Abstract
Glaucoma is the leading cause of irreversible blindness and vision loss in the world. Although intraocular pressure (IOP) is no longer considered the only risk factor for glaucoma, it is still the most important one. In most cases, high IOP is secondary to trabecular meshwork dysfunction. High IOP leads to compaction of the lamina cribrosa and subsequent damage to retinal ganglion cell axons. Damage to the optic nerve head is evident on funduscopy as posterior bowing of the lamina cribrosa and increased cupping. Currently, the only documented method to slow or halt the progression of this disease is to decrease the IOP; hence, accurate IOP measurement is crucial not only for diagnosis, but also for the management. Due to the dynamic nature and fluctuation of the IOP, a single clinical measurement is not a reliable indicator of diurnal IOP; it requires 24-hour monitoring methods. Technological advances in microelectromechanical systems and microfluidics provide a promising solution for the effective measurement of IOP. This paper provides a broad overview of the upcoming technologies to be used for continuous IOP monitoring.
Keywords: Continuous Monitoring, Glaucoma, Implantable Pressure Sensor, Intraocular Pressure, Microelectromechanical Systems, Microfluidics
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide,[1] and is the second most common cause of blindness after cataracts.[2] In 2013, 64.3 million people had glaucoma, and the prevalence is estimated to become as high as 111.8 million by 2040.[3] Glaucoma is considered to be an intraocular pressure (IOP)-associated optic neuropathy with characteristic funduscopic findings.[1] Although it is a multifactorial disease, high IOP is considered to be the primary risk factor.[4] IOP is the result of a balance between aqueous humor production and drainage, and a malfunction in the drainage system leads to a pressure increase in the eye. Thus, monitoring IOP is key for the diagnosis and treatment of glaucoma.
There are several methods used to estimate IOP, and several devices to evaluate IOP are emerging, but the perfect instrument to detect absolute IOP has yet to be developed. In fact, as the eye is not a dry, thin-walled balloon, there is no exact value for IOP that can be detected by any conventional device. The IOP is the pressure simultaneously perceived by each tissue in the eye globe, and the pressure is different for the cornea, sclera, and lamina cribrosa. To complicate the matter further, the IOP has a dynamic nature; its fluctuation, based on endogenous and exogenous factors, make “snapshot” measurements even less reliable. Factors such as corneal thickness, body posture and liquid consumption, time of measurement, blood pressure, and stress influence measurements performed by conventional methods, and the obtained results cannot be considered an exact estimate.[4,5,6,7] Underestimation of IOP causes missed or delayed detection of glaucoma.[8] Thus, continuous measurement of IOP is crucial to detect fluctuations and to understand its role in glaucoma.
Miniaturization of sensor technology has provided new opportunities for IOP monitoring and glaucoma diagnosis in early stages.[9] Using the miniaturization advantages of microelectromechanical systems (MEMS) technology and microfluidics systems, new implantable IOP sensors can be developed for continuous IOP monitoring. The upcoming sensors can be implanted in the anterior chamber,[10,11,12,13,14] embedded intraocularly or in a contact,[15,16,17,18,19,20,21,22] or integrated into a tonometry device for better measurement accuracy.[23] Currently, some continuous pressure monitoring systems such as Triggerfish® (Sensimed AG, Switzerland) and EYEMATE® (Implandata Ophthalmic Products GmbH, Germany) have been used in clinical applications, but further development or the availability of an optimal implantable IOP sensor are necessary before these systems can be used in clinical practice.
This article provides an overview of the ongoing research that will provide the underlying technology for future IOP monitoring devices. We also discuss a new trend in the development of an integrated MEMS/microfluidics system, which can be used as an IOP monitoring system along with an artificial drainage system to treat glaucoma. First, the required characterization for IOP sensors from engineering and clinical points of view is discussed. Afterwards, the underlying technologies are investigated. Existing sensors are reviewed, and the future directions are discussed.
