Abstract
Objectives
The injured anterior cruciate ligament (ACL) is thought to exhibit an impaired healing response, and attempts at surgical repair have not been successful. Connective tissue growth factor (CTGF) is reported to be associated with wound healing, probably through transforming growth factor beta 1 (TGF-β1).
Methods
A rabbit ACL injury model was used to study the effect of CTGF on ligament recovery. Quantitative real-time PCR (qRT-PCR) was performed for detection of changes in RNA levels of TGF-β1, type 1 collagen (COL1), type 2 collagen (COL2), SRY-related high mobility group-box gene9 (SOX9), tissue inhibitor of metalloproteinase-1 (TIMP-1) and matrix metallopeptidase 13 (MMP-13). Expression of related proteins was detected by Western blotting.
Results
The current study showed that CTGF could promote the recovery of an injured anterior cruciate ligament. It can upregulate mRNA and expression of TGF-β1, COL1, COL2, SOX9, and tissue inhibitor of TIMP-1, and downregulate mRNA and expression of MMP-13, suggesting that the curative effect of CTGF on injured rabbit ligaments is through regulation of these cellular factors.
Conclusions
This finding revealed the healing role of CTGF in injured tissues and provides new possibilities of treating injured tissues and wound healing by using CTGF.
Cite this article: X. Sun, W. Liu, G. Cheng, X. Qu, H. Bi, Z. Cao, Q. Yu. The influence of connective tissue growth factor on rabbit ligament injury repair. Bone Joint Res 2017;6:399–404. DOI: 10.1302/2046-3758.67.BJR.2016-0255.R1.
Keywords: Anterior cruciate ligament, Connective tissue growth factor, TGF-β1
Article focus
Our study aimed to determine whether connective tissue growth factor improves ligament recovery in a rabbit anterior cruciate ligament injury model.
Key messages
Our results suggest that connective tissue growth factor could promote the recovery of injured anterior cruciate ligament.
Strengths and limitations
Strengths: This finding revealed the healing role of CTGF in injured tissues and provides new possibilities of treating injured tissues and wound healing by using CTGF.
Limitations: further study required considering the effects on knee injuries caused by the ACL.
Introduction
The anterior cruciate ligament (ACL) has long been seen as the primary passive restraint to anterior translation of the tibia with respect to the femur.1 Furthermore, ACL contributes to knee rotational stability in both frontal and transverse planes because of its specific orientation.2,3 It has been the focus of extensive biomechanical/anatomical research, and is one of the most frequently studied structures of the human musculoskeletal system over the past decades. ACL injuries are one of the most common and devastating knee injuries and are mainly sustained as a result of participation in sports.4
It was known that ACL had poor healing capacity, with a substantially high rate of failure (40% to 100%), even after surgical repair using suture.5,6 The reconstruction of ACL has remained the benchmark of care for ACL injuries, especially for young individuals, and some athletes who aim to return to high-level sporting activities.4,7 However, surgical treatment of ACL injury remains expensive, with variable outcomes,8 and patients often do not return to their pre-injury activity level.9 New treatment methods for ACL injury are to be explored, aiming at higher efficiency and lower cost. One potential method uses connective tissue growth factor (CTGF), which has been shown to play an important role in many biological processes such as cell adhesion, migration, proliferation, angiogenesis, skeletal development, and tissue wound repair, and is also critically involved in treating fibrotic disease and several forms of cancers.10,11
CTGF, also known as CCN family member 2 (CCN2),10,11 is a matricellular protein of the CCN family of extracellular matrix-associated heparin-binding proteins.12,13 CTGF is known to act in cell adhesion, migration, proliferation, angiogenesis, vascular differentiation and myofibroblast formation, all of which can lead to tissue remodelling and changes in organ structure.14 It has also been reported that CTGF is associated with wound healing and virtually all fibrotic pathology.13,15 Recently, it has been found that TGF-β1, associated with the increased expression of CTGF, induces the hypertrophy of the ligamentum flavum (LF) through the p38 MAPK pathway.16 CTGF has also been reported to regulate TGF-β1 in the TGF-β1-induced invasion and migration of hepatocellular carcinoma,17 so it is reasonable to consider the possibility that CTGF is also associated with TGF-β1 in ACL healing.
