Abstract
Acetaminophen can adversely affect the liver especially when overdosed. We used whole blood as a surrogate to identify genes as potential early indicators of an acetaminophen-induced response. In a clinical study, healthy human subjects were dosed daily with 4g of either acetaminophen or placebo pills for 7 days and evaluated over the course of 14 days. Alanine aminotransferase (ALT) levels for responders to acetaminophen increased between days 4 and 9 after dosing and 12 genes were detected with expression profiles significantly altered within 24 hrs. The early responsive genes separated the subjects by class and dose period. In addition, the genes clustered patients who overdosed on acetaminophen apart from controls and also predicted the exposure classifications with 100% accuracy. The responsive genes serve as early indicators of an acetaminophen exposure and their gene expression profiles can potentially be evaluated as molecular indicators for further consideration.
Introduction
Acetaminophen (Tylenol®) is widely used to relieve pain and reduce fever. Currently, the maximum U.S. FDA approved daily dose is 4g. Acetaminophen poisoning is common world-wide and is potentially fatal 1. The majority of clinical cases present in the emergency room with acute liver failure. Previously, the Centers for Disease Control and Prevention and the U.S. FDA indicated that about 55 000 to 80 000 people in the U.S. overdose from acetaminophen each year, of which at least 500 die particularly from liver failure 2–6. The U.S. FDA has issued a warning for manufactures of acetaminophen to indicate the potential risk of sudden liver failure. More recently, the manufacture of Tylenol®, an over-the-counter product, has included a warning on the label that the drug content contains acetaminophen. Physicians have been asked by the U.S. FDA to avoid prescribing pharmaceuticals with a high dose of acetaminophen as drug-induced liver failure is a growing concern 7, 8.
The early detection of acetaminophen liver injury and determination of prognosis at presentation are critical to clinicians but can be challenging when using serum enzymes as an indicator of liver damage 9. Liver biopsies to obtain material for histopathological evaluations are invasive and are a significant risk to the patient. Furthermore, at times serum acetaminophen levels shortly after overdose are undetectable 10. Thus, there is a need for novel diagnostic and prognostic indicators using biospecimens that can be easily obtained with minimal invasion and are efficient at early detection.
We used blood as a surrogate to identify gene expression profiles as potential early indicators of an acetaminophen response. In a clinical study, “Responders” to dosing of acetaminophen were classified apart from “non-responders” based on both ALT levels greater than 2x the upper limit of normal and 2x their individual baseline ALT values. We detected 12 genes in the blood with expression profiles significantly altered within 24 hrs of the beginning of dosing, three days before the responder group’s ALT marker elevated to levels that would be classified as responders. Using independent gene expression data from the blood of five human acetaminophen overdose patients, the panel of early response genes clustered the samples from the overdosed patients apart from controls and also predicted exposure to acetaminophen within this independent data set with 100% accuracy. We conclude that this panel of blood gene expression profiles can potentially serve as candidates for early biomarkers of an acetaminophen response.
Materials and Methods
Study Design
The randomized, single-blind, placebo-controlled, clinical study objective was to identify gene expression from whole blood of healthy human subjects receiving 4g of acetaminophen daily or placebo that are early indicators of an acetaminophen response, detectable well before ALT elevations. Healthy male and female individuals from 18 – 58 years old weighing 55 kg to 85 kg volunteered as subjects in the study (See Supplementary Table 1). The study was instituted only once. The protocol (#2265) was approved by the University of North Carolina-Chapel Hill Institutional Review Board and informed consent was obtained from each patient. There were 66 subjects enrolled for 14 days each and were acclimated for 3 days on a controlled, standardized whole-food diet in order to assure a uniform nutritional intake. Starting on day 0 and until day 7 relative to the start of dosing, each subject received daily repeat dosing every 6 hrs (i.e. 4x daily) of either 1g of acetaminophen (two 500-mg Tylenol® Extra-Strength tablets, Orth-McNeil Pharmaceuticals, Titusville, NJ, USA) or placebo pills orally. Blood was collected at 8 a.m. on each day of the clinical study for ALT measurement and complete blood counts (CBC). Furthermore, each day peripheral blood (7.5 mls) was drawn into PAXgene™ (PreAnalytiX/QIAGEN, Hilden, Germany) blood RNA collection tubes (3 tubes @ 2.5 mls). Samples were mixed and RNA was isolated according to the manufacturer’s protocol, including the optional on-column DNase digestion. RNA quality was assessed with an Agilent Bioanalyzer™ (Palo Alto, CA) and only samples with intact 18S and 28S ribosomal RNA peaks were used for microarray analysis. The RNA quality from 3 subjects (#s 1, 2 and 3) were poor and subjects #s 12 and 21 had no day 0 samples. Therefore, the samples from these subjects were not processed further.
