Abstract
The purpose of this review is to examine whether a contribution of social exclusion to the pathogenesis of psychosis is compatible with the dopamine hypothesis and/or the neurodevelopmental hypothesis. Humans experience social exclusion as defeating. An animal model for defeat is the resident-intruder paradigm. The defeated animal shows evidence of an increased sensitivity to amphetamine, increased dopamine release in the nucleus accumbens and prefrontal cortex, and increased firing of dopaminergic neurons in the ventral tegmental area. As for humans, one study showed that amphetamine-induced striatal dopamine release was significantly greater among nonpsychotic young adults with severe hearing impairment than among normal hearing controls. Two other studies reported an association between childhood trauma and increased dopamine function in striatal subregions. Several studies have suggested that the perigenual anterior cingulate cortex (pgACC) may play a role in the processing of social stress. Importantly, the pgACC regulates the activity of the ventral striatum through bidirectional interconnections. We are not aware of studies in humans that examined whether (proxies for) social exclusion contributes to the structural brain changes present at psychosis onset. Animal studies, however, reported that long-term isolation may lead to reductions in volume of the total brain, hippocampus, or medial prefrontal cortex. Other animal studies reported that social defeat can reduce neurogenesis. In conclusion, the answer to the question as to whether there are plausible mechanisms whereby social exclusion can contribute to the pathogenesis of psychosis is cautiously affirmative.
Keywords: dopamine, neurodevelopment, social exclusion, social defeat, etiology, schizophrenia, psychosis, pathogenesis
Introduction
Although social exclusion is a common consequence of psychosis, several epidemiological findings suggest that social exclusion is also a co-participating cause. Indeed, there are consistent reports of increased risks for migrants from developing countries, members of discriminated ethnic minority groups, subjects raised in urban areas, and individuals with a low IQ, a hearing impairment, or a history of victimization in childhood.1
The first attempt to find a common denominator for these findings is the social defeat hypothesis of psychosis.2–5 This hypothesis posits that social defeat, defined as “subordinate position or outsider status”2,3 or as “the negative experience of being excluded from the majority group,”4,5 leads to an increased baseline activity and/or sensitization of the mesolimbic dopamine system and that these dopamine changes, in turn, place the individual at an increased risk of developing the disorder. Because a prevailing definition of social exclusion is “an enforced lack of social participation,” the concepts of social exclusion and social defeat overlap.6 The authors of the social defeat hypothesis emphasized that the variable of interest in their hypothesis is a subjective experience, which depends on the subject’s interpretation of events.
An important model for social defeat stress in animals is the resident-intruder paradigm, whereby a male rodent (the intruder) is placed into the home cage of another male (the resident). The intruder is aggressively attacked and forced to display submissive behavior. This experience is not equivalent to the experience of social defeat in humans, because the defeat of the intruder is aimed at establishing a hierarchy, not at social exclusion. By social isolation of the animal after defeat, the experience of the animal would resemble more the experience of social exclusion in humans. Nonetheless, the resident-intruder paradigm is relevant for our understanding of the experience of social exclusion in humans, because humans experience social exclusion as defeating.
The principal question for this article is whether there are any plausible neurobiological mechanisms whereby social exclusion could lead to the development of a psychosis. Because this is a question about pathogenesis, not etiology, one could reformulate the question as to whether a contribution of social exclusion is compatible with the existing knowledge about pathogenesis. The pathogenic mechanisms of psychoses, however, are uncertain and a large number of hypotheses have been proposed. Consequently, for practical purposes, this review examined whether a contribution of social exclusion is compatible with 3 important hypotheses on pathogenesis discussed in authoritative reviews: the social-cognition hypothesis, the dopamine hypothesis, and the neurodevelopmental hypothesis.1,7
Briefly, the social-cognition hypothesis posits that an impaired capacity to mentalize, ie, to understand one’s own and others’ behavior in terms of mental states, such as intentions, wishes, beliefs, and emotions, plays an important role in the development of psychosis.1 The dopamine hypothesis, version III, postulates that multiple “hits” interact to result in dopamine dysregulation, the final common pathway to psychosis.8 Finally, the neurodevelopmental hypothesis posits that nonaffective psychotic disorder is due to an abnormal brain development, already manifest in childhood and youth, on account of delays in motor, social and intellectual development, and later, at disease onset, in structural brain deficits.
As for this review, we included important studies that used concepts related to social exclusion or social defeat, such as discrimination, negative social evaluation, social adversity, social fragmentation, or social disadvantage.