To investigate the healing effect of CTGF on ACL injury, and to elucidate the mechanism of this effect, we constructed an ACL injury model with rabbits and considered some TGF-β1-associated cellular factors involved in tissue regeneration and wound recovery. It showed that CTGF could promote the recovery of injured anterior cruciate ligament. It can upregulate the mRNA and expression of TGF-β1, type 1 collagen (COL1), type 2 collagen (COL2) and SOX9, as well as the tissue inhibitor of metalloproteinase-1 (TIMP-1), and downregulate the mRNA and expression of Matrix metalloproteinase 13 (MMP-13). These results confirmed the curative effect of CTGF on injured rabbit ACL, and that the mechanism comes via the regulation of these TGF-β1-associated cellular factors.
Materials and Methods
A total of 30 healthy male New Zealand White rabbits were obtained from the Medical Experimental Animal Center of Guangdong Province at the age of six months. The animals were housed in a specific-pathogen-free (SPF) animal housing facility at our hospital, which was controlled at a temperature of 22°C to 24°C with 50% to 60% humidity. The animals had access to food and water before being used in experiments. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Yantaishan Hospital.
Anterior cruciate ligament surgery
The rabbits were provided with water but no food for 12 hours before the surgery. 160 000 units of penicillin were administered through buttock injections to prevent bacterial infection before surgery. Using anaesthetic with an intravenous injection of 3% pentobarbital sodium solution (30 mg/kg), the rabbits were secured on the operating table, and the left hind leg of the rabbit was shaved, disinfected and draped. A longitudinal incision about 3 cm in length was made at the medial border of the patellar tendon to expose the knee joint. The fascia and the muscle were carefully separated, and the wound was washed with saline to prevent it from drying out. Half of the anterior cruciate ligament was cut, while the other half remained connected to the tibia and femur.
The 30 New Zealand White rabbits were randomly divided into two groups: A (control group) and B (treatment group), each with 15 rabbits. Rabbits in group A were given 0.5 ml fibrin gel which was injected between the bone and ligament, near the entrance of the femoral tunnel. Rabbits in group B were given 0.5 ml fibrin gel containing 15 ng CTGF, which was injected into the same position. The wound was then sutured in layers and cleaned with iodine, followed by wrapping in bondage.
Post-operative care
The rabbits were given penicillin at a dose of 160 000 units per day for three continuous days after the surgery to prevent infection in the operated knee, which was immobilised in extension using an elastic bandage for a period of five days post-operatively. All rabbits could move freely and resumed normal activity two days after the surgery. General examination of these animals was performed daily to detect any clinical sign of pain or other complications such as anorexia, abnormal cry, decreased activity, or leg-dressing problems. Dermal sutures were removed seven days after surgery.
Anatomical observation
Two weeks after the operation, the rabbits were killed by air embolism at the edge of the ear vein, and the knee joints of groups A (control) and B (treatment) rabbits were obtained for examination.
Specimen collection
In order to determine the blood concentration of basic fibroblast growth factor (bFGF) and TGF-β1, 1 cc ear vein blood was collected from rabbits in each group on the 15th day following model construction. The supernatants in the blood samples were collected after high-speed centrifugation, and the levels of bFGF and TGF-β1 were determined using the appropriate ELISA kits (R&D Systems, Inc., Minneapolis, Minnesota), following the manufacturer’s instructions.