PRINCIPLES OF PRESSURE MEASUREMENTS
A sensor is a transducer that measures a physical quantity and converts it into a signal that can be read by an observer or an electronic instrument. In other words, a sensor transfers some physical quantities that cannot be observed directly as readable data by a human or by computing for further analysis. Several conditions and diseases are linked to the deviation of IOP from a healthy, normal range, motivating the need for chronic implantable pressure sensors. Differential (gauge) pressure measurement is the prevailing method of pressure measurement for medical applications; atmospheric pressure is designated as the baseline or “zero” for that measurement.[24] Most available pressure sensors are based on flexible components with integrated sensor and manometer devices. In the first method, there is an elastic membrane under pressure; the shape change of the elastic membrane is characterized by a displacement that can then be measured using a suitable sensor. This displacement results in a change in an electrical quantity of the sensor, such as capacitance or resistance, which can be read and recorded using a suitable electrical circuit. Manometer-based methods comprise a column of liquid in a tube whose ends are exposed to different pressures. The fluid flows from the higher-pressure end to the lower-pressure end and can be visually perceived and measured using calibration on the tube. It can also be equipped with an image processing system to be machine-readable. Figure 1 shows the typical applications of pressure sensors based on the principles discussed.
Figure 1.
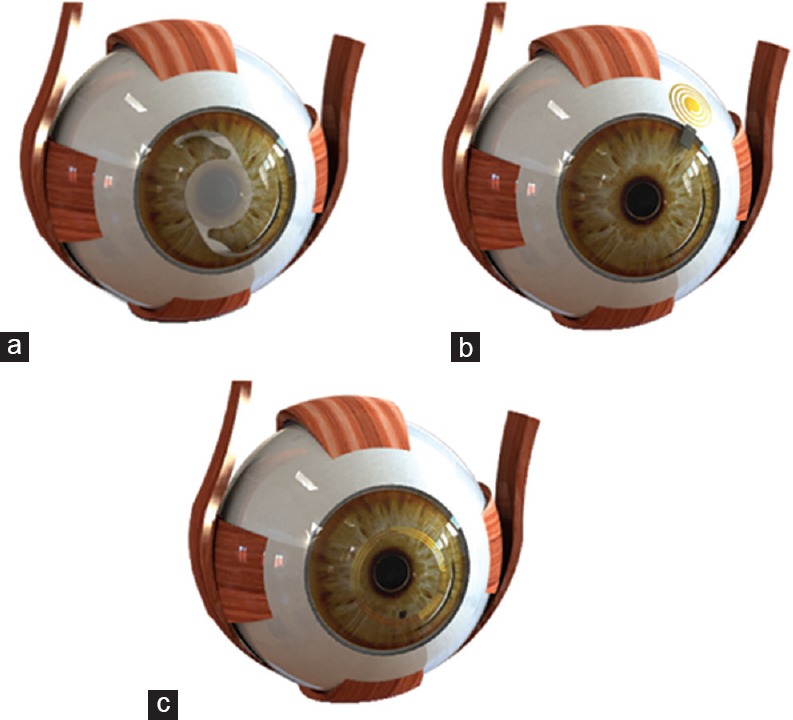
Manometer-based sensor integrated into an implantable lens (a), Capacitive sensor measuring IOP directly (b), Resistive sensor on a contact lens (c). IOP, intraocular pressure
CHARACTERIZATIONS OF CONTINUOUS IOP MONITORING SENSORS
As is implied by the name, a continuous IOP monitoring sensor should continuously measure and record pressure. Suitable solutions include wearable or implantable IOP sensors placed in a specific region of the eye. While wearable sensors are safer and easier to design, they cause tissue changes and are less accurate, as they measure pressure indirectly. Implantable sensors are more accurate but require a surgical procedure, which is constrained by sensor size and biocompatibility. In addition, the in vivo environment has bioengineering challenges that must be considered before the development of such a sensor. The required code of conduct for the development of implantable IOP monitoring has higher restrictions for the eye. Clinically, a suitable implantable pressure sensor should ensure pressure measurement for a long period of time with minimum discomfort to the patient.[25] Engineers consider precision, power consumption, calibration, sensitivity, resolution, and maintenance of the sensor. From both medical and engineering perspectives, there are several other concerns of great importance, such as biocompatibility, patient safety, size restrictions, and clear vision. These devices should be biocompatible and not incite an immune reaction inside the eye.[26] A potential solution is the use of biocompatible materials that have proven safe for intraocular use.