Clinical Chemistry
Clinical chemistry evaluations of serum samples were performed using a Roche Cobas Fara chemistry analyzer (Roche Diagnostic Systems, Westwood, NJ) to numerically measure serum ALT enzyme levels (See Supplementary Table 2).
Classification of Subjects
Individuals given placebos were classified as within the Placebo group. In a randomized control trial, elevated levels of serum ALT have been associated with healthy adult human subjects receiving 4 g of acetaminophen daily 9. In our study, we classified subjects receiving acetaminophen as follows. Responders: Individuals with a 2-fold increase in serum ALT (See Supplementary Table 2) from their respective baseline value and greater than 2x the upper limit of normal (as defined by historical data from the University of North Carolina-Chapel Hill hospital: females = 30 IU/L; males = 40 IU/L) in response to acetaminophen. Non-responders: Individuals receiving acetaminophen but not meeting the “Responder” criteria. The baseline for normal was defined as the mean of the daily ALT measurements obtained in the days before the start of acetaminophen dosing (the acclimation period). The baseline ALT levels for subjects #11 and #18 were elevated and thus, the data from these subjects were removed from further analysis. The classification assignments of the subjects are in Supplementary Table 3.
Microarray Analysis
Gene expression profiling was conducted using Agilent-012097 Human 1A (V2) microarrays (Agilent Technologies, Palo Alto, CA). 500 ng of total RNA from a human universal reference (Stratagene, La Jolla, CA) and subject samples were amplified using the Agilent Low RNA Input Fluorescent Linear Amplification Kit and labeled with Cy3 and Cy5 cytofluors respectively according to manufacturer’s protocol. For each two-color comparison, 750 ng of each Cy3 and Cy5 cRNA were mixed and fragmented using the Agilent In Situ Hybridization Kit protocol. Hybridizations were performed for 17 hrs in a rotating hybridization oven according to the Agilent 60-mer oligo microarray processing protocol prior to washing and scanning. Gene expression pixel intensities were extracted by scanning the microarrays with an Agilent Scanner (Agilent Technologies, Wilmington, DE). The log base 2 pixel intensity values from the Cy3 and Cy5 channels were background subtracted, corrected for dye bias and normalized across arrays 11. The preprocessing of the data from subjects #s 13, 49 and 63 (Placebo, Non-responder and Responder classes respectively) revealed them as being outliers and thus were excluded from further analysis. Finally, log base 2 ratio values were generated (subject sample to universal reference) and corrected for batch effects (see Supplementary Table 4 for batches) using an empirical Bayes model with the classification of the subjects and before or after dosing as covariates and parametric estimation of priors12. The microarray data for subject #62 at day -1 and day 4 and for subject #64 at day -1 were missing and hence these subjects were excluded from further analysis. Statistical analysis was performed on the preprocessed data from the remaining 54 subjects (Responders n = 12, Non-responders n= 32 and Placebo n= 10).