Quantitative real-time PCR
The rabbits in both groups were killed by air embolism on the 15th day following surgery. Six of the 15 rabbits in each group were selected for further analysis. The ligament tissue samples were isolated and frozen in liquid nitrogen (N2). At the appropriate point in the experiment, they were removed from the liquid N2, washed with phosphate-buffered saline (PBS) and sliced into small pieces. Total RNA (2 μg) was extracted using ISOGEN (Nippon Gene, Tokyo, Japan) and underwent real-time (RT)-PCR. Quantitative RT-PCR was performed with the IQ5 System (Bio-Rad, Hercules, California). PCR reactions and SYBR Green Real-Time PCR Master were performed in 25 μl solutions Mix (Toyobo Co. Ltd, Osaka, Japan) and 0.2 μmol/L specific primers. Primer sequences are shown in Table I. PCR was performed by incubation for two minutes at 95°C, followed by 50 amplification cycles with a 20-second denaturation at 95°C, 30-second annealing and 30 second extension at 72°C.
Table I.
Primer sequences used for real-time PCR analysis
| Gene | Forward primer | Reverse primer |
|---|---|---|
| TGF-β1 | GTGCGGCAGTGGTTG AGC | GGTAGTGAACCCGTTGATGTCC |
| COL1 | CGACCTGGTGAGAGAGGAGTTG | AATCCATCCAGACCATTGTGTCC |
| COL2 | AACGGTGGCTTCCACTTC | GCAGGAAGGTCATCTGGA |
| TIMP-1 | GGCTTCTGGCATCCTGTTGTTG | AAGGTGGTCTGGTTGACTTCTGG |
| MMP-13 | AGGAAGACCTCCAGTTTGCAGAG | GCTGCATTCTCCTTCAGGATTC |
Western blotting
Rabbit ligament tissue lysates were fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and detected by immunoblotting. Briefly, proteins separated with 7% SDS-PAGE (Alpha Innotech, San Leandro, California) were transferred onto polyvinyl difluoride (PVDF) membranes for 45 to 80 minutes at 100 V. The membranes were blocked with TBST (TBS/0.5% Tween-20), containing 5% non-fat dry milk, for three hours at room temperature. The samples were closed with5% fat-free milk for one hour and added to 50g/L BSA diluted each antibody (1:500). These antibodies included rabbit TGF-β1, COL1, COL2, TIMP-1 and MMP-13. The membrane was washed with TBST (1ml/L Tween-20) three times (five minutes/time) after incubating at 4°C overnight. The membranes were washed four times with TBST, and incubated with 1:3000 diluted horse radish peroxidase (HRP, Santa Cruz Biotechnology, Santa Cruz, California)-conjugated corresponding secondary antibodies (Boster Biological Technology, Ltd, Wuhan, China) at 37°C for one hour. After being washed four times with TBST, the immunoreactive traces were detected with an enhanced chemiluminescence (ECL) Kit (Merck Millipore, Billerica, Massachusetts). β-actin was used as a control to determine the sample loading size and to validate the experiment settings. The intensity of each protein expression on the membranes was scanned by AlphaImager scanner and analysed by AlphaEaseFC image process software (Alpha Innotech Corp., San Leandro, California).
Haematoxylin and eosin staining and observation
The frozen tissue is sectioned in a cryostat (a sectioning microtome in a freezing chamber) and placed on a microscope slide for staining. The section is fixed immediately using 10% buffered formalin, before it begins to decay, and is then stained using haematoxylin and eosin stain (H&E).18 After H&E staining, the section was carefully observed with an automated digital system, Cytation 5 Cell Imaging Multi-Mode Reader (BioTek Instruments Inc., Winooski, Vermont).
SEM analysis
The ligament tissues of rabbits in both groups were fixed with 2.5% glutaraldehyde phosphoric acid buffer and dehydrated with ethanol, then vacuum dried. The surface of the specimen was sprayed with gold and observed by scanning electron microscope (SEM) (XL30E; Philips, Amsterdam, The Netherlands).