A suitable force sensor for IOP monitoring should be small enough to be implantable in a specified section of the eye without inducing vision loss. Smaller sensors can be implanted more easily and have a lower risk of tissue damage. Although IOP sensors can be placed on the cornea, sclera, and other locations, the anterior chamber has the benefit of pressure measurement independent of globe biomechanical properties, and the accuracy of the measurement is not affected by previously performed eye operations such as keratoplasty and keratoprosthesis. However, implants placed in the vitreous cavity have a higher risk of infection, retinal detachment, and encapsulating fibrosis.[27] The available space for placement of an intraocular implant in the anterior chamber is a cylinder with an axial length of 3–4 mm[28] and an average width of 12.5 mm,[29] which must be used effectively. The mean corneal thickness is 520 μm at the center and progressively increases toward the periphery.[30] The pressure range and resolution are also crucial for pressure measurement, and the implantable pressure sensor must cover the possible range of eye pressure for various circumstances with good resolution and precision. The required resolution for IOP monitoring that is clinically relevant is 2 mmHg.[31] The IOP normally fluctuates by approximately 4–6 mmHg throughout the day, and may be as high as 15 mmHg in glaucomatous eyes.[32]
Other important aspects are repeatability, drift, and long-term accuracy, and the sensor response must be stable over a long period of time. Another aspect is the frequency response of the sensor, which is important due to the dynamic behavior of the IOP. In this regard, the designed sensor should cover the required frequency response compatible with eye pressure dynamics. It has been reported that the bandwidth of the IOP sensor should be 0–30 Hz.[33]
An important quality of the sensor is energy consumption which should be as efficient as possible. The method of power transmission is a major concern; wireless power transmission is an ultimate solution, as the sensor can be recharged wirelessly without any physical connection. The sensors may also utilize photovoltaic cells for long-term power within this environment.[24] However, a data acquisition system powered by a battery is essential for continuous recording of IOP. The battery should be small enough for implantation using a minimally invasive procedure while having a sufficient lifetime.
Another issue is data recording, and the corresponding data acquisition method and telemetry. The sensor can be equipped with a wireless module to transmit data, or a suitable visualization method such as image processing could be used to obtain the measured pressure. Another concern regarding these devices is the induced current and voltage transmitted to the patient and heat generated in the body, or physical harm during any unexpected accident. Electronic circuits are prone to ionic contamination due to interaction with the warm, electrolytic components inside the eye.[34]
In addition, the implant should be easily retrievable in emergency cases,[35] and frequent movement of the eye must be considered to prevent possible tissue damage. Before clinical application of the sensor, the standard limits must also be respected and required risk management procedures must be employed.[36,37]
UNDERLYING TECHNOLOGIES
Based on the nature of IOP, which is a hydraulic pressure, and the structure of the eye, both flexible component- and manometer-based devices can be utilized for IOP measurement. Intraocular fluids can be manipulated in micron-sized channels to measure IOP using a microfluidic system. These microchannels can be inserted into the eye as an artificial drainage system.[38] However, MEMS technology allows miniaturization of pressure sensors for IOP measurement. These unique characteristics of MEMS and microfluidic systems qualify these methods as a remarkable solution with medical applications.
MICROELECTROMECHANICAL SYSTEMS
MEMS technology enables the development of implantable micron-sized sensors to monitor physiological parameters inside the human body. MEMS sensors are light, small, and consume low amounts of energy, which makes them ideal for implantation.[25] They have flexibility in choosing pressure response ranges, bandwidth, sensitivity, and economic costs.[39] MEMS sensors encompass resistive, capacitive, piezoelectric, piezoresistive, electromagnetic, and optical characteristics used in miniature sensors. The sensor can be passive or active based on power harvesting, and the output data of the sensor can be logged, or can be accessed on demand. Due to their ability to provide wireless and on-demand readouts using a resonant circuit, capacitive sensors have been the focus of substantial attention for IOP measurement. However, as resistive and piezoresistive sensors require an electric circuit to read pressure and are coupled to a logging device to save the pressure information, they are bulky.
