Rem2 signaling promotes Slc7a1 expression while KCl stimulation causes a switch, perhaps in signaling pathways upstream of Rem2, such that Rem2 signaling represses Slc7a1 expression. Taken together, these data indicate that depletion of Rem2 modifies the neuronal transcriptome in a complex manner in response to changes in neuronal activity.
Rem2 signaling promotes the expression of genes that encode membrane targeted proteins
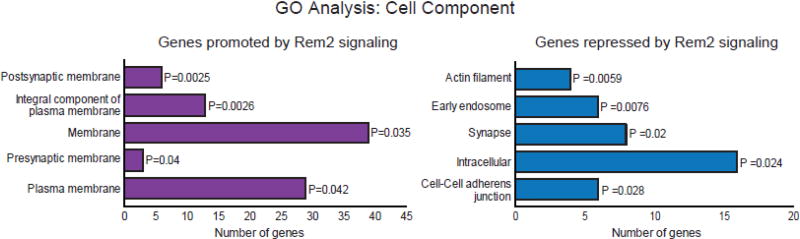
We next utilized the DAVID functional annotation classification tool [38, 39] to further analyze the subset of genes whose expression is promoted by Rem2 signaling (Fig. 4B, yellow circle). The DAVID algorithm groups genes from large datasets together by their known biological process, cell component, or molecular function [38, 39], by identifying enriched gene ontology (GO) terms in a given dataset. We began our gene ontology analysis by determining the cell component classification for genes whose expression is promoted by Rem2 signaling (Fig. 5; purple bars, Table. S5). Only GO terms that were significantly enriched (P < 0.05) using Fisher’s exact test were considered in the analysis. We found that enriched GO terms for genes promoted by Rem2 signaling encode a multitude of membrane protein classifications including: postsynaptic membrane, integral component of plasma membrane, membrane, presynaptic membrane and plasma membrane (Fig. 5; purple bars, Table. S5). Further, we observed that a large proportion of these genes encode ion channels and receptors including Kcna1, Grm2 and Gabrb3 (Table. S5). We validated the expression profiles of Grm2 and Gabrb3, two genes identified by the GO analysis as membrane targeted, confirming that Rem2 signaling does promote the expression of genes that code for membrane localized proteins (Fig. 4C).
Figure 5. Rem2 signaling promotes the expression of genes encoding membrane targeted proteins and represses the expression of genes encoding cytoplasmic proteins that regulate the cytoskeleton.
DAVID Gene Ontology (GO) Cell Component analysis for genes whose expression is promoted by Rem2 signaling (Left, purple) or repressed by Rem2 signaling (Right, blue). Only the GO terms with a p value <0.05 by Fisher’s Exact test were analyzed. The number of genes identified for each GO term are shown by each bar. The P value for each GO term is shown next to the corresponding bar.
One well-established role for Rem2 is to promote the formation of excitatory and inhibitory synapses [16–18]. Therefore, finding that Rem2 signaling promotes the expression of genes which encode membrane localized protein. A number of well-described synaptogenic factors are membrane associated proteins [11], suggesting that these genes might represent additional molecules through which Rem2 mediates synapse formation. Interestingly, the DAVID GO analysis also suggests a role for Rem2 signaling in synaptic transmission, a previously unexplored output of Rem2 signaling. We found that Rem2 signaling promotes the expression of Snap25 and Gabrb3, which encode the presynaptically localized protein SNAP25 and postsynaptically localized β3 subunit of the GABAA receptor. SNAP25 is part of the SNARE complex which is critical for vesicle fusion and neurotransmitter release at both excitatory and inhibitory synapses [40–42]. In addition, the GABAA receptor is a ligand-gated channel that is a crucial receptor responsible for mediating inhibitory neurotransmission in the brain [43, 44]. Mutations in Gabrb3 have been linked to childhood absence epilepsy [45]. Further, Kcna1, identified here as a gene whose expression is promoted by Rem2 signaling, encodes the potassium channel Kv1.1, which localizes to axons and dendrites and is specifically enriched in synaptosomes [46]. Taken together, these data suggest that in response to neuronal depolarization, Rem2 signaling promotes the expression of membrane targeted genes required for multiple aspects of synaptic function, a previously unsuspected role for this protein.
Rem2 signaling represses the expression of genes that encode proteins that regulate the cytoskeleton
As above, we used the DAVID functional annotation classification tool [38, 39] to further analyze the subset of genes whose expression is repressed by Rem2 signaling (Fig. 4B; blue circle). Using cell component analysis, we identified uniquely enriched GO terms in our list of genes whose expression is repressed by Rem2 signaling: actin filament, early endosome, synapse, intracellular and cell-cell adherens junction classifications (Fig. 5; blue bars, Table. S6). We found that many of the genes repressed by Rem2 signaling encoded proteins that localized to the cytoplasm or specifically associate with actin filaments including Cpeb2, Rhoq and Dlc1. Using q-PCR, we validated the expression profile of Cpeb2 using RNA from all three biological replicates to confirm our result that Rem2 signaling represses its expression in an activity-dependent manner (Fig. 4C).
Another known function of Rem2 is as an activity-dependent negative regulator of dendritic branching [14–16]. Interestingly, we find that Rem2 signaling represses the induction of Cpeb2; the closely related family member Cpeb1 promotes dendritic elaboration [47]. Both Cpeb2 and Cpeb1 are RNA-binding molecules and while little is known about the function of Cpeb2, Cpeb1 was identified in the Xenopus laevis visual system to be required for proper dendritic outgrowth in vivo [47]. Thus, Rem2 repression of Cpeb2 could represent one molecular mechanism by which Rem2 inhibits activity-dependent dendritic branching.