Statistical Analysis
We used a discontinuous piecewise linear regression (DPLR) model (1st order autoregressive process) 13 to analyze the gene expression data by day. The model is similar to a classical linear regression. However, with respect to the DPLR model, the regression function is in two pieces, separated at the time point at which acetaminophen is administered. For each gene, let Yt denote the preprocessed log base 2 ratio value for the tth day. The regression model is:
where βo is the Y intercept of the regression line, and where:
When Xt1 ≤ Xp, Xt2 = 0 and Xt3 = 0 as indicator variables, then the response function for the regression model becomes βo + β1Xt1 (the 1st piece). Similarly, when Xt1 > Xp, Xt2 = 1 and Xt3 = 1 as indicator variables, then the regression model becomes (βo - Xpβ2+ β3)+(β1+ β2)Xt1 (the 2nd piece). Thus, if β3 = 0, the piecewise regression is continuous and there is no difference in the mean responses for the two regression lines at Xp.
Subject is the random effect in the model and the restricted maximum likelihood (REML) method for estimation of variance within each group was used. We assume that the gene expression response at different days in the subjects prior to dosing follows a different linear relationship after dosing at day Xp (i.e., the linear response is in two pieces) and that the linear response may not only change slope at Xp, but may also be discontinuous at Xp. In this study, Xp is t = 0 and β3 represents the difference in the mean responses for the two regression lines at Xp. For each gene and given the model, we perform a two-sided t-test for the null hypothesis Ho: β3 = 0 and reject it for the alternative hypothesis Ha: β3 ≠ 0 at α = 0.05. We account for multiple testing of the genes using a false discovery rate (FDR) 14, 15 q-value ≤ 0.05. Genes with cyclic expression patterns were identified using the sine and cosine of X in the following trigonometric model:
where a is the amplitude = 1, 2π/b is the period where b = 1, π = 180, Xt is the day of dosing with t = −3, −2, −1, 0, 1, 2, 3, 4, h the horizontal shift = 0 and k is the vertical shift = 0. Genes with a significant fit to the model (i.e., a correlation coefficient R2 ≥ 0.2) were considered cyclic and thus not appropriate for the discontinuous piecewise linear model.
Code availability: The SAS code for the discontinuous piecewise linear regression mixed regression model and trigonometric model are available upon request.
Overdosed Patients
Five individuals (2 males and 3 females) of Caucasian or African-American ethnicity who overdosed on acetaminophen were admitted to the University of North Carolina-Chapel Hill emergency room. The individuals ages ranged from 19 – 59 years, their ALT (U/I) ranged between 435 – 6446 and peripheral blood was drawn 2 or 5 days after admittance for microarray analysis. Blood was collected using PAXgene vacutainer tubes (PreAnalytiX; Qiagen), and RNA was isolated as described by the manufacturer and labeled as described above for microarray analysis. Briefly, each sample was hybridized against a human universal RNA control (Stratagene, La Jolla, CA) and processed as described above for microarray analysis. Hybridizations to Agilent-012097 Human 1A (V2) microarrays (Agilent Technologies, Palo Alto, CA) were performed for 17 hrs in a rotating hybridization oven according to the Agilent 60-mer oligo microarray processing protocol prior to washing and scanning with an Agilent Scanner (Agilent Technologies, Wilmington, DE). The raw pixel intensity gene expression data were loaded into the Rosetta Resolver database (build 5.1.0.1.23, Rosetta Biosoftware, a unit of Rosetta Inpharmatics; Palo Alto, CA) and error weighted ratio values computed from the normalized and background-subtracted pixel intensity values 16. The log base 10 ratio data were generated as follows. Overdose patients: average of baseline group [day -3 human blood] to patient and Baseline group: Reference to day -3 human blood.
Predictions
The fold change (samples to universal reference) blood gene expression data of the 12 early responsive genes from the placebo and responder subjects in the present study at day 1 (24 hrs after receiving acetaminophen) were used in a support vector machine 17, 18 (SVM) three degrees radial basis gamma kernel to build a predictive model (Partek Genomics Suite v. 6.6, St. Louis, MO). Following a comparison of prediction accuracies based on surveying various parameter settings and leave-one-out-cross-validation, the parameters with the best accuracy were with cost-based shrinking, tolerance of 0.001, nu = 0.5, and gamma = 0.01. The model was then applied to the fold change (subject samples to universal reference) blood gene expression data of the 12 early responsive genes from the five acetaminophen overdose patients and three controls. Prediction accuracy was based on the number of subjects predicted correctly (control as Placebo and overdosed as Responder).