Impaired Social Cognition
The concepts of social cognition, theory of mind, and mentalizing capacity are closely related. Because positive symptoms often involve misinterpretations of behavior observed in others, it is conceivable that processes that interfere with the normal acquisition of mentalizing ability increase the risk for psychosis. Because the big start in the development of mentalizing is made in relationship with the mother and during preschool years, neglect and abuse during these years are particularly harmful.9 However, the development of mentalizing capacity continues in later years, in relation with peers, teachers, and other members of society. The evidence to support the notion that social exclusion interferes with this development comes from studies showing delays in the development of mentalizing in children with a severe hearing impairment (SHI).10 Furthermore, it is conceivable that growing up as a member of a discriminated ethnic minority is damaging to the capacity to correctly infer the intentions of others, in particular those from the dominant group.11 The focus of this review, however, is on biological mechanisms.
Dopamine Dysregulation
There are consistent reports of increased dopamine synthesis capacity, increased dopamine release, and increased baseline synaptic dopamine concentrations.12 An important topic here is sensitization, a process whereby repeated exposures to a given stimulus results in an enhanced response at subsequent exposure, in this example excess release of dopamine. Although there is a conspicuous lack of longitudinal studies in humans, the findings suggest that the mesolimbic dopamine system is sensitized. The question here is whether social exclusion, or exposures to stimuli related to social exclusion, leads to increased dopamine synthesis capacity, increased dopamine release, increased baseline synaptic dopamine concentrations, and/or sensitization of the dopamine system.
Animal studies provide ample evidence for this. Firstly, after one or more episodes of defeat within the resident-intruder paradigm, the defeated animal shows evidence of an increased sensitivity to amphetamine (ie, increased locomotor activity), increased dopamine release in the nucleus accumbens (part of the ventral striatum [VS]) and prefrontal cortex (homologous to the medial prefrontal cortex [mPFC] in humans), and increased firing of dopaminergic neurons in the ventral tegmental area (VTA).13–16 Lengthy social isolation after the defeat amplifies the changes in dopamine activity, whereas return to the group mitigates them.17 Secondly, an interesting experiment in cynomolgus macaques showed that a return to the social group after individual housing produced an increase in the amount or availability of dopamine D2 receptors in the dominant monkeys, not in the subordinate ones.18 These findings suggest that place in hierarchy could influence the dopaminergic system. Thirdly, rat pups reared in isolation following weaning, develop, in adulthood, increased striatal synaptic dopamine concentrations and increased striatal dopamine release in response to cocaine or amphetamine.19,20
As for humans, neuroreceptor imaging studies have assessed the impact of hearing impairment and childhood trauma on dopamine function in nonpsychotic individuals. Gevonden et al21 used Single Photon Emission Computed Tomography (SPECT) to compare dopamine function in young adults with a SHI to that in healthy controls. The participants underwent 2 SPECT scans with the dopamine D2/3 antagonist [123I]iodobenzamide, which is, like [11C]raclopride, sensitive to detect changes in synaptic dopamine concentrations after an amphetamine challenge. There were no significant differences in baseline striatal D2/3 receptor binding. However, amphetamine-induced striatal dopamine release was significantly greater among the participants with SHI than among the healthy controls. Reports of social exclusion were not associated with dopamine release after amphetamine, perhaps because self-reports of this phenomenon are biased by a tendency to give socially desirable replies.
Oswald et al22 exposed a general population sample of young adults to 2 [11C]raclopride Positron Emission Tomography (PET) scans. The first scan was preceded by saline, the second by amphetamine. The results showed a positive association between reports of childhood trauma and baseline D2/3 receptor availability in the VS of males, not in females. Further, there was a positive association between trauma and amphetamine-induced dopamine release in the VS.
Egerton et al23 used [18F]-DOPA PET to investigate the impact of childhood adversity on individuals at ultra-high risk for psychosis and healthy volunteers. The results showed that sexual and physical abuse (Cohen’s d = 0.75) and unstable family arrangements (d = 0.86) were associated with increased dopamine synthesis capacity in the associative striatum. Interestingly, there was no relationship between dopamine synthesis and events (ie, parental loss or separation) that do not necessarily involve intentional harm to the child.
