Abstract
Pregnancy leads to significant changes in the body, which potentially affect the retina. Pregnancy can induce disease, such as that seen in hypertensive retinopathy and choroidopathy. It can cause exudative retinal detachments in the HELLP syndrome (hemolysis, elevated liver enzymes and low platelets), disseminated intravascular coagulation (DIC), and thrombotic thrombocytopenic purpura (TTP), and provoke arterial and venous retinal occlusive disease. Pregnancy may also exacerbate pre-existing retinal disease, such as idiopathic central serous chorioretinopathy (ICSC) and diabetic retinopathy. Special consideration needs to be exercised when treating pregnant patients in choosing medications, as well as in selecting diagnostic modalities and surgical methods.
Keywords: Pregnancy, Retina, Exudative Retinal Detachment
INTRODUCTION
The pregnant body undergoes several physiologic, hormonal, and metabolic changes that potentially affect the retina[1,2]. Blood pressure typically decreases early in pregnancy and then increases, occasionally to super-normal levels, in the third trimester. During the second and third trimesters, insulin resistance increases, resulting in decreasing glycemic control. Blood volume increases, reaching its peak in the second trimester, and serum cortisol levels increase as well. Finally, pregnancy induces a relative hypercoagulable state, rendering pregnant women susceptible to pathologic clot formation. These alterations can cause new retinal diseases or exacerbate pre-existing retinal disorders. Herein, we present a literature review as well as our own clinical experience with pregnant patients suffering from retinal disease.
RETINAL AND CHOROIDAL DISEASES THAT MAY BE INDUCED BY PREGNANCY
Hypertensive Retinopathy and Choroidopathy
Signs of acute hypertensive retinopathy on fundus examination should prompt immediate referral for evaluation and treatment of the pregnancy-induced hypertension syndromes, pre-eclampsia and eclampsia. Pre-eclampsia is characterized by hypertension, peripheral edema and proteinuria, while eclampsia is defined as pre-eclampsia plus seizures. Fundus findings include arteriolar constriction, retinal hemorrhages, cotton wool spots, retinal edema, and lipid exudates [Figure 1]. More severe cases may include subretinal fluid resulting from choriocapillaris infarction and/or optic disc swelling and ischemia. In most cases, the changes are reversible as blood pressure normalizes after delivery, although focal areas of retinal thinning and/or chorioretinal atrophy may persist following retinal and/or choroidal infarctions [Figure 2].
Figure 1.
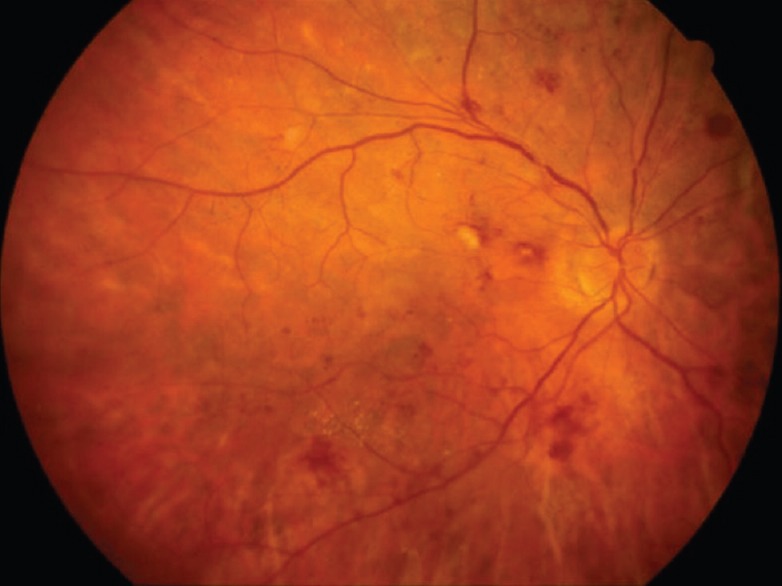
Color fundus photograph of a patient with pregnancy-induced hypertension. Findings include arteriolar constriction, retinal hemorrhages, cotton-wool spots and lipid exudates.