Additionally, Rem2 signaling negatively regulates the expression of the actin cytoskeleton regulating genes Rhoq and Dlc1. Dlc1 can act as a GTPase activating protein (GAP) for several Rho GTPases including CDC42 and Rho A–C which have been implicated in cytoskeleton regulation [48]. Furthermore, the Rho GTPase Rhoq promotes neurite outgrowth through a Rab11 and Exo70 dependent mechanism [49]. Therefore, repression of Rhoq and Dlc1 expression by Rem2 signaling could represent another mechanism by which Rem2 restricts dendritic complexity.
Taken together, our genetic analysis suggests a formerly unappreciated mechanism where Rem2 signaling promotes the expression of genes that encode membrane proteins in response to increased neuronal depolarization, while inhibiting the expression of genes that encode cytosolic signaling proteins in this same context (Fig. 5). We postulate that these results represent a functional “division of labor” of Rem2 signaling that matches previously identified Rem2 cell biological outputs: promoting the expression of genes that build synapses, while repressing the expression of genes that promote dendrite growth and branching.
Rem2 regulates the activation of activity-dependent transcription factors
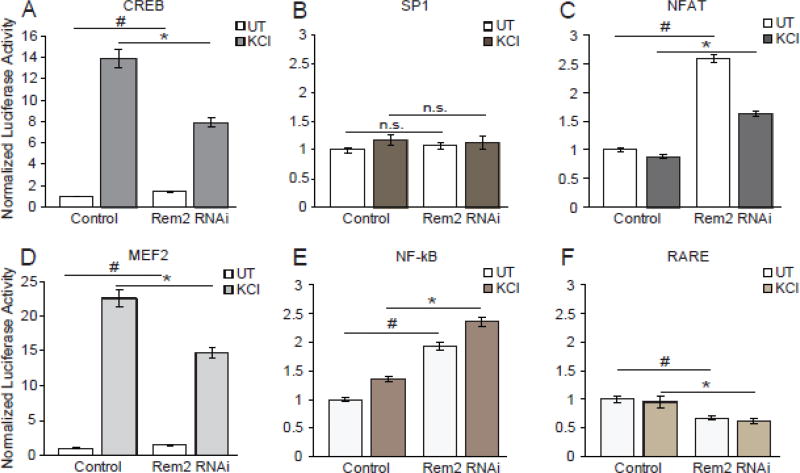
One known output of CaM kinase signaling is the activation of the activity-regulated transcription factor CREB [50, 51]. Given our RNA-seq data, coupled with our previously published results demonstrating that Rem2 functions in a signaling network with CaM kinases [14], we sought to determine if Rem2 knockdown affected the activity-dependent activation of CREB. To address this issue, we quantified CREB transcriptional activity using CRE (CREB response element) reporter dual luciferase assays. We transiently transfected cultured neurons with a reporter construct containing 4× CRE sites upstream of a minimal promoter driving firefly luciferase [52]. As a control, we transfected an SP1 reporter construct containing GC box promoter elements upstream of a minimal promoter driving firefly luciferase [53]; SP1 activation should not be dependent on neuronal depolarization. Rat cortical neurons were chosen for this experiment as they are particularly robust to both the culture and transfection paradigm used in this experiment. Neurons were transfected at 4 DIV with the CREB or SP1 luciferase reporter plus a plasmid expressing renilla-luciferase under control of a thymidine kinase promoter, and either a control shRNA (Control) and our verified shRNA targeting Rem2 (Rem2 RNAi) [16–18]. At 6 DIV, all neurons were treated with TTX overnight, thereby lowering the baseline activation of activity-dependent transcriptional programs in our cultures. At 7 DIV, a subset of neurons from each condition were left untreated, or treated with 55mM KCl to depolarize the neurons and activate CREB [52]. After 6 hours, cell lysates were harvested and a dual luciferase assay performed to quantify firefly and renilla luminescence in each condition [54].
As expected, we found that KCl stimulation was sufficient to induce CREB activation (Fig. 6A). Importantly, we found that RNAi-mediated knockdown of Rem2 led to a significant decrease in the KCl-induced activation of CREB (Fig. 6A, filled bars). This result suggests that Rem2 normally functions to promote the activation of CREB in response to neuronal depolarization. In addition, close examination of the untreated condition revealed that RNAi-mediated knockdown of Rem2 increases CREB activity, suggesting that in the absence of neuronal activity, Rem2 functions to repress transcriptional activation of CREB (Fig. 6A, hollow bars). Taken together, these data imply that Rem2 has differential effects on CREB transcriptional pathways, depending on the activity state of the cell. As predicted, neither Rem2 knockdown nor KCl stimulation had a significant effect on the activation of SP1 (Fig. 6B).
Figure 6. Rem2 specifically regulates the activation of activity-dependent transcription factors.
Quantification of firefly/renilla luciferase luminescence acquired from cortical neuron lysate from (A) CREB (B) SP1, (C) NFAT, (D) MEF2, (E) NF-κB or (F) RARE reporter transfected cells. Filled bars represent conditions treated with 55mM KCl for 6 hours before harvesting cell lysate while hollow bars represent untreated (UT) conditions. For each reporter, all conditions were normalized to the control UT condition within each biological replicate and averaged between replicates. Data is presented as mean of normalized luciferase activity ± SEM. * indicates p<0.05 by Student’s t-test between KCl treated conditions. # indicates p<0.05 by Student’s t-test between UT conditions. Each bar represents at least 3 biological replicates assayed in triplicate.