To summarize, the findings of the 2 studies on baseline D2/3 receptor binding were mixed. Further, although the use of a cross-sectional design does not permit definitive conclusions, the results on dopamine release and synthesis are compatible with the idea of dopamine sensitization. Future studies could examine other groups with a history of exposure to social exclusion (eg, immigrants from developing countries or subjects with a nonheterosexual orientation), preferably using a longitudinal design. Ideally, the first assessment is made before the exposure (eg, when the migrant arrives in the new country), the second thereafter (eg, after the migrant has spent several years in the country of destination). Without the use of a longitudinal design, it remains uncertain when the sensitization has developed.
Neural Mechanisms in Humans Linking Social Exclusion and Stress to Dopamine Dysfunction
The dopaminergic VS receives inputs from the VTA, the mPFC, anterior cingulate cortex (ACC), amygdala, and hippocampus, which are critical for salience and reward signaling. These circuits are strongly implicated in social cognition.1
Three networks center on subregions of the amygdala,24 a key structure for the integration of emotion and social processing. A social-perceptive network connects ventrolateral amygdala (lateral nucleus, which receives rapid sensory inputs across all modalities) to sensory association areas of the temporal cortex and the orbitofrontal cortex (OFC). It has been implicated in decoding and interpreting social signals from others in the context of past experience and current goals. A social-affiliative network is anchored in the medial sector of the amygdala, which contains nuclei (especially the basal nucleus) that share anatomical connections with mesolimbic, reward-related areas of the ventromedial prefrontal cortex, medial temporal lobe, ventromedial striatum, and hypothalamus. This network relates to appetitive, prosocial and trusting interactions. A social aversion network is centered on the dorsal sector of the amygdala, which contains nuclei (such as the central nucleus) that project to dorsal ACC, insula, ventrolateral striatum, hypothalamus, and brainstem. These regions are implicated in fear, fright or flight, and avoidant behavior.
Two other networks are relevant. The mentalizing network connects mPFC with the temporoparietal junction, superior temporal sulcus, precuneus, and anterior temporal lobe (ATL). It serves social reasoning, social knowledge, actively thinking about others, reflecting on oneself, and theory of mind. It overlaps with the so-called default mode or resting-state network. Finally, the empathy and mirror network is engaged when vicariously experiencing states (such as pain) of others or in action observation and includes parts of the dorsal cingulate and anterior insula.
Recent work has shown that social risk factors such as socioeconomic status,25 urban upbringing and living,26 and ethnic minority status27 have convergent effects on social stress processing in a neural system centered on perigenual anterior cingulate cortex (pgACC)28 (figure 1).
Fig. 1.
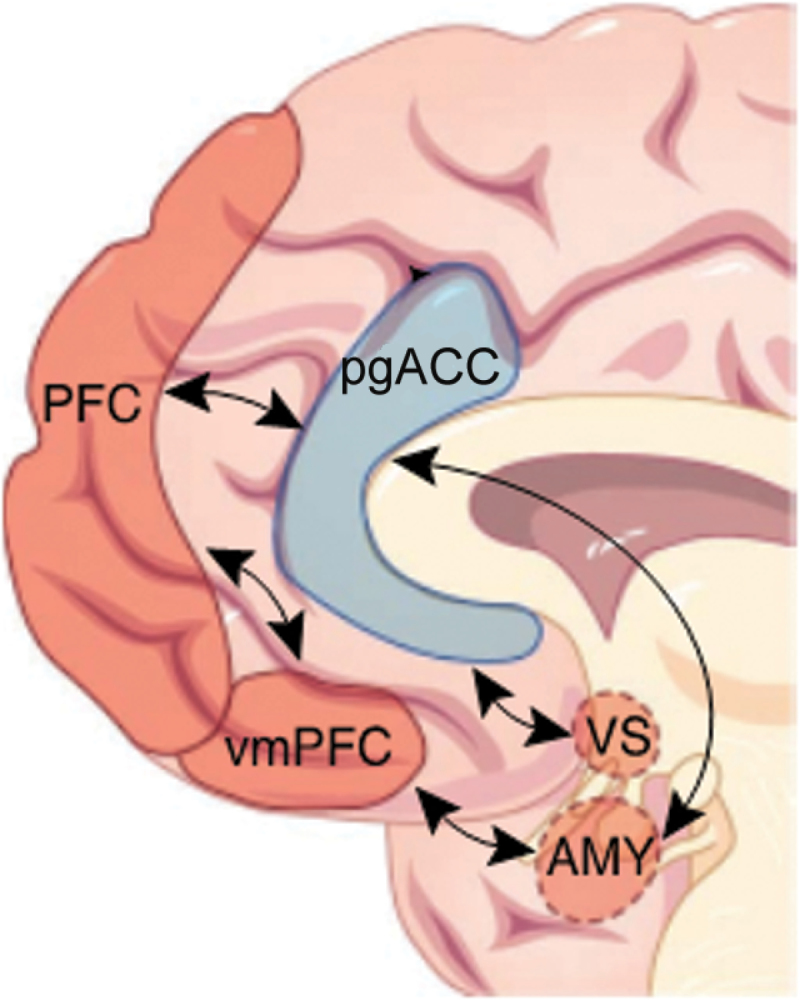
A circuit related to ethnic minority risk for mental illness. At the core of the proposed mechanism is pgACC (blue), which regulates subcortical structures such as ventral striatum (VS) and amygdala (AMY) and is in turn modulated by ventromedial prefrontal cortex (vmPFC) and medial prefrontal cortex (PFC) regions. From Meyer-Lindenberg and Tost,29 with permission. pgACC, perigenual anterior cingulate cortex.
We have also implicated the pgACC in the regulation of the human hypothalamic-pituitary-adrenal stress response system itself.30 Furthermore, genome-wide significant common31 and rare32 susceptibility genes for psychosis impact the same network. Taken together, these data suggest a central role for a stress-associated convergent “risk circuit” for psychosis29: at the center of this network is pgACC, which regulates key limbic structures such as amygdala, hippocampus, and VS and in turn participates in regulatory interactions with higher-order lateral and mPFC structures such as Brodmann’s areas 46 and 10. Taken together with the animal experimental evidence, this would suggest that striatal dopaminergic dysfunction is a consequence of prefrontal dysregulation of that region, in part through the strong bidirectional interactions between pgACC and VS, a hypothesis that could be tested using hybrid PET-fMRI.
Recent advances in social neuroscience link these networks up more specifically with processes linked to social exclusion in humans: prejudice and stereotype.33,34 The social neuroscience of prejudice, or (usually negative) attitudes and emotional reactions to individuals based on (out) group membership, maps across several networks. Prominently implicated is the social aversion network, where amygdala supports threat-based associations and anterior insula signals negative affect (which often accompanies a prejudiced response). In the social-perceptive network, amygdala signals initial responses to salient positive or negative cues, including cues regarding group membership. The mPFC, a prominent component of both the social-affiliative and mentalizing networks, is engaged more strongly toward in-group than out-group members. Finally, appetitive responses such as positive attitudes and approach-related behavioral tendencies, which are often expressed toward in-group members, map on the striatum. Stereotypes (characteristics ascribed to a social group through (over)generalization) are likely represented in the ATL, which feeds into the mPFC. In this way, social stereotypes are thought to influence social attributions in dorsal mPFC activity. The interaction of these networks is critical for integrated social behavior. Neural projections from the amygdala and insula to the mPFC may support the integration of affective responses with mentalizing and empathy processes. Amygdala and OFC connect to the ATL via the uncinate fasciculus to support bidirectional interactions between affective responses and stereotype concepts. Signals from amygdala, insula, striatum, OFC, and ATL converge in regions of the mPFC, where information seems to be integrated in support of elaborate person representations. Finally, the joint influences of prejudiced affect and stereotype concepts on behavior are likely to converge in the dopaminergic striatum, which receives inputs from the amygdala, OFC, mPFC, lateral PFC, rostral ACC, ATL, and midbrain.
Abnormal Neurodevelopment
The central question, here, is whether social exclusion contributes to (1) delays in motor, social, and intellectual development, or to (2) structural brain deficits at the onset of psychosis.
As for the first question, the relationship between social exclusion and these developmental delays may be circular. While nobody will doubt that motor, social, and cognitive delays may lead to social exclusion and that exposure to social exclusion contributes to an impaired development of social skills, there is more debate on the issue as to whether the experience of social exclusion contributes to an impaired motor and intellectual development.35 A consideration of this interesting discussion, however, is beyond the scope of this article.
An answer to the second question, as to whether the human experience of social exclusion contributes to the grey matter reduction, white matter disruption, or ventricular enlargement observed at the onset of schizophrenia, is also uncertain. There have been reports of a decreased pgACC volume in males raised in cities36 and in male second-generation immigrants,37 but no studies of the relationship between (proxies for) social exclusion and widespread grey matter deficits, ventricular enlargement, or white matter disruption.
Some animal studies, however, did examine whether long-term social isolation or exposure to social defeat leads to the aforementioned brain changes. Fabricius et al38 applied stereological volume estimates to rats isolated after weaning and found that isolated males had significantly smaller brains and smaller hippocampi than group-housed controls and larger ventricles than controls. However, this was not seen in female rats. Schubert et al39 applied magnetic resonance volumetry to the limbic system of isolated rats and observed no volume reduction in the hippocampi, but a 5% reduction in the volume of the mPFC, a region that is strongly directly and indirectly (through ACC) connected to the VS.
Although it is uncertain whether decreased neurogenesis plays a role in the pathogenesis of psychosis, it is of interest that social defeat affects this phenomenon. Czeh et al40 exposed adult rats to 5 weeks of daily social defeat and found that this led to decreased gliogenesis in the mPFC and to decreased neurogenesis in the gyrus dentatus, while there was only a minor impact on nonlimbic structures. Other studies showed that acute social defeat stress suppressed hippocampal cell proliferation transiently up to 50%–75%,41 while chronic defeat resulted in a more subtle decrease of only 29%–33%.42,43 Lack of neutrophil support and impaired neuronal vascular supply have been offered as explanations.44 Interestingly, several postmortem studies have described decreased numbers of hippocampal neurons in schizophrenia patients.45
Taken together, these results indicate that it is important to examine whether social exclusion contributes to the development of the anatomic changes already present at the time of the first psychotic episode. For example, one could compare the development of the brain over years between excluded and nonexcluded adolescents. Adolescents can be excluded on account of various features, such as an ethnic or sexual minority status, a hearing impairment, or an odd appearance.
Conclusion
There is evidence to suggest that social exclusion has an impact on human dopaminergic functioning and thereby influences the risk of developing psychosis. Studies of animals suggest that it is important to examine whether social exclusion contributes to the abnormal brain development in nonaffective psychotic disorder. Consequently, future studies, preferentially with a longitudinal design, should examine dopaminergic functioning and structural brain development in various socially excluded groups.
Acknowledgment
The authors thank Elizabeth Cantor-Graae for advice. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 2. Selten JP, Cantor-Graae E. Social defeat: risk factor for schizophrenia? Br J Psychiatry. 2005;187:101–102. [DOI] [PubMed] [Google Scholar]
- 3. Selten JP, Cantor-Graae E. Hypothesis: social defeat is a risk factor for schizophrenia? Br J Psychiatry Suppl. 2007;51:s9–s12. [DOI] [PubMed] [Google Scholar]
- 4. Selten JP, van der Ven E, Rutten BP, Cantor-Graae E. The social defeat hypothesis of schizophrenia: an update. Schizophr Bull. 2013;39:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Selten JP, van Os J, Cantor-Graae E. The social defeat hypothesis of schizophrenia: issues of measurement and reverse causality. World Psychiatry. 2016;15:294–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morgan C, Burns T, Fitzpatrick R, Pinfold V, Priebe S. Social exclusion and mental health: conceptual and methodological review. Br J Psychiatry. 2007;191:477–483. [DOI] [PubMed] [Google Scholar]
- 7. Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colvert E, Rutter M, Kreppner J, et al. Do theory of mind and executive function deficits underlie the adverse outcomes associated with profound early deprivation?: findings from the English and Romanian adoptees study. J Abnorm Child Psychol. 2008;36:1057–1068. [DOI] [PubMed] [Google Scholar]
- 10. Peterson CC, Siegal M. Deafness, conversation and theory of mind. J Child Psychol Psychiatry. 1995;36:459–474. [DOI] [PubMed] [Google Scholar]
- 11. Branscombe NR, Schmitt MT. Perceiving pervasive discrimination among African Americans: implications for group identification and well-being. J Personality Soc Psychol. 1999;77:135–149. [Google Scholar]
- 12. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. [DOI] [PubMed] [Google Scholar]
- 14. de Jong JG, Wasilewski M, van der Vegt BJ, Buwalda B, Koolhaas JM. A single social defeat induces short-lasting behavioral sensitization to amphetamine. Physiol Behav. 2005;83:805–811. [DOI] [PubMed] [Google Scholar]
- 15. Trainor BC. Stress responses and the mesolimbic dopamine system: social contexts and sex differences. Horm Behav. 2011;60:457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isovich E, Engelmann M, Landgraf R, Fuchs E. Social isolation after a single defeat reduces striatal dopamine transporter binding in rats. Eur J Neurosci. 2001;13:1254–1256. [DOI] [PubMed] [Google Scholar]
- 18. Morgan D, Grant KA, Gage HD, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. [DOI] [PubMed] [Google Scholar]
- 19. Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33:13–29. [DOI] [PubMed] [Google Scholar]
- 20. Kosten TA, Zhang XY, Kehoe P. Chronic neonatal isolation stress enhances cocaine-induced increases in ventral striatal dopamine levels in rat pups. Brain Res Dev Brain Res. 2003;141:109–116. [DOI] [PubMed] [Google Scholar]
- 21. Gevonden M, Booij J, van den Brink W, Heijtel D, van Os J, Selten JP. Increased release of dopamine in the striata of young adults with hearing impairment and its relevance for the social defeat hypothesis of schizophrenia. JAMA Psychiatry. 2014;71:1364–1372. [DOI] [PubMed] [Google Scholar]
- 22. Oswald LM, Wand GS, Kuwabara H, Wong DF, Zhu S, Brasic JR. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology (Berl). 2014;231:2417–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egerton A, Valmaggia LR, Howes OD, et al. Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophr Res. 2016;176:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci. 2012;32:14729–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lederbogen F, Kirsch P, Haddad L, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. [DOI] [PubMed] [Google Scholar]
- 27. Akdeniz C, Tost H, Streit F, et al. Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry. 2014;71:672–680. [DOI] [PubMed] [Google Scholar]
- 28. Tost H, Champagne FA, Meyer-Lindenberg A. Environmental influence in the brain, human welfare and mental health. Nat Neurosci. 2015;18:1421–1431. [DOI] [PubMed] [Google Scholar]
- 29. Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–668. [DOI] [PubMed] [Google Scholar]
- 30. Boehringer A, Tost H, Haddad L, et al. Neural correlates of the cortisol awakening response in humans. Neuropsychopharmacology. 2015;40:2278–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Erk S, Meyer-Lindenberg A, Schnell K, et al. Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch Gen Psychiatry. 2010;67:803–811. [DOI] [PubMed] [Google Scholar]
- 32. Stefansson H, Meyer-Lindenberg A, Steinberg S, et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505:361–366. [DOI] [PubMed] [Google Scholar]
- 33. Amodio DM. The neuroscience of prejudice and stereotyping. Nat Rev Neurosci. 2014;15:670–682. [DOI] [PubMed] [Google Scholar]
- 34. Kubota JT, Banaji MR, Phelps EA. The neuroscience of race. Nat Neurosci. 2012;15:940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baumeister RF, Twenge JM, Nuss CK. Effects of social exclusion on cognitive processes: anticipated aloneness reduces intelligent thought. J Pers Soc Psychol. 2002;83:817–827. [DOI] [PubMed] [Google Scholar]
- 36. Haddad L, Schäfer A, Streit F, et al. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia. Schizophr Bull. 2015;41:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akdeniz C, Schäfer A, Streit F, et al. Sex/dependent association of perigenual anterior cingulate cortex volume and migration background, an environmental risk factor for schizophrenia [published online ahead of print October 8, 2016]. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fabricius K, Helboe L, Steiniger-Brach B, Fink-Jensen A, Pakkenberg B. Stereological brain volume changes in post-weaned socially isolated rats. Brain Res. 2010;1345:233–239. [DOI] [PubMed] [Google Scholar]
- 39. Schubert MI, Porkess MV, Dashdorj N, Fone KC, Auer DP. Effects of social isolation rearing on the limbic brain: a combined behavioral and magnetic resonance imaging volumetry study in rats. Neuroscience. 2009;159:21–30. [DOI] [PubMed] [Google Scholar]
- 40. Czeh B, Müller-Keuker JI, Rygula R, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. [DOI] [PubMed] [Google Scholar]
- 41. Lagace DC, Donovan MH, DeCarolis NA, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Czéh B, Michaelis T, Watanabe T, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Czéh B, Welt T, Fischer AK, et al. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52:1057–1065. [DOI] [PubMed] [Google Scholar]
- 44. Hammels C, Pishva E, De Vry J, et al. Defeat stress in rodents: From behavior to molecules. Neurosci Biobehav Rev. 2015;59:111–140. [DOI] [PubMed] [Google Scholar]
- 45. Falkai P, Malchow B, Wetzestein K, et al. Decreased oligodendrocyte and neuron number in anterior hippocampal areas and the entire hippocampus in schizophrenia: a stereological postmortem study. Schizophr Bull. 2016;42(suppl 1):S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]