MEMS sensors can also be classified as invasive or noninvasive. Noninvasive sensors have the advantages of easy placement and removal on the surface of the eye and less foreign body reaction, but they are not able to monitor the absolute IOP due to differences in corneal thickness. While invasive methods provide absolute IOP measurement, they require surgical implantation. Most implantable IOP sensors measure pressure variation using capacitance changes caused by pressure acting on a miniaturized capacitive-sensing chamber. It includes an electric circuit that enables a wireless pressure readout through the resonance frequency of an RLC [a resistor (R), an inductor (L), and a capacitor (C)] circuit, which is affected by the pressure change. The circuit can be integrated into the sclera, while the capacitive MEMS sensor is placed into the anterior chamber, providing a compact size system with no vision loss. A suitable wireless system for this application should provide a precise data readout without any contact with the eye. The working principle of a capacitive IOP sensor is depicted in Figure 2.
Figure 2.

A three-dimensional model of the eye showing an implantable capacitive IOP sensor integrated into the anterior chamber; the antenna of the sensor is placed in the sclera. IOP, intraocular pressure
Another approach for the development of IOP sensors is to use strain gauge sensors. This type of sensor may be integrated into contact lenses to measure variations in eyeball curvature due to IOP. In this method, pressure sensing is affected by corneal thickness and mechanical properties. It requires a power source and a data-logging circuit, resulting in a bulky sensor set.[20] However, it is noninvasive and facilitates telemetry. Figure 3 shows a resistive contact lens IOP sensor.
Figure 3.

Diagram of a contact lens IOP sensor that includes a strain gauge sensor placed circumferentially, an antenna for telemetry and wireless powering, and a microprocessor. IOP, intraocular pressure
Although the above-mentioned methods are prevalent, several other approaches, such as piezoresistive and optical sensors, have also been investigated.
MICROFLUIDICS
Microfluidics is the science and technological application of the manipulation and processing of small amounts of fluids in microchannels.[40] Applications of microfluidics include cell separation and sorting. An early application of microfluidics inside the eye is IOP measurement using the manometer principle, which has been successfully tested in a rabbit model.[15] A representation of this type of sensor is shown in Figure 4.
Figure 4.

An implantable sensor integrating a microfluidic channel and a gas chamber that allows visual IOP readout using a phone camera. IOP, intraocular pressure
The sensor includes a gas chamber connected to a microchannel filled with a colored liquid. The other end of the channel is connected to the anterior chamber. If the IOP increases, the fluid transfers through the microchannel. Therefore, the IOP can be evaluated by measuring fluid displacement. The measuring device can be a mobile camera and a corresponding mobile app used by the patient. A Bourdon tube has also been used for IOP measurement; the tube was implanted without sutures through the cornea and secured on the iris. This sensor measured IOP based on the degree of deformation of compliant spiral-tube structures.[41]
COMPARISON OF MEMS AND MICROFLUIDIC SENSORS
Most IOP sensors are based on MEMS technology. However, several microfluidics-based systems have also been developed. MEMS-based sensors require an external power supply, which increases the complexity of the design to include a wireless or battery power supply and a mechanism to read or record the IOP data. However, microfluidics-based devices require no power and work using the pressure itself, and the IOP readout can be easily obtained. These devices have a low production cost. The ability of microfluidics to manipulate small volumes of fluids is unique, as it could be used to actively reduce the IOP in cases of fluctuation.[38] A brief overview of the ongoing research into continuous IOP monitoring systems is presented in Table 1.
Table 1.