Given the effect of Rem2 RNAi on CREB activation, we asked whether depletion of Rem2 altered the activation of other activity-regulated transcriptional pathways by examining the activation of the transcription factors MEF2, NFAT, RARE and NF-κB using dual luciferase assays (see methods for details about these plasmids). Interestingly, in untreated conditions, Rem2 negatively regulates MEF2, NFAT and NF-κB pathways and positively regulates the RARE pathway (Fig. 6C–F). One interpretation of these data is that Rem2 acts upstream in a common signaling pathway that regulates multiple activity-dependent transcriptional programs. Alternatively, it could be that Rem2 plays a modulatory role in a number of independent signaling pathways that regulate activity-dependent transcriptional programs. Interestingly, a recent publication identified another RGK family member, Rad, as a modulator of the NF-κB pathway [55]. This study demonstrated that Rad binds to the RelA/P65 subunit of NF-κB causing decreased promoter binding and subsequently decreased expression of NF-kB target genes [55]. Thus, we hypothesize that regulation of gene expression is an unappreciated role for RGK family members.
CREB-dependent genes are not particularly enriched in the activity-dependent, Rem2-dependent gene set
Since our data demonstrates that Rem2 affects CREB transcriptional activity, and our previous work implicated Rem2 in a CaMK signaling pathway [14], we sought to determine if Rem2 preferentially modulated the expression of genes regulated by CREB. To accomplish this, we compared our dataset of genes regulated by Rem2 with two previously published datasets examining CREB or CBP (CREB binding protein) occupancy on the promoters of genes using ChIP-seq [33, 56]. Interestingly, we found that approximately 80% of genes promoted or repressed by Rem2 signaling had been identified in the ChIP-seq datasets to have either CREB or CBP occupancy at their promoters. These genes include the previously characterized CREB-regulated genes Grm2, Npas2 and Per1 [57–59]. Somewhat surprisingly, we failed to observe a change in Fos expression with Rem2 knockout using our RNA-seq approach (Fig. 4A), despite the fact that we observe a change in CREB transcriptional activation using a luciferase reporter assay (Fig. 6A). We postulate that this discrepancy is due to the fact that the endogenous Fos promoter contains numerous binding sites for other transcription factors which contribute to Fos activation. Conversely, the CRE-luciferase reporter construct (Fig. 6A) only interrogates CREB activation without the influence of additional factors. Consequently, changes in Rem2 signaling that may modulate CREB activity are detectable in our 4× CRE-luciferase reporter assay, but are not dramatic enough to produce a change in Fos expression at its endogenous locus.
Next, we compared the Rem2-independent genes (green overlap, Fig. 4B) to the genes identified in the CREB or CBP ChIP-seq and found that 86% and 88% of these genes had CREB or CBP occupancy respectively. For comparison, we also determined the CREB occupancy of a subset of genes that were neither regulated by neuronal depolarization nor Rem2 signaling. To accomplish this, we randomly picked 100 protein-coding genes whose expression was unchanged with Rem2 KO or neuronal depolarization and cross referenced that list to the genes from the CREB or CBP ChIP-seq analyses. We found that 74% and 63% of the activity and Rem2-independent genes were identified as having CREB or CBP binding sites in their promoters, respectively. Taken together, these analyses suggest that neuronal depolarization upregulates CREB-dependent genes both in the presence and absence of Rem2; however, CREB-dependent genes are not particularly enriched in the subset of genes whose expression is both activity-and Rem2 dependent. These findings favor our hypothesis that Rem2 plays a modulatory role in a number of independent signaling pathways that regulate activity-dependent transcriptional programs.
Conclusions
The proper development, maintenance, and function of brain circuits depends critically on both genes and activity. The molecular programs that alter neural circuits in response to activity are still poorly understood. Our lab discovered that the activity-regulated GTPase Rem2 is a key molecule linking sensory experience to changes in synapse and dendrite development. Specifically, Rem2 promotes synapse formation, yet inhibits activity-dependent increases in dendritic complexity [14–16, 18]. However, the signaling mechanisms by which Rem2 and other RGK proteins mediate these processes are not well-understood. Our exploration of changes in gene expression in response to Rem2 deletion represents a critical first step towards uncovering these signaling networks.
Our unbiased genetic analysis of Rem2-dependent changes in gene expression led to the finding that expression of known synaptogenic factors Lrrtm4 and Gpc5 are regulated by Rem2 signaling (Fig. 1, 2). Thus, we have uncovered a novel signaling pathway that further elucidates the genetic pathway by which neurons regulate synapse formation (Fig. 3). Further, we found that Rem2 signaling both promotes and represses the expression of non-overlapping sets of genes in the context of neuronal depolarization (Fig. 4), indicating that the role of Rem2 signaling in the regulation of the transcriptional response to neuronal depolarization is complex. Additionally, we were surprised to discover that Rem2 modulates the activity of several activity-dependent transcription factors (Fig. 6), suggesting a general role for Rem2 as a modulator of activity-dependent transcriptional programs.
Interestingly, inhibition of high-voltage activated Ca2+ channels (VGCCs; Cav1 and Cav2 families) by overexpression of Rem2 and other RGK family members has been reported in numerous cell types [17, 60, 61], suggesting that Rem2-dependent effects on gene expression could be due to increased calcium flux through VGCCs that occurs upon Rem2 deletion. However, a number of lines of evidence undermine this model. First, a previously published electrophysiological study from our lab [17, 62] and others [17, 62] using verified RNAi reagents to knock down Rem2 in cultured neurons, failed to uncover a role for Rem2 loss of function in modulation of calcium currents. In addition, we found no difference in the resting Ca2+ levels of cortical neurons obtained from Rem2−/− mice compared to WT controls using ratiometric Ca2+ imaging [25]. The Ikeda lab also reports a similar result using an electrophysiological approach: no change in calcium currents in dissociated cultures of striatal medium spiny neurons in which Rem2 was deleted [63]. Taken together, existing data do not support the hypothesis that loss of Rem2 affects calcium flux into the cell.