Figure 2.
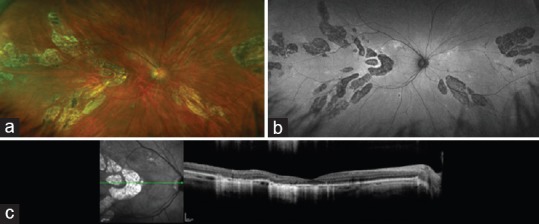
Images of the right eye of a 34-year-old woman with reduced vision since pregnancy complicated by pre-eclampsia and renal failure 10 months earlier. Multifocal, discrete areas of atrophy involving the outer retina, retinal pigment epithelium and inner choroid are seen in the color photograph (a), fundus autofluoresence image (b), and optical coherence tomography (OCT) image (c). (Images courtesy of David N. Zacks, MD, PhD).
Exudative Retinal Detachment
Extensive exudative retinal detachment induced by pregnancy can be seen in the HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), disseminated intravascular coagulation (DIC), and thrombotic thrombocytopenic purpura (TTP). The HELLP syndrome occurs in up to 15% of women with pre-eclampsia and is associated with a high rate of infant mortality. Patients can present with bilateral exudative retinal detachments and yellow-white subretinal deposits. Currently, the only effective treatment is prompt delivery. In DIC, thrombi can occlude vessels in the choriocapillaris, leading to exudative retinal detachment. Once DIC has been treated and retinal detachment has resolved, patients may be left with prominent retinal pigment epithelial changes.[3] TTP has been associated with exudative retinal detachment as well as retinal vascular occlusions and retinal hemorrhages.[3]
Retinal Vascular Occlusive Disease
Arterial
Post-partum Purtscher-like retinopathy typically occurs within 24 hours of delivery and often causes severe and permanent bilateral vision loss due to multifocal areas of retinal infarction involving the macular area[4] [Figure 3]. Pregnancies associated with Purtscher-like retinopathy are usually complicated. There is no known treatment for this condition, since retinal infarction has already occurred when visual symptoms develop. Although amniotic fluid embolism can also occlude retinal arterioles in pregnant patients, this condition is very rare and usually fatal.
Figure 3.
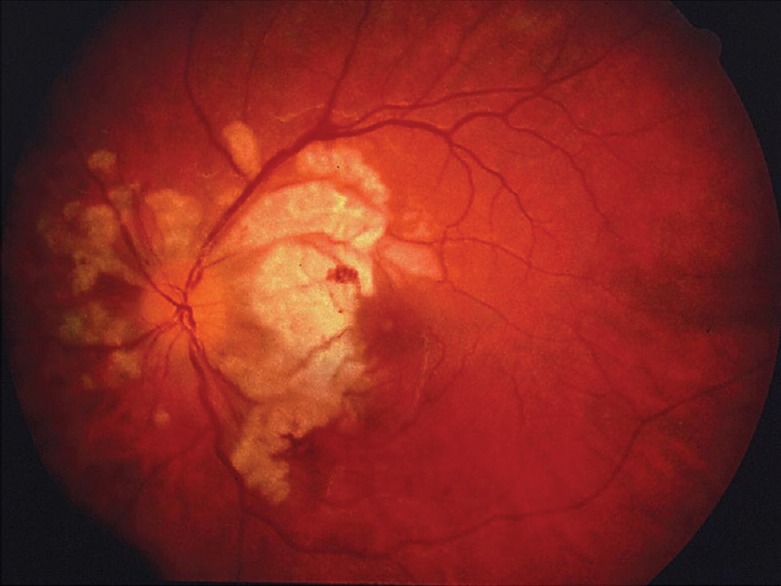
Fundus photograph of the left eye of a patient with severe bilateral vision loss occurring immediately after childbirth. There are multiple, large, near-confluent patches of ischemic retinal whitening involving the posterior retina.