Overview of developed continuous intraocular pressure monitoring sensors

PERSPECTIVE TRENDS
All of the topics discussed here are related to the diagnosis of glaucoma using MEMS and microfluidics technology. The future demand will be for a miniaturized system for both the measurement and treatment of glaucoma using MEMS and microfluidics. Such a system could be a passive artificial drainage mechanism that regulates intraocular pressure. The unique capabilities of microfluidics systems to manipulate small volumes of fluid along with miniaturized MEMS fabrication methods provide a reliable solution for a new generation of implants. Ongoing research in this field is in its infancy, but the advantages are promising. In the near future, an implantable lens embedded with an artificial drainage system may be developed which addresses both glaucoma and cataracts without manipulation of the conjunctiva, to treat glaucoma.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.McCann P, Hogg RE, Fallis R, Azuara-Blanco A. The effect of statins on intraocular pressure and on the incidence and progression of glaucoma: A systematic review and meta-analysis statins and IOP, glaucoma incidence and progression. Invest Ophthalmol Vis Sci. 2016;57:2729–2748. doi: 10.1167/iovs.15-18595. [DOI] [PubMed] [Google Scholar]
- 2.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82:887–888. [PMC free article] [PubMed] [Google Scholar]
- 3.Tham Y, Li X, Wong TY, Quigley HA, Aung T, Cheng C. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Jindal V. Glaucoma A multifactorial disease and its multidimensional management. International Journal of Scientific and Research Publications. 2013;3:1–3. [Google Scholar]
- 5.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38:1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
- 6.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol. 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 7.Buddle R. A day in the life of IOP. Review Optometry. 2014;151:26–32. [Google Scholar]
- 8.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis. J Cataract Refract Surg. 2005;31:146–155. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Varel Ç, Shih Y, Otis BP, Shen TS, Böhringer KF. A wireless intraocular pressure monitoring device with a solder-filled microchannel antenna. J Micromech Microengineering. 2014;24:045012. [Google Scholar]
- 10.Chen PJ, Rodger DC, Saati S, Humayun MS, Tai YC. MEMS. IEEE 21st International Conference on 2008 Jan 13, IEEE; 2008. Implantable parylene-based wireless intraocular pressure sensor. In Micro Electro Mechanical Systems, 2008; pp. 58–61. [Google Scholar]
- 11.Chen P, Rodger DC, Humayun MS, Tai Y. Unpowered spiral-tube parylene pressure sensor for intraocular pressure sensing. Sensors Actuators A Physical. 2006;127:276–282. [Google Scholar]
- 12.Haque RM, Wise KD. In Micro Electro Mechanical Systems (MEMS) IEEE 24th International Conference on 2011 Jan 23, IEEE; 2011. A 3D implantable microsystem for intraocular pressure monitoring using a glass-in-silicon reflow process; pp. 995–998. [Google Scholar]
- 13.Demeng L, Niansong M, Zhaofeng Z. An ultralow power wireless intraocular pressure monitoring system. J Semiconductors. 2014;35:105014. [Google Scholar]
- 14.Lin KM, Sant HJ, Ambati BK, Gale BK. Intraocular pressure sensors: New approaches for real-time intraocular pressure measurement using a purely microfluidic chip. In 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2012. Chemical and Biological Microsystems Society [Google Scholar]
- 15.Araci IE, Su B, Quake SR, Mandel Y. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat Med. 2014;20:1074–1078. doi: 10.1038/nm.3621. [DOI] [PubMed] [Google Scholar]
- 16.Rosengren L, Rangsten P, Bäcklund Y, Hök B, Svedbergh B, Selén G. A system for passive implantable pressure sensors. Sensors Actuators A Physical. 1994;43:55–58. [Google Scholar]
- 17.Stangel K, Kolnsberg S, Hammerschmidt D, Hosticka B, Trieu H, Mokwa W. A programmable intraocular CMOS pressure sensor system implant. IEEE J Solid State Circuits. 2001;36:1094–1100. [Google Scholar]
- 18.Schnakenberg U, Walter P, Vom Bögel G, Krüger C, Lüdtke-Handjery H, Richter H, et al. Initial investigations on systems for measuring intraocular pressure. Sensors Actuators A Physical. 2000;85:287–291. [Google Scholar]
- 19.Leonardi M, Leuenberger P, Bertrand D, Bertsch A, Renaud P. First steps toward noninvasive intraocular pressure monitoring with a sensing contact lens. Invest Ophthalmol Vis Sci. 2004;45:3113–3117. doi: 10.1167/iovs.04-0015. [DOI] [PubMed] [Google Scholar]
- 20.Leonardi M, Pitchon EM, Bertsch A, Renaud P, Mermoud A. Wireless contact lens sensor for intraocular pressure monitoring: Assessment on enucleated pig eyes. Acta Ophthalmol. 2009;87:433–437. doi: 10.1111/j.1755-3768.2008.01404.x. [DOI] [PubMed] [Google Scholar]
- 21.Chiou J, Huang Y, Yeh G. A capacitor-based sensor and a contact lens sensing system for intraocular pressure monitoring. J Micromech Microengineering. 2015;26:015001. [Google Scholar]
- 22.Mansouri K. The road ahead to continuous 24-hour intraocular pressure monitoring in glaucoma. J Ophthalmic Vis Res. 2014;9:260–268. [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KH, Kim BH, Seo YH. A noncontact intraocular pressure measurement device using a micro reflected air pressure sensor for the prediagnosis of glaucoma. J Micromech Microengineering. 2012;22:035022. [Google Scholar]
- 24.Yu L, Kim BJ, Meng E. Chronically implanted pressure sensors: Challenges and state of the field. Sensors. 2014;14:20620–20644. doi: 10.3390/s141120620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clausen I, Glott T. Development of clinically relevant implantable pressure sensors: Perspectives and challenges. Sensors. 2014;14:17686–17702. doi: 10.3390/s140917686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotzar G, Freas M, Abel P, Fleischman A, Roy S, Zorman C, et al. Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials. 2002;23:2737–2750. doi: 10.1016/s0142-9612(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 27.Katuri KC, Ramasubramanian MK, Asrani S. In Mechatronics and Embedded Systems and Applications (MESA) IEEE/ASME International Conference on 2010 Jul 15, IEEE; 2010. A surface micromachined capacitive pressure sensor for intraocular pressure measurement; pp. 149–154. [Google Scholar]
- 28.Bhardwaj V, Rajeshbhai GP. Axial length, anterior chamber depth-a study in different age groups and refractive errors. J Clin Diagn Res. 2013;7:2211–2212. doi: 10.7860/JCDR/2013/7015.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldsmith JA, Li Y, Chalita MR, Westphal V, Patil CA, Rollins AM, et al. Anterior chamber width measurement by high-speed optical coherence tomography. Ophthalmology. 2005;112:238–244. doi: 10.1016/j.ophtha.2004.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruberti JW, Sinha Roy A, Roberts CJ. Corneal biomechanics and biomaterials. Annu Rev Biomed Eng. 2011;13:269–295. doi: 10.1146/annurev-bioeng-070909-105243. [DOI] [PubMed] [Google Scholar]
- 31.Chihara E. Assessment of true intraocular pressure: The gap between theory and practical data. Surv Ophthalmol. 2008;53:203–218. doi: 10.1016/j.survophthal.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Wilensky JT. Diurnal variations in intraocular pressure. Trans Am Ophthalmol Soc. 1991;89:757–790. [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper RL, Beale DG, Constable IJ, Grose GC. Continual monitoring of intraocular pressure: Effect of central venous pressure, respiration, and eye movements on continual recordings of intraocular pressure in the rabbit, dog, and man. Br J Ophthalmol. 1979;63:799–804. doi: 10.1136/bjo.63.12.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang G. Design challenges of implantable pressure monitoring system. Front Neurosci. 2010;4:2. doi: 10.3389/neuro.20.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrasco FG, Alonso DD, Niño-de-Rivera L. Biocompatibility and implant of a less invasive intraocular pressure sensor. Microelectronic Engineering. 2016;159:32–37. [Google Scholar]
- 36.Mark T. Medical electrical equipment Part 1: General requirements for basic safety and essential performance. 2005 [Google Scholar]
- 37.International Organization for Standardization. ISO 14971: medical devices-application of risk management to medical devices. ISO. 2000 [Google Scholar]
- 38.Gunn NM, inventor; Novartis Ag., assignee. Osmotically actuated fluidic valve. United States patent US 9,572,712. 2017 Feb 21. [Google Scholar]
- 39.Abeysinghe DC, Dasgupta S, Boyd JT, Jackson HE. A novel MEMS pressure sensor fabricated on an optical fiber. IEEE Photon Technol Lett. 2001;13:993–995. [Google Scholar]
- 40.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 41.Chen P, Rodger DC, Agrawal R, Saati S, Meng E, Varma R, et al. Implantable micromechanical parylene-based pressure sensors for unpowered intraocular pressure sensing. J Micromech Microengineering. 2007;17:1931. [Google Scholar]
- 42.Rendón-Nava A, Nino-de-Rivera-yO L. Intraocular pressure sensor design. In Electrical and Electronics Engineering. 3rd International Conference on 2006 Sep 6, IEEE; 2006. pp. 1–4. [Google Scholar]
- 43.Todani A, Behlau I, Fava MA, Cade F, Cherfan DG, Zakka FR, et al. Intraocular pressure measurement by radio wave telemetry. Invest Ophthalmol Vis Sci. 2011;52:9573–9580. doi: 10.1167/iovs.11-7878. [DOI] [PubMed] [Google Scholar]
- 44.Kouhani MH, Weber A, Li W. InMicro Electro Mechanical Systems (MEMS) IEEE 30th International Conference on 2017 Jan 22, IEEE; 2017. Wireless intraocular pressure sensor using stretchable variable inductor; pp. 557–560. [Google Scholar]
- 45.Zeng P, Cui Q, Wu M, Chen PY, Cheng MM. Wireless and continuous intraocular pressure sensors using transparent graphene. In SENSORS, 2016 IEEE 2016 Oct 30 (pp. 1-3) IEEE [Google Scholar]
- 46.Yeh GT, Wu TW, Tsai SW, Hsu SH, Chiou JC. Toward a wireless contact lens sensor system with a micro-capacitor for intraocular pressure monitoring on in-vitro porcine eye. In SENSORS, 2015 IEEE 2015 Nov 1 (pp 1-4) IEEE [Google Scholar]
- 47.Shin K, Jang C, Kim MJ, Yun K, Park KH, Kang JY, et al. Development of novel implantable intraocular pressure sensors to enhance the performance in in vivo tests. J Microelectromech Syst. 2015;24:1896–1905. [Google Scholar]
- 48.Chen G, Chan I, Leung LK, Lam DC. Soft wearable contact lens sensor for continuous intraocular pressure monitoring. Med Eng Phys. 2014;36:1134–1139. doi: 10.1016/j.medengphy.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Chitnis G, Maleki T, Samuels B, Cantor LB, Ziaie B. A minimally invasive implantable wireless pressure sensor for continuous IOP monitoring. IEEE Trans Biomed Eng. 2013;60:250–256. doi: 10.1109/TBME.2012.2205248. [DOI] [PubMed] [Google Scholar]
- 50.Huang YC, Yeh GT, Yang TS, Chiou JC. A contact lens sensor system with a micro-capacitor for wireless intraocular pressure monitoring. In SENSORS, 2013 IEEE 2013 Nov 3 (pp 1-4) IEEE [Google Scholar]
- 51.Bhamra H, Tsai JW, Huang YW, Yuan Q, Irazoqui P. In Solid-State Circuits Conference (ISSCC) IEEE International 2017 Feb 5, IEEE; 2017. 21.3 A sub-mm 3 wireless implantable intraocular pressure monitor microsystem; pp. 356–357. [DOI] [PubMed] [Google Scholar]
- 52.Donida A, Di Dato G, Cunzolo P, Sala M, Piffaretti F, Orsatti P, et al. A circadian and cardiac intraocular pressure sensor for smart implantable lens. IEEE Trans Biomed Circuits Syst. 2015;9:777–789. doi: 10.1109/TBCAS.2015.2501320. [DOI] [PubMed] [Google Scholar]
- 53.Ghannad-Rezaie M, Gulari MN, de Melo Franco R, Mian SI, Chronis N. In Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII) Transducers & Eurosensors XXVII: The 17th International Conference on 2013 Jun 16, IEEE; 2013. A powerless optical microsensor for monitoring intraocular pressure with keratoprostheses; pp. 2708–2711. [Google Scholar]
- 54.Bello SA, Malavade S, Passaglia CL. Development of a smart pump for monitoring and controlling intraocular pressure. Ann Biomed Eng. 2017;45:990–1002. doi: 10.1007/s10439-016-1735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Araci IE, Baday M. Contact lens with a microfluidic channel to monitor radius of curvature of cornea. 2016 [Google Scholar]