In summary, we propose that Rem2 functions at the nexus of signaling pathways that sense and respond to changes in neuronal activity to modulate both structural and functional plasticity, in part by regulating changes in gene expression. Our gene ontology analysis suggests that Rem2 signaling primarily promotes the expression of genes that code for membrane localized proteins that regulate synaptic transmission and represses the expression of genes that encode cytoplasmic regulator proteins that affect the cytoskeleton, consistent with Rem2 function in promoting synapse formation and repressing dendritic branching (Fig. 5). Therefore, we favor a model where Rem2 functions as a cytosolic signaling molecule to mediate changes in gene expression in the nucleus, perhaps by interacting with the CaMK signaling pathways [14, 64].
Materials and Methods
Ethics Statement
All experimental procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee at Brandeis University.
Neuronal cell culture and transfection
Hippocampal neurons from E18 Long Evans rats of either sex were cultured in Neurobasal media with B27 Supplement (Invitrogen) at a low density (8×104) cells/well in a 24 well dish on a confluent astrocyte feeder layer, as previously described [16]. AraC (Sigma) was added 24 hours after neuron plating to a final concentration of 5µM. For synapse assays, neurons were transfected at 4 DIV using the calcium phosphate method [65]. All conditions were transfected with a CMV-GFP plasmid at 500ng/well. For control conditions, neurons were also transfected with an empty pSuper plasmid at either 33 or 100ng/well. For Rem2 RNAi, neurons were also transfected with a pSuper plasmid containing an shRNA targeting Rem2 [18] at 33ng/well. For siRNA knockdown of Lrrtm4 and Gpc5, neurons were also transfected with pools of 4 siRNAs targeting the indicated gene at 20nM. For Lrrtm4 or Gpc5 RNAi, neurons were also transfected with a pSuper plasmid containing an shRNA targeting Gpc5 or Lrrtm4 [26] expressed at 33ng or 100ng/well respectively. It has been previously reported that co-transfection efficiency of up to three plasmids is 93% [14, 16]. On DIV 8 and 11, half of the neuronal media is removed and replaced with fresh Neurobasal with B27 supplement and 5µM AraC.
Synapse assays
At 14 DIV, the neuronal media was replaced with 1× PBS and fixed using 4% paraformaldehyde/4% sucrose for 8 minutes at room temperature. The coverslips were then washed three times with 1× PBS and moved to a humidified chamber. Neurons were co-stained with mouse anti PSD-95 (1:500; Neuromab) and rabbit anti Synapsin I (1:500; Fisher) diluted in 1× GDB (0.1% gelatin, 0.3% Triton X-100, 4.2% of 0.4M phosphate buffer and 9% 5M NaCl). The coverslips were incubated with primary antibodies overnight at 4°C and then washed three times with 1× PBS. Cy3- or Cy5-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) were diluted 1:500 in 1× GDB and incubated on coverslips for two hours at room temperature. Coverslips were then washed three times with 1× PBS and mounted on glass slides using Aqua-mount mounting media (Lerner Laboratories).
12-bit images of GFP transfected neurons were taken on an Olympus Fluoview300 confocal microscope using a 60× oil objective. Identical settings within biological replicates were used for laser power, amplifier offset and detector gain. Z-stacks of 5–10 optical sections with a step size of .5µm were taken for each neuron. Images of GFP-expressing neurons from three separate coverslips were taken for each condition for each biological replicate. Maximum intensity projections were made from the z-stacks of each neuron using ImageJ (NIH). Synapse density was quantified using Metamorph image analysis software and was defined as the overlap of GFP, anti-PSD-95 and anti-Synapsin staining. For each biological replicate, a threshold was set for Synapsin and PSD-95 staining using a control transfected neuron. The set threshold was then applied to every neuron within that particular experiment. The average synapse density for each condition was then calculated. At least two biological replicates were analyzed for each condition.
HEK 293T cell culture and western blotting
HEK 293T cells were grown in a 12 well plate and transfected using the calcium phosphate method [65] with 200 ng GFP (GFP), or 200 ng GFP and either 100 ng myc-Lrrtm4 (Lrrtm4 OE), 100 ng myc-Lrrtm4 and 1 µg shRNA targeting Lrrtm4 (Lrrtm4 RNAi), 1 µg shRNA targeting Lrrtm4 and 100 ng RNAi resistant Lrrtm4 cDNA (Lrrtm4 Rescue), 100 ng myc-Gpc5 (Gpc5 OE), 100 ng myc-Gpc5 and 1 µg shRNA targeting Gpc5 (Gpc5 RNAi), or 1 µg shRNA targeting Gpc5 and 100 ng RNAi resistant Gpc5 cDNA (Gpc5 Rescue). For the Western blot, the HEK 293T cells were lysed in 3× Sample Buffer (SDS, bromphenol blue, 1M Tris pH 6.8, glycerol) and proteins were separated by electrophoresis on a 10% SDS/PAGE gel. Proteins were then transferred to a nitrocellulose membrane and blocked for one hour in a solution containing 5% milk in TBST. The membrane was then incubated overnight at 4°C with anti-myc (Santa Cruz Biotechnologies; 1:500) and anti- β-actin (Abcam; 1:5000) primary antibodies. The membrane was washed three times for 15 minutes in TBST and incubated in appropriate secondary antibodies (Licor; 1:5000) for two hours at room temperature. The membrane was washed three times for 15-minutes with TBST and developed using the Odyssey Western Blotting system (Licor).