Venous
Pregnancy induces a hypercoagulable state that can lead to retinal venous occlusions, such as branch or central retinal vein occlusion. Retinal vein occlusions most often occur in the third trimester or post partum period, and appear similar to those seen outside of pregnancy. The risk of retinal venous occlusion during pregnancy has been estimated to range from 0.05 to 1.8%.[5]
RETINAL AND CHOROIDAL DISEASES THAT MAY BE EXACERBATED BY PREGNANCY
Idiopathic Central Serous Chorioretinopathy
Pregnancy is a known trigger for active episodes of idiopathic central serous chorioretinopathy (ICSC), likely related in part to elevated serum cortisol levels. Of clinical interest is the fact that subretinal fibrin deposition occurs in up to 90% of pregnant patients with ICSC compared to 20% of non-pregnant patients.[6] Management of these patients can be challenging, as fluorescein angiography and photodynamic therapy are not well studied in pregnancy, and most physicians therefore prefer to avoid them. We recommend that if no fibrin is present in or near the fovea, and the patient is near term, the patient can be observed with an expectation of resolution after delivery. For a patient whose fovea is threatened by fibrin, it is reasonable to consider thermal laser photocoagulation guided by fundus examination and optical coherence tomography (OCT) imaging [Figure 4].
Figure 4.
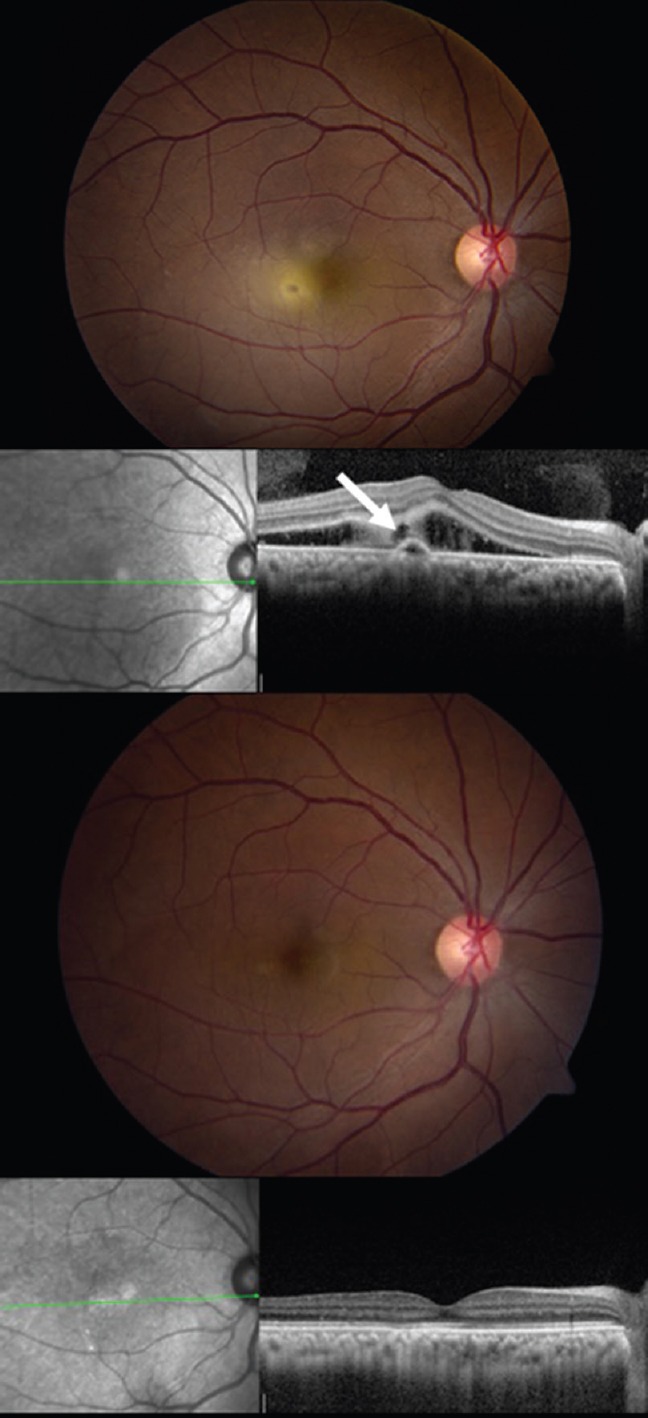
Fundus photograph (top) and OCT image (second row) of the right eye of a pregnant woman in the late second trimester with progressive subretinal fibrin deposition and VA of 20/60. The white arrow indicates the presumed location of the responsible leak, which was treated with mild-intensity laser photocoagulation. Five months after treatment, there is complete resolution of subretinal fluid and fibrin by fundus photography (third row) and OCT imaging (fourth row).
Diabetic Retinopathy
Diabetic retinopathy often progresses during pregnancy—the rate of retinopathy progression in pregnant patients in a prospective study comparing patients with Type I Diabetes was twice that of non-pregnant patients in the study[7]. After adjusting for hemoglobin A1C levels, the study found that pregnancy itself was associated with progression.[7] The Diabetes in Early Pregnancy Study showed that, in patients with moderate nonproliferative diabetic retinopathy (NPDR) at baseline, 55% experienced a 2-step ETDRS progression in their retinopathy level, and 29% progressed to proliferative diabetic retinopathy (PDR).[8] The Diabetes Control and Complications Trial (DCCT) found that although the retinopathy of type 1 diabetic patients progressed at a faster rate during pregnancy, the long-term risk of progression of early retinopathy was unlikely to be increased by pregnancy.[9]
Patients planning to become pregnant should be counseled to maximize glycemic control prior to conception. The American Academy of Ophthalmology Preferred Practice Patterns[10] recommend that pregnant patients undergo dilated fundus examination in the first trimester, with subsequent follow-up determined by the severity of retinopathy. Individuals with moderate NPDR should be re-examined every 3-6 months and those with severe NPDR or worse should be examined every 1-3 months. Heightened surveillance should continue during the first year post-partum. Gestational diabetes does not require eye examinations during pregnancy.
In patients who present with proliferative retinopathy during pregnancy, pan-retinal photocoagulation (PRP) should generally be performed at the time of diagnosis. As discussed below, it is prudent to avoid anti-VEGF therapy given fetal safety concerns. Observation is a reasonable management option for pregnant patients with mild diabetic macular edema (DME), since the edema may well resolve after delivery. For DME requiring treatment, we believe for safety reasons that anti-VEGF therapy should be avoided in favor of focal laser photocoagulation or intravitreal corticosteroid injections.
GENERAL MANAGEMENT RECOMMENDATIONS
Intravitreal Agents
Triamcinolone is likely safe in pregnancy. Due to obvious ethical constraints, there is no adequate fetal safety data for anti-VEGF agents. However, several small case series reported no harmful effects on the fetus when mothers inadvertently received up to 6 intravitreal anti-VEGF injections.[11,12,13,14] Based on theoretical risks to the fetus and the paucity of good safety data, it is generally recommended that anti-VEGF agents be used in pregnant patients only if absolutely necessary. Furthermore, physicians are advised to consider pregnancy testing in women of childbearing age prior to administering intravitreal anti-VEGF injections.
Common Ocular Medications
Many commonly prescribed ocular medications are potentially deleterious in pregnancy. Beta blockers can be teratogenic, and acetazolamide has been associated with neonatal acidosis. Prostaglandin analogues are considered pregnancy category C (insufficient research data to determine safety), although no adverse events have been seen in limited ophthalmic research.[15]
Angiography
Fluorescein dye crosses the placenta and is present in breast milk for up to 72 hours. However, to our knowledge there are no reports of adverse fetal or infant effects from the dye. Indocyanine green (ICG) dye does not cross the placenta, and is sometimes used in pregnant women for non-ophthalmic indications without reports of adverse fetal effects. It is nevertheless prudent in pregnant patients to use OCT and/or OCT angiography, where possible, in place of invasive angiography.
Vitreoretinal Surgery
Elective surgery should generally be avoided during pregnancy. When invasive procedures are unavoidable, we recommend that the obstetrical team be involved in surgical planning and, ideally, be present at the bedside for late-term procedures. Local anesthesia should be utilized instead of general anesthesia wherever possible. Lidocaine is considered safe for use during pregnancy. However, bupivacaine is considered a category C drug, and may lead to fetal bradycardia.[7]
CONCLUSION
The body undergoes several important physiologic and hormonal changes during pregnancy that may exacerbate or induce retinal complications. Ophthalmologists and optometrists, as well as all physicians treating these patients, should be aware of these changes and prepared to help detect and manage pregnancy-related retinal disease.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Petraglia F, D’Antona D. Maternal adaptations to pregnancy: Endocrine and metabolic changes. UpToDate, 2017. Available from: www.uptodate.com/contents .
- 2.Foley MR. Maternal adaptations to pregnancy: Cardiovascular and hemodynamic changes. UpToDate, 2017. Available from: www.uptodate.com/contents .
- 3.Sheth BP, Mieler WF. Ocular complications of pregnancy. Curr Opin Ophthalmol. 2001;12:455–463. doi: 10.1097/00055735-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Blodi BA JM, Gass JDM, Fine SL, Joffe LM. Purtscher’s-like retinopathy after childbirth. Ophthalmology. 1990;97:1654–1659. doi: 10.1016/s0161-6420(90)32365-5. [DOI] [PubMed] [Google Scholar]
- 5.Errera MH, Kohly RP, da Cruz L. Pregnancy-associated retinal diseases and their management. Surv Ophthalmol. 2013;58:127–142. doi: 10.1016/j.survophthal.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Sunness JS, Haller JA, Fine SL. Central serous chorioretinopathy and pregnancy. Arch Ophthalmol. 1993;111:360–364. doi: 10.1001/archopht.1993.01090030078043. [DOI] [PubMed] [Google Scholar]
- 7.Klein BE, Moss SE, Klein R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care. 1990;13:34–40. doi: 10.2337/diacare.13.1.34. [DOI] [PubMed] [Google Scholar]
- 8.Chew EY, Mills JL, Metzger BE, Remaley NA, Jovanovic-Peterson L, Knopp RH, et al. Metabolic control and progression of retinopathy. The Diabetes in Early Pregnancy Study. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care. 1995;18:631–637. doi: 10.2337/diacare.18.5.631. [DOI] [PubMed] [Google Scholar]
- 9.Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084–1091. doi: 10.2337/diacare.23.8.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice pattern® Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2016. Available from: www.aao.org/ppp . [Google Scholar]
- 11.Polizzi S, Ferrara G, Restaino S, Rinaldi S, Tognetto D. Inadvertent use of bevacizumab in pregnant women with diabetes mellitus type 1. J Basic Clin Physiol Pharmacol. 2015;26(2):161–163. doi: 10.1515/jbcpp-2014-0058. [DOI] [PubMed] [Google Scholar]
- 12.Polizzi S, Mahajan VB. Intravitreal Anti-VEGF Injections in Pregnancy: Case Series and Review of Literature. J Ocul Pharmacol Ther. 2015;31(10):605–610. doi: 10.1089/jop.2015.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan L, Kelly SP, Glenn A, Williams CP, McKibbin M. Intravitreal bevacizumab injection in unrecognised early pregnancy. Eye (Lond) 2014;28(4):492–494. doi: 10.1038/eye.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarantola RM, Folk JC, Boldt HC, Mahajan VB. Intravitreal bevacizumab during pregnancy. Retina. 2010;30(9):1405–1411. doi: 10.1097/IAE.0b013e3181f57d58. [DOI] [PubMed] [Google Scholar]
- 15.Schultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16:308–314. doi: 10.1097/01.icu.0000179803.42218.cc. [DOI] [PubMed] [Google Scholar]