Gene Interaction Analysis
Ingenuity Pathway Analysis (IPA) software build 355958M (Qiagen, Redwood City, CA) was used to create a gene interaction network from 9 of the 12 early responsive genes (focus genes) detected in the responders only and that were mapped to pathways in the IPA knowledgebase content version 21901358. The network was scored according to the –log base 10 p-value from a Fisher’s exact test assessing the significance of the proportion of highly connected 9 focus genes within, or not in the network to all other genes in the knowledgebase within, or not in the network. Finally, the network was pruned to highlight the interactions of the focus genes with the central hub.
Results
A Subset of Subjects Respond to Acetaminophen
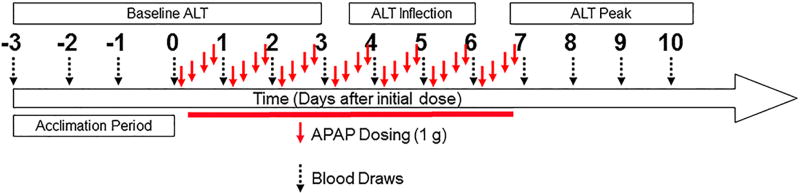
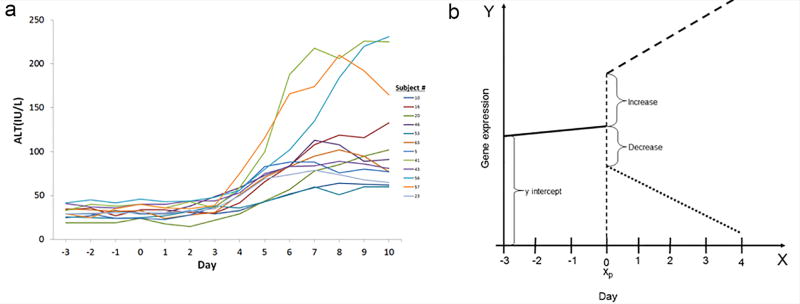
Fifty-four male and female human subjects (Table 1) were evaluated over the course of a 14 day acetaminophen response clinical study to monitor changes in serum enzyme levels and profile gene expression from their blood. As illustrated in Figure 1, starting on day 0 and until day 7, subjects received daily, repeated (4x) dosing of either 1g of acetaminophen or placebo pills. As shown in Figure 2a, the ALT levels for the responders to acetaminophen are at baseline level between day 0 and 3, they increase between day 3 and 6, and then peak between day 7 and 10.
Table 1.
Demographics of subjects in the clinical study with gene expression measurements.
| Classes | Number | |
|---|---|---|
| Placebos: | 10 | |
| Non-responders | 32 | |
| Responders: | 12 | |
| Ethnicities | ||
| Caucasian: | 20 | |
| Hispanic: | 23 | |
| African American: | 10 | |
| Asian: | 1 | |
| Age range (years) | 18–58 | |
| Genders | ||
| Males: | 32 | |
| Females: | 22 | |
Figure 1.
Study design. Blood draws from subjects are represented by black arrows. Red arrows represent acetaminophen doses. The numbers represent the days relative to the initial dose of acetaminophen.
Figure 2.
Response to acetaminophen dosing. (a) Plot of alanine aminotransferase (ALT) in IU/L for each of the responder subjects at a given day. (b) Discontinuous piecewise regression. Representation of the response function for a discontinuous piecewise regression on genes expression (y-axis) dependent on the day of blood draw (x-axis). Xp is the jump point between the two regression lines and denotes the point at which acetaminophen is given. The solid line represents a gene expression response during the acclimation period. The dashed line represents an increase in gene expression following dosing of acetaminophen. The slanted dotted line represents a decrease in gene expression following dosing of acetaminophen.