Luciferase assays
Cortical neurons from E18 Long Evans rats of either sex were dissociated and plated at a high density (5×105/well) in a 24 well dish. At 4 DIV, the neurons were transfected using the calcium phosphate method as described above. One well was transfected with a GFP plasmid at 500ng/well which was used as a transfection control. The firefly and renilla readings from the GFP well were subtracted from the firefly and renilla readings to remove background luminescence. All remaining wells were transfected with a thymidine kinase renilla plasmid at 200ng/well which was used as an internal control for transfection variability between wells in an experiment. All of the firefly reporters (CRE-Luc [52], MRE-Luc [66], SP1-Luc [53], NFAT-Luc [67], RARE-Luc [68] or NF-κB-Luc [69]) were transfected into neurons at 500ng/well. In conditions where Rem2 was knocked down (Rem2 RNAi), a pSuper plasmid containing a shRNA targeting Rem2 was also transfected into neurons at 33ng/well. To make sure the effect we were seeing was from knocking down Rem2 and not from activating the RNAi machinery, a control shRNA was transfected into control neurons at 33ng/well. At 6DIV, neurons were quieted overnight with 1µM TTX. On DIV 7, a subset of neurons was treated with 55mM KCl for 6 hours, lysed with passive lysis buffer (Promega) and assayed using a dual luciferase assay. Firefly luciferase was measured by adding 100µl of 75mM HEPES pH 8.0, 5mM MgSO4, 20mM DTT, 100mM EDTA and 530µM ATP, .5mM D-luciferin and .5mM coenzyme A. Renilla luciferase was measured using 100µl of 25mM Na4PPi, 10mM NaOAc, 15mM EDTA, .5M Na2SO4, 1M NaCl, and .1mM Coelenterazine, pH 5.0. All conditions were assayed at least three times in triplicate and normalized to renilla luciferase then to the control untreated condition for each firefly reporter.
Cell culture, infection, stimulation and harvesting of RNA for expression analysis by qPCR
Cortical neurons from E16 Rem2flx/flx mice of either sex with either black or agouti coat color were harvested and dissociated as previously described. They were plated at a high density (1.5×107)/ 10cm dish in glia conditioned Neurobasal media with B27 supplement (Invitrogen). At 1DIV, neurons were infected with either AAV1.hSyn.eGFP.WPRE.bGH or AAV1.hSyn.HI.eGFP-Cre.WPRE.SV40 (UPenn virus core). On DIV 5, the neurons were quieted overnight with 1µM TTX and then on DIV6 a subset of neurons were treated with 55mM KCl for 4 hours. RNA was extracted using Trizol Reagent (Invitrogen) following the manufacturer’s protocol and DNAse free RNA was then reverse transcribed using Random Primer Mix (New England Biologicals). Quantitative PCR was run using IQ-SYBR Green Supermix (Quanta Biologicals, VWR) on a Rotor Gene thermal cycler (Roche). Bands from genes of interest were normalized to β-actin (Actb) and shown as fold change over baseline using the delta-delta TT method [70]. Primers for Rem2 and Fos were previously validated [15] and the additional primers listed in Table 1 were validated by analyzing melting curves for reproducibility.
Table 1.
Primers used for qPCR
| Gene targeted | Sequence | |
|---|---|---|
| Actb | Forward | ACGGTCAGGTCATCACTATCG |
| Reverse | AGCCACCAATCCACACAGA | |
| Fos | Forward | ATGGGCTCTCCTGTCAACACAC |
| Reverse | ATGGCTGTCACCGTGGGGATAAAG | |
| Rem2 | Forward | TGAGAGACGGATCATGGTGGACAA |
| Reverse | AGCCGAAGAAGGGTTTCTGGAACT | |
| Lrrtm4 | Forward | AGAAGAAAGCTGAGAGAGGGG |
| Reverse | CATCTGCAGTTCTTTGGGCA | |
| Slc7a1 | Forward | CTCCTGGCTTACTCTTTGGTGG |
| Reverse | GATCTAGCTCCTCGGTGGTTCT | |
| Npnt | Forward | TGGGTCCTACAAGTGCCAAT |
| Reverse | ATATGGTGTCTTCAGGGGCC | |
| Dcn | Forward | CTGGCCAATGTTCCTCATCT |
| Reverse | AAGTCATTTTGCCCAACTGC | |
| Gpc5 | Forward | TGCTGGAATGGGGAGGAAAT |
| Reverse | CTTGTTGGGCTTGGGTGATC | |
| Exo5 | Forward | GCTTCCTGGTTCGTTGACACCT |
| Reverse | CCCAAGCATCTTCTTTTGTGGCG | |
| Ntf3 | Forward | CTACTACGGCAACAGAGACGCT |
| Reverse | GGTGAGGTTCTATTGGCTACCAC | |
| Renbp | Forward | CGTGTTGAGCTTCTGGATGCAG |
| Reverse | AAATGGTCCGCTGCACCTTCAC | |
| Rasl12 | Forward | CCAAGGAGACACAACGTGGCTA |
| Reverse | TCAAAGTCCAGGCAGGCAGAGA | |
| Glis3 | Forward | CCATACTTGTGCCAGCATCC |
| Reverse | TCTCGGGAAGAATGTGCCTT | |
| Klhl4 | Forward | GTGGGAGGAAGAGATGGCTT |
| Reverse | TAGCTCCATCCATCGTGACC | |
| Gabrb3 | Forward | CCTCACGCTTGACAATCGAG |
| Reverse | CGTAGTGATCCTGAGCCCAT | |
| Grm2 | Forward | TATCATCTGGCTGGCTTTCC |
| Reverse | GGCTCACCACGTTCTTCTGT | |
| Lin54 | Forward | GATCCAGCCCAACCTCACTA |
| Reverse | TGTGTCTGGATGCCAGATGT | |
| Klhl14 | Forward | CCCATGCAAGAAAGAAGAGC |
| Reverse | GCTGAGGCAGAGATGACACA | |
| Pard6a | Forward | CTGCTTGGCTATACGGATGC |
| Reverse | CCCTTTCTTGCGCCTTTGTA | |
| Pacsin3 | Forward | GAGCATTGAGGCTGCCAGTGAT |
| Reverse | GCTCTCTGTGTGTCTAACGACC | |
| Kctd6 | Forward | CCATCACCACAAAGGTCCACTC |
| Reverse | GGTGGTAATCGCACGGTCCAAA | |
| Cpeb2 | Forward | AGCTCAGTCCAGGCACTCAT |
| Reverse | TTCCAAGGACGGATTTGAAC | |
RNA-sequencing and Analysis
500ng of total RNA from each condition (Control, Rem2 KO, Control + KCl, Rem2 KO + KCl) was used to make mRNA libraries. mRNA was isolated, fragmented into regions about 300bp in length and ligated to condition specific adaptors using the TruSeq RNA library prep kit (Illumina). The quality of the libraries from each biological replicate were assayed using a 2100 BioAnalyzer (Agilent technologies). The libraries were sequenced on an Illumina HiSeq 2000 deep sequencer following the standard protocol for 75bp single-end non-stranded reads. The libraries were then analyzed using Galaxy (usegalaxy.org) [71]. The reads were mapped to the mm10 mouse genome using TopHat (Galaxy version 2.1.0) and FPKM for known protein-coding genes was determined using Cufflinks (Galaxy version 2.2.1.0). Fold changes in gene expression between conditions in each biological replicate were calculated manually using Microsoft Excel. The RNA-sequencing data from this publication has been deposited to GEO. GEO accession number: GSE97891.
Statistics
Within each biological replicate, the average synapse density value was obtained for control and each experimental condition. The normalized synapse density for each experiment represents the experimental average synapse density divided by the control synapse density. The standard error of this ratio is then determined using the following equation:
The normalized synapse density values from each biological replicate were averaged for each condition and the error was calculated as follows:
Statistical analysis was determined comparing each experimental condition to control using combined raw data from all biological replicates using SPSS Software (version 24) to run a two-way between- effect ANOVA followed by Tukey’s post-hoc test for significance.
Supplementary Material
Fold change in Klhl4 mRNA expression by qPCR from biological replicate number one. mRNA levels were normalized to Actb and then to the control condition. Data is presented as mean ± SEM. Rem2 KO is not significantly different from control by Student’s t-test.
A) Immunostaining for PSD-95 (red) and Synapsin I (blue) in the dendrites of transfected neurons. A synapse is defined as the overlap of red and blue puncta (shown in white) on a GFP positive dendrite. Scale bar = 5µm. B) Quantification of the density of PSD-95/Synapsin I positive puncta for neurons transfected with a pSuper empty vector (Control), shRNA targeting Rem2 (Rem2 RNAi), or siRNA smartpool targeting the indicated gene product. Data is presented as mean of normalized PSD-95/Synapsin I puncta density ± SEM. Asterisks indicate p<0.05, # indicates P = 0.072 compared to control by two-way ANOVA with Tukey post-hoc test. Normalized values are the average of three biological replicates and n ≥ 37 neurons for each condition. C) Validation of shRNAs targeting Lrrtm4 and Gpc5 and RNAi resistant Gpc5 or Lrrtm4 cDNAs. Western blot with anti-Myc antibody of HEK 293T cell lysate with anti-Actin is used as a loading control. The Western blot was developed using the Odyssey IR system.
Fold changes in mRNA expression by qPCR for each indicated gene averaged between two biological replicates. Hollow bars represent untreated conditions while yellow bars represent KCl treated conditions for genes promoted by Rem2 signaling and blue bars represent KCl treated conditions for genes repressed by Rem2 signaling. First, mRNA levels were normalized to Actb and then to the control untreated (UT) condition within each biological replicate. Data represents the average of two biological replicates. Data is presented as mean ± SEM.
Highlights.
Rem2 signaling regulates gene expression.
Rem2 signaling promotes the expression of known synaptogenic factors Lrrtm4 and Gpc5.
The Gpc5 ligand functions in the postsynaptic neuron to regulate excitatory synapse formation.
Rem2 both promotes and represses gene expression in the context of neuronal depolarization.
Rem2 knockdown modulates activity-dependent transcriptional pathways.
Acknowledgments
We would like to thank the members of the Paradis lab, Marr lab and Rosbash lab for helpful comments and suggestions throughout the project. We also thank the Rosbash and Marr labs for technical assistance. This work was supported by the NIH grant R01NS065856 (S.P) and NIH Career Development K01 award K01MH101639 (A.R.M.), R21GM117034 (M.T.M), and R01GM117034 (M.T.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
KK, MTM and SP designed the experiments. KK, XC, ARM and LR performed the experiments. KK, XC, LR and MTM analyzed the data. ARM created the Rem2flx/flx mouse line used in this study. KK and SP wrote the manuscript. KK, SP, MTM, LR, ARM and XC edited the manuscript.
References
- 1.Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- 2.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of physiology. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiesel TN, Hubel DH. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. Journal of neurophysiology. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 4.West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nature reviews. Neuroscience. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 6.Flavell SW, Kim TK, Gray JM, Harmin DA, Hemberg M, Hong EJ, Markenscoff-Papadimitriou E, Bear DM, Greenberg ME. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron. 2008;60:1022–1038. doi: 10.1016/j.neuron.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loebrich S, Nedivi E. The function of activity-regulated genes in the nervous system. Physiological reviews. 2009;89:1079–1103. doi: 10.1152/physrev.00013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, Elde R, Zimmer A, He C, Pei G, Bao L, Zhang X. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science (New York, N.Y.) 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 10.Biederer T, Stagi M. Signaling by synaptogenic molecules. Current opinion in neurobiology. 2008;18:261–269. doi: 10.1016/j.conb.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia PH, Li P, Shen K. Cell biology in neuroscience: cellular and molecular mechanisms underlying presynapse formation. The Journal of cell biology. 2013;203:11–22. doi: 10.1083/jcb.201307020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annual review of neuroscience. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 14.Ghiretti AE, Kenny K, Marr MT, 2nd, Paradis S. CaMKII-dependent phosphorylation of the GTPase Rem2 is required to restrict dendritic complexity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6504–6515. doi: 10.1523/JNEUROSCI.3861-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiretti AE, Moore AR, Brenner RG, Chen LF, West AE, Lau NC, Van Hooser SD, Paradis S. Rem2 is an activity-dependent negative regulator of dendritic complexity in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:392–407. doi: 10.1523/JNEUROSCI.1328-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiretti AE, Paradis S. The GTPase Rem2 regulates synapse development and dendritic morphology. Developmental neurobiology. 2011;71:374–389. doi: 10.1002/dneu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore AR, Ghiretti AE, Paradis S. A loss-of-function analysis reveals that endogenous Rem2 promotes functional glutamatergic synapse formation and restricts dendritic complexity. PloS one. 2013;8:e74751. doi: 10.1371/journal.pone.0074751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, Zhu L, Brass LF, Chen C, Greenberg ME. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlin BS, Shao H, Kadono-Okuda K, Guo N, Andres DA. Rem2, a new member of the Rem/Rad/Gem/Kir family of Ras-related GTPases. The Biochemical journal. 2000;347(Pt 1):223–231. [PMC free article] [PubMed] [Google Scholar]
- 20.Sasson Y, Navon-Perry L, Huppert D, Hirsch JA. RGK family G-domain:GTP analog complex structures and nucleotide-binding properties. Journal of molecular biology. 2011;413:372–389. doi: 10.1016/j.jmb.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Reymond P, Coquard A, Chenon M, Zeghouf M, El Marjou A, Thompson A, Menetrey J. Structure of the GDP-bound G domain of the RGK protein Rem2. Acta crystallographica. Section F, Structural biology and crystallization communications. 2012;68:626–631. doi: 10.1107/S1744309112013541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Tseng YH, Kantor JD, Rhodes CJ, Zetter BR, Moyers JS, Kahn CR. Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:14911–14918. doi: 10.1073/pnas.96.26.14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang T, Colecraft HM. Regulation of voltage-dependent calcium channels by RGK proteins. Biochimica et biophysica acta. 2013;1828:1644–1654. doi: 10.1016/j.bbamem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqui TJ, Tari PK, Connor SA, Zhang P, Dobie FA, She K, Kawabe H, Wang YT, Brose N, Craig AM. An LRRTM4-HSPG complex mediates excitatory synapse development on dentate gyrus granule cells. Neuron. 2013;79:680–695. doi: 10.1016/j.neuron.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 25.RS Moore AR, Kenny K, Royer L, Chan U, Flavahan K, Van Hooser SD, Paradis S. Rem2 regulates distinct homeostatic mechanisms in visual circuit plasticity. Neuron. 2017 DOI Under review. [Google Scholar]
- 26.de Wit J, O'Sullivan ML, Savas JN, Condomitti G, Caccese MC, Vennekens KM, Yates JR, 3rd, Ghosh A. Unbiased discovery of glypican as a receptor for LRRTM4 in regulating excitatory synapse development. Neuron. 2013;79:696–711. doi: 10.1016/j.neuron.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain research. Brain research reviews. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 29.Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Progress in neurobiology. 2011;94:259–295. doi: 10.1016/j.pneurobio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 31.Ataman B, Boulting GL, Harmin DA, Yang MG, Baker-Salisbury M, Yap EL, Malik AN, Mei K, Rubin AA, Spiegel I, Durresi E, Sharma N, Hu LS, Pletikos M, Griffith EC, Partlow JN, Stevens CR, Adli M, Chahrour M, Sestan N, Walsh CA, Berezovskii VK, Livingstone MS, Greenberg ME. Evolution of Osteocrin as an activity-regulated factor in the primate brain. Nature. 2016;539:242–247. doi: 10.1038/nature20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong EJ, West AE, Greenberg ME. Transcriptional control of cognitive development. Current opinion in neurobiology. 2005;15:21–28. doi: 10.1016/j.conb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, Markenscoff-Papadimitriou E, Kuhl D, Bito H, Worley PF, Kreiman G, Greenberg ME. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science (New York, N.Y.) 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 37.Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science (New York, N.Y.) 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- 38.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 39.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMahon HT, Sudhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. The Journal of biological chemistry. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- 41.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 42.Tafoya LC, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7826–7838. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacological reviews. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor PM, Thomas P, Gorrie GH, Connolly CN, Smart TG, Moss SJ. Identification of amino acid residues within GABA(A) receptor beta subunits that mediate both homomeric and heteromeric receptor expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:6360–6371. doi: 10.1523/JNEUROSCI.19-15-06360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurba KN, Hernandez CC, Hu N, Macdonald RL. GABRB3 mutation, G32R, associated with childhood absence epilepsy alters alpha1beta3gamma2L gamma-aminobutyric acid type A (GABAA) receptor expression and channel gating. The Journal of biological chemistry. 2012;287:12083–12097. doi: 10.1074/jbc.M111.332528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science (New York, N.Y.) 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 47.Bestman JE, Cline HT. The RNA binding protein CPEB regulates dendrite morphogenesis and neuronal circuit assembly in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20494–20499. doi: 10.1073/pnas.0806296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim TY, Vigil D, Der CJ, Juliano RL. Role of DLC-1, a tumor suppressor protein with RhoGAP activity, in regulation of the cytoskeleton and cell motility. Cancer metastasis reviews. 2009;28:77–83. doi: 10.1007/s10555-008-9167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujita A, Koinuma S, Yasuda S, Nagai H, Kamiguchi H, Wada N, Nakamura T. GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70. PloS one. 2013;8:e79689. doi: 10.1371/journal.pone.0079689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Molecular and cellular biology. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes & development. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- 52.Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33:383–395. doi: 10.1016/s0896-6273(01)00561-x. [DOI] [PubMed] [Google Scholar]
- 53.Briggs MR, Kadonaga JT, Bell SP, Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science (New York, N.Y.) 1986;234:47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- 54.Sherf BA, Navarro SL, Hannah RR, Wood KV. Dual-Luciferase® reporter assay: An advanced co-reporter technology integrating firefly and Renilla luciferase assays. Promega Notes. 1996;57 [Google Scholar]
- 55.Hsiao BY, Chang TK, Wu IT, Chen MY. Rad GTPase inhibits the NFkappaB pathway through interacting with RelA/p65 to impede its DNA binding and target gene transactivation. Cellular signalling. 2014;26:1437–1444. doi: 10.1016/j.cellsig.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Lesiak A, Pelz C, Ando H, Zhu M, Davare M, Lambert TJ, Hansen KF, Obrietan K, Appleyard SM, Impey S, Wayman GA. A genome-wide screen of CREB occupancy identifies the RhoA inhibitors Par6C and Rnd3 as regulators of BDNF-induced synaptogenesis. PloS one. 2013;8:e64658. doi: 10.1371/journal.pone.0064658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. The Journal of biological chemistry. 2003;278:718–723. doi: 10.1074/jbc.M209241200. [DOI] [PubMed] [Google Scholar]
- 58.Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7728–7733. doi: 10.1073/pnas.102075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen H, Puhl HL, 3rd, Niu SL, Mitchell DC, Ikeda SR. Expression of Rem2, an RGK family small GTPase, reduces N-type calcium current without affecting channel surface density. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9762–9772. doi: 10.1523/JNEUROSCI.3111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Correll RN, Botzet GJ, Satin J, Andres DA, Finlin BS. Analysis of the Rem2 - voltage dependant calcium channel beta subunit interaction and Rem2 interaction with phosphorylated phosphatidylinositide lipids. Cell Signal. 2008;20:400–408. doi: 10.1016/j.cellsig.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang HG, Wang C, Pitt GS. Rem2-targeted shRNAs reduce frequency of miniature excitatory postsynaptic currents without altering voltage-gated Ca(2)(+) currents. PloS one. 2011;6:e25741. doi: 10.1371/journal.pone.0025741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liput D, Puhl H, Ikeda S. Electrophysiological and behavioral characterization of a mutant mouse lacking Rem2 protein in striatal medium spiny neurons. 2017 Neuroscience Meeting Planner, Neuroscience 2017. 2017 Abstracts. [Google Scholar]
- 64.Royer L, Herzog JJ, Kenny K, Tzvetkova B, Cochrane JC, Marr MT, Paradis S. The Ras-like GTPase Rem2 is a potent endogenous inhibitor of calcium/calmodulin-dependent kinase II activity. bioRxiv. 2017 doi: 10.1101/148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science (New York, N.Y.) 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 67.Ichida M, Finkel T. Ras regulates NFAT3 activity in cardiac myocytes. The Journal of biological chemistry. 2001;276:3524–3530. doi: 10.1074/jbc.M004275200. [DOI] [PubMed] [Google Scholar]
- 68.Hoffman LM, Garcha K, Karamboulas K, Cowan MF, Drysdale LM, Horton WA, Underhill TM. BMP action in skeletogenesis involves attenuation of retinoid signaling. The Journal of cell biology. 2006;174:101–113. doi: 10.1083/jcb.200604150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson AA, Kwok LW, Porter EL, Payne JG, McElroy GS, Ohle SJ, Greenhill SR, Blahna MT, Yamamoto K, Jean JC, Mizgerd JP, Kotton DN. Lentiviral delivery of RNAi for in vivo lineage-specific modulation of gene expression in mouse lung macrophages. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:825–833. doi: 10.1038/mt.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 71.Afgan E, Baker D, van den Beek M, Blankenberg D, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Eberhard C, Gruning B, Guerler A, Hillman-Jackson J, Von Kuster G, Rasche E, Soranzo N, Turaga N, Taylor J, Nekrutenko A, Goecks J. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic acids research. 2016;44:W3–w10. doi: 10.1093/nar/gkw343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fold change in Klhl4 mRNA expression by qPCR from biological replicate number one. mRNA levels were normalized to Actb and then to the control condition. Data is presented as mean ± SEM. Rem2 KO is not significantly different from control by Student’s t-test.
A) Immunostaining for PSD-95 (red) and Synapsin I (blue) in the dendrites of transfected neurons. A synapse is defined as the overlap of red and blue puncta (shown in white) on a GFP positive dendrite. Scale bar = 5µm. B) Quantification of the density of PSD-95/Synapsin I positive puncta for neurons transfected with a pSuper empty vector (Control), shRNA targeting Rem2 (Rem2 RNAi), or siRNA smartpool targeting the indicated gene product. Data is presented as mean of normalized PSD-95/Synapsin I puncta density ± SEM. Asterisks indicate p<0.05, # indicates P = 0.072 compared to control by two-way ANOVA with Tukey post-hoc test. Normalized values are the average of three biological replicates and n ≥ 37 neurons for each condition. C) Validation of shRNAs targeting Lrrtm4 and Gpc5 and RNAi resistant Gpc5 or Lrrtm4 cDNAs. Western blot with anti-Myc antibody of HEK 293T cell lysate with anti-Actin is used as a loading control. The Western blot was developed using the Odyssey IR system.
Fold changes in mRNA expression by qPCR for each indicated gene averaged between two biological replicates. Hollow bars represent untreated conditions while yellow bars represent KCl treated conditions for genes promoted by Rem2 signaling and blue bars represent KCl treated conditions for genes repressed by Rem2 signaling. First, mRNA levels were normalized to Actb and then to the control untreated (UT) condition within each biological replicate. Data represents the average of two biological replicates. Data is presented as mean ± SEM.