Statistical analysis
Statistical analysis was performed with SPSS statistical software, version 19.0 (IBM Corp., Armonk, New York). Data were expressed as the mean and standard deviation (sd). Statistical analyses were performed with a two-tailed Student’s t-test for two-group comparison. Statistical significance was assumed at p < 0.05.
Results
Concentration of bFGF and TGF-β1 in the blood
The amount of bFGF and TGF-β1 in the blood of both group A and group B rabbits was detected by ELISA, and the results are summarised in Table II. Both the bFGF and TGF-β1 in the blood of group B rabbits exhibited much higher concentrations than those in the blood of group A rabbits (p < 0.05), indicating that CTGF treatment increased the release of both bFGF and TGF-β1 in the blood.
Table II.
Amount of bFGF and TGF-β1 detected by ELISA (ng/ml)
Compared with the value in group A, the difference is significant (p < 0.05)
mRNA level of TGF-β1, COL1, COL2, SOX9, TIMP-1 and MMP-13
Using RT-PCR, we measured the mRNA level of the important cellular factors including TGF-β1, COL1, COL2, SOX9, TIMP-1 and MMP-13. As shown in Figure 1, compared with the mRNA levels of rabbits in group A, the mRNA levels of TGF-β1, COL1, COL2, SOX9 and TIMP-1 in group B were all significantly higher (p < 0.05), and came to 1.32 (sd 0.64), 0.57 (sd 0.14), 0.94 (sd 0.12), 0.87 (sd 0.07) and 0.98 (sd 0.16), respectively, which suggests that CTGF can upregulate the mRNA levels of these genes. In contrast, the mRNA levels of MMP-13 in the ligament tissue of group B rabbits (1.06, sd 0.28) were significantly lower than those in group A rabbits (1.84, sd 0.36). This indicates that CTGF can downregulate the mRNA of MMP-13.
Fig. 1.
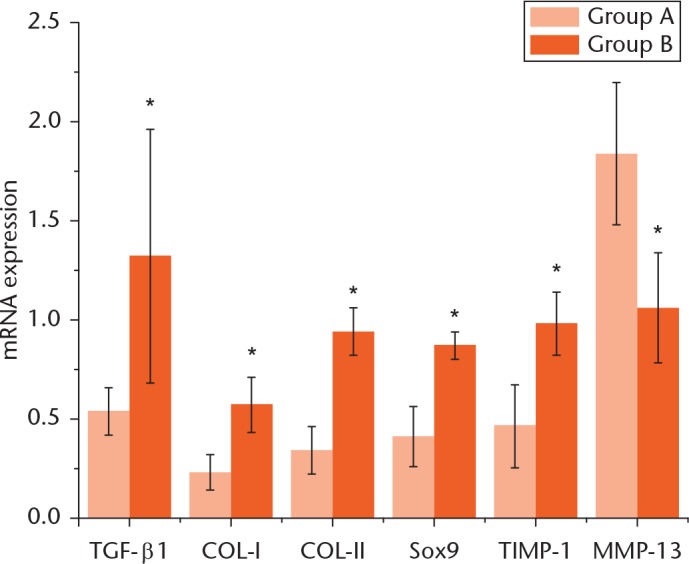
mRNA expression of TGF-β1, COL1, COL2, SOX9, TIMP-1 and MMP-13 in the ligament tissue of rabbits in both control (A) and treatment (B) groups. The line segments on each bar chart indicate a 95% confidence interval (CI) for that value. *Indicates a significant difference compared with the value in group A (p < 0.05).
Protein levels of TGF-β1, COL1, COL2, TIMP-1 and MMP-13
Western blotting was used to measure the protein levels of the important cellular factors including TGF-β1, COL1, COL2, TIMP-1 and MMP-13. As shown in Table III and Figure 2, the protein expression levels of TGF-β1, COL1, COL2 and TIMP-1 in the ligament tissue of the rabbits in group B were all higher than those in group A (p < 0.05), demonstrating that CTGF increased the protein expression of these four factors. On the other hand, however, the protein level of MMP-13 in the ligament tissue of group B was lower than those in group A (p < 0.05), which indicates that CTGF can lower the protein expression of MMP-13.
Table III.
Western Blotting of TGF-β1, COL1, COL2, TIMP-1 and MMP-13 expression levels
| Group | TGF-β1 | COL1 | COL2 | TIMP-1 | MMP-13 |
|---|---|---|---|---|---|
| A | 0.68 ± 0.21 | 0.42 ± 0.08 | 0.32 ± 0.08 | 0.49 ± 0.18 | 1.21 ± 0.26 |
| B | 1.15 ± 0.17* | 0.84 ± 0.12* | 0.71 ± 0.13* | 0.93 ± 0.14* | 0.72 ± 0.21* |
Compared with the value in group A, the difference is significant (p < 0.05)
Fig. 2.
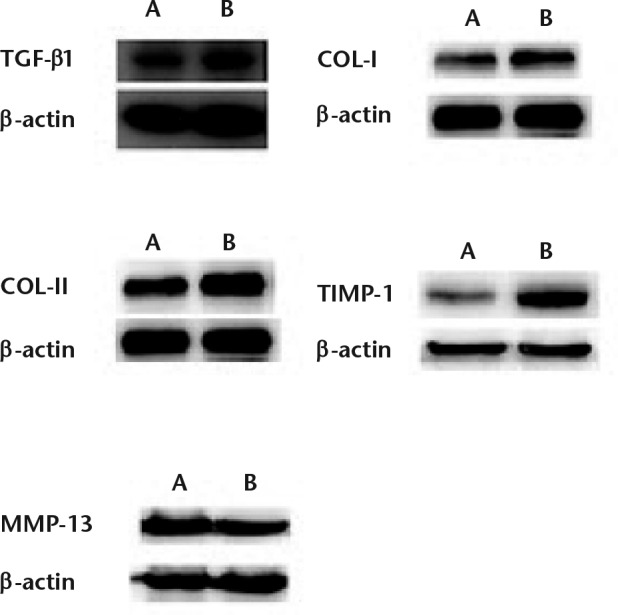
Protein levels of TGF-β1, COL1, COL2, TIMP-1 and MMP-13 in the ligament tissue of rabbits in both control (A) and treatment (B) groups. β-actin was used as a control.
Pathological change observed by H&E staining
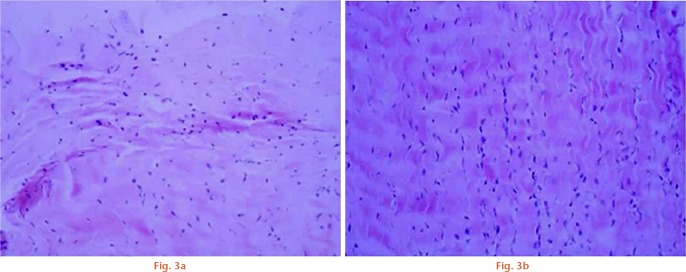
Pathological changes in the tissues in the rabbits in each group were compared after H&E staining, seen in Figure 3. The collagen fibres in the ligament tissue of group A rabbits exhibited a disordered arrangement, with a loosened microstructure and many broken collagen fibres. Fibroblasts were distributed unevenly, with a significantly increased number of nuclei, which underwent some shrinkage change. However, the collagen fibre in the ligament tissue of group B rabbits formed a wave-like structure with no broken collagen fibres. Fibroblasts increased in number, but there were no visible changes in the nucleus. Fibroblasts exhibited a regular oval shape and were evenly distributed, which represents a recovered ligament tissue.
Pathological changes in the tissues in group A and B rabbits were compared after haematoxylin and eosin staining (x100). Compared with group A, the collagen fibre in the ligament tissue of group B rabbits formed a wave-like structure with no broken collagen fibres. Fibroblasts increased in number, but there were no visible changes in the nucleus. Fibroblasts exhibited a regular oval shape and were evenly distributed, which represents a recovered ligament tissue.
SEM images
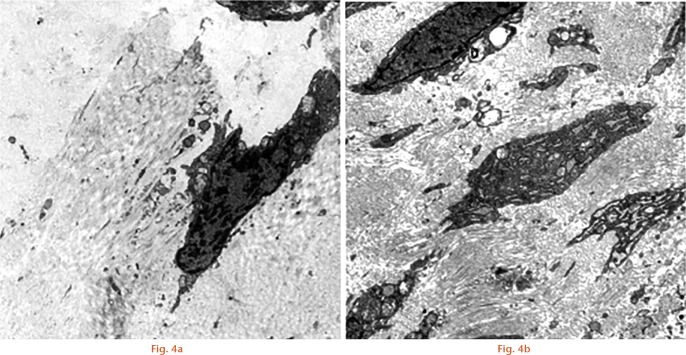
Further pathological changes in the tissues in the rabbits in each group were compared by scanning electron microscope, seen in Figure 4. We observed that there were fewer fibroblasts and only a small number of collagen fibrils in the ligament tissue of those in group A, which suggests that cell proliferation ability was weak. However, for those in group B, fibroblasts and collagen fibrils were significantly increased because of strong proliferative ability, which represents a recovered ligament tissue.
SEM images showed pathological changes in the tissues in group A and B rabbits (scale is 2 μm). Compared with group A, the group B rabbits, fibroblasts and collagen fibrils were significantly increased because of strong proliferative ability, which represents a recovered ligament tissue.
Discussion
Basic fibroblast growth factor (bFGF), also referred to as FGF-2, is a representative growth factor which has shown the effect on the repair and regeneration of tissues.19,20 It has been reported that TGF-β1 could induce proliferation in human renal fibroblasts by means of induction of bFGF.21 In our study, we observed that CTGF treatment in the injured ACL increased the blood concentration of both TGF-β1 and bFGF. It is most likely that CTGF first stimulated the secretion of TGF-β1, which then further induced the production of bFGF.
The repair and remodelling of connective tissue involves not only chondrocyte reproduction and activity, but also the formation of collagen fibres and ground substance. Both COL1 and COL2 are important natural resources for tissue regeneration and wound healing.22 In our study, we found that CTGF stimulated the production of COL1 and COL2 in the injured ACL, which is needed for ACL reconstruction and ligament recovery.
Matrix metallopeptidase 13, also known as collagenase 3, is a member of the matrix metalloproteinase (MMP) family involved in the breakdown of the extracellular matrix.23 The preferred substrate for MMP-13 is collagen 2, which cleaved five times faster than collagen 1 and six times faster than collagen 3,24 and more readily by MMP-13 than by other collagenases. In our study, we found that COL1 and COL2 were both increased in group B, which is in line with the fact that the MMP-13 level was greatly reduced in group B.
The tissue inhibitor of metalloproteinase-1 (TIMP-1), a 28.5 kDa glycoprotein that belongs to the TIMP family, is one of the endogenous inhibitors of MMP.25 We found that both the mRNA level and the protein level of TIMP-1 were increased in group B, and the level of mRNA and protein level of MMP-13 was decreased. This confirms the inhibitory role of TIMP-1 on MMP-13, which is helpful in maintaining sufficient collagen for ACL reconstruction and ligament recovery.
In conclusion, our current study showed that connective tissue growth factor can promote the recovery of injured ACL. It can upregulate the mRNA and expression of TGF-β1, COL1, COL2 and TIMP-1, and downregulate the mRNA and expression of MMP-13. Our findings demonstrated that the curative effect of CTGF on injured rabbit ligament is through regulating these cellular factors.
Footnotes
Author Contribution: X. Sun: Study design, data analysis, writing the paper.
W. Liu: Study design, data analysis, writing the paper.
G. Cheng: Data analysis, interpretation of data.
X. Qu: Data analysis, interpretation of data.
H. Bi: Interpretation of data, writing the paper.
Z. Cao: Study design, data analysis, manuscript preparation.
Q. Yu: Interpretation of data.
Conflicts of Interest Statement: None declared.
Funding Statement
None declared.
References
- 1. Kiapour AM, Wordeman SC, Paterno MV, et al. Diagnostic value of knee arthrometry in the prediction of anterior cruciate ligament strain during landing. Am J Sports Med 2014;42:312-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levine JW, Kiapour AM, Quatman CE, et al. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med 2013;41:385-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quatman CE, Kiapour AM, Demetropoulos CK, et al. Preferential loading of the ACL compared with the MCL during landing: a novel in sim approach yields the multiplanar mechanism of dynamic valgus during ACL injuries. Am J Sports Med 2014;42:177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hewett TE, Di Stasi SL, Myer GD. Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. Am J Sports Med 2013;41:216-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg 2005;125:217-221. [DOI] [PubMed] [Google Scholar]
- 6. Taylor SA, Khair MM, Roberts TR, DiFelice GS. Primary Repair of the Anterior Cruciate Ligament: A Systematic Review. Arthroscopy 2015;31:2233-2247. [DOI] [PubMed] [Google Scholar]
- 7. Musahl V, Becker R, Fu FH, Karlsson J. New trends in ACL research. Knee Surg Sports Traumatol Arthrosc 2011;19:S1-S3. [DOI] [PubMed] [Google Scholar]
- 8. Kiapour AM, Murray MM. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res 2014;3:20-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. Am J Sports Med 2011;39:538-543. [DOI] [PubMed] [Google Scholar]
- 10. Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 2011;10:945-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hall-Glenn F, Lyons KM. Roles for CCN2 in normal physiological processes. Cell Mol Life Sci 2011;68:3209-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci 2008;33:461-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci 2006;119:4803-4810. [DOI] [PubMed] [Google Scholar]
- 14. Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012;5(Suppl 1):S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal 2010;4:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao YL, Duan Y, Zhu LX, et al. TGF-β1, in association with the increased expression of connective tissue growth factor, induce the hypertrophy of the ligamentum flavum through the p38 MAPK pathway. Int J Mol Med 2016;38:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H, Wang S, Ma W, Lu Y. Transforming Growth Factor β1 Promotes Migration and Invasion of Human Hepatocellular Carcinoma Cells Via Up-Regulation of Connective Tissue Growth Factor. Cell Biochem Biophys 2015;73:775-781. [DOI] [PubMed] [Google Scholar]
- 18. Chan JK. The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology. Int J Surg Pathol 2014;22:12-32. [DOI] [PubMed] [Google Scholar]
- 19. Delgado-Rivera R, Harris SL, Ahmed I, et al. Increased FGF-2 secretion and ability to support neurite outgrowth by astrocytes cultured on polyamide nanofibrillar matrices. Matrix Biol 2009;28:137-147. [DOI] [PubMed] [Google Scholar]
- 20. Hankemeier S, Keus M, Zeichen J, et al. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng 2005;11:41-49. [DOI] [PubMed] [Google Scholar]
- 21. Strutz F, Zeisberg M, Renziehausen A, et al. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int 2001;59:579-592. [DOI] [PubMed] [Google Scholar]
- 22. Oliveira SM, Ringshia RA, Legeros RZ, et al. An improved collagen scaffold for skeletal regeneration. J Biomed Mater Res A 2010;94:371-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hijova E. Matrix metalloproteinases: their biological functions and clinical implications. Bratisl Lek Listy 2005;106:127-132. [PubMed] [Google Scholar]
- 24. Mitchell PG, Magna HA, Reeves LM, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest 1996;97:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu ZS, Wu Q, Yang JH, et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer 2008;122:2050-2056. [DOI] [PubMed] [Google Scholar]