Identification of an Early Gene Expression Response to Dosing
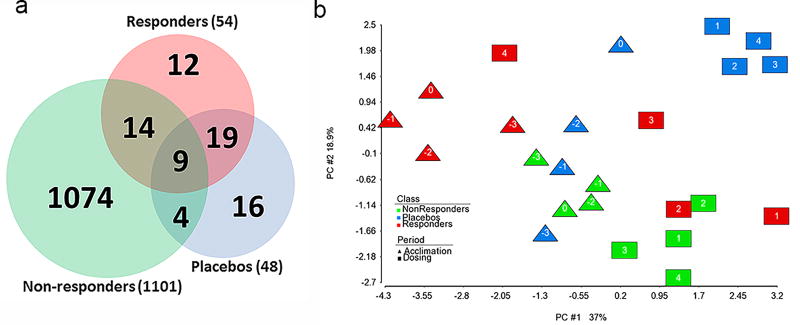
To identify gene expression profiles from the blood of the subjects that respond early during the dosing period, we used a discontinuous piecewise regression (Figure 2b) to model the data from the subjects between day -3 and day 0 separately from day 1 to day 4 and detect a significant change in gene expression. If there is no expression response of a particular gene following a subject receiving acetaminophen, then there would be no statistically significant difference between the expression of the gene during the acclimation period and the dosing period. As displayed in the Venn diagram in Figure 3a, the Placebo and the “Non-responder” classes has 48 and 754 genes (1101 probes) respectively with significant differences (FDR ≤ 0.05) in their gene expression profiles between the two periods whereas the “Responder” class has 54 genes significantly different (FDR ≤ 0.05) and responded to the dosing within 24 hrs. Nine genes overlap between the three classes, but 12 genes are unique to responder class (Table 2). The greater number of genes detected from the Non-responders is primarily due to the larger sample size for that class. Hence, there is more power of detection to identify genes with significant differences between their expression during the acclimation period and the dosing period.
Figure 3.
Overlap of responsive genes and separation of classes. (a) Venn diagram of early responsive genes (q-value ≤ 0.05). (b) Principal component analysis showing the separation of classes by period based on the average of the 12 mean responsive genes according to class and day. The numbers in the data points represent the day relative to the start of dosing. The percent of variation captured by each principal component (PC) is denoted on each axis.
Table 2.
Genes with a significant difference in responder group mean gene expression following dosing
| Probe ID | Gene ID | Description | AR | SE | TS |
|---|---|---|---|---|---|
| A_23_P128333 | NM_002824 | Parathymosin (PTMS) | 0.0468 | 0.0095 | 4.94 |
| A_23_P76799 | NM_013448 | Bromodomain adjacent to zinc finger domain, 1 A (BAZ1A) | 0.1790 | 0.0401 | 4.47 |
| A_23_P104804 | NM_006006 | Zinc finger and BTB domain containing 16 (ZBTB16) | 0.2989 | 0.0719 | 4.16 |
| A_23_P13065 | AK001831 | Zinc finger, DHHC-type containing 13 (ZDHHC13) | 0.3003 | 0.0745 | 4.03 |
| A_23_P58321 | NM_001237 | Cyclin A2 (CCNA2) | 0.2885 | 0.0726 | 3.98 |
| A_23_P11507 | NM_015534 | Zinc finger, ZZ-type containing 3 (ZZZ3) | 0.2408 | 0.0608 | 3.96 |
| A_23_P128613 | NM_024089 | KDEL (Lys-Asp-Glu-Leu) containing 1 (KDELC1) | 0.5092 | 0.1286 | 3.96 |
| A_23_P120533 | NM_007219 | Ring finger protein 24 (RNF24) | −0.6434 | 0.1618 | −3.98 |
| A_23_P211180 | NM_000211 | Integrin, beta 2 (ITGB2) | −1.3192 | 0.3270 | −4.03 |
| A_23_P212180 | A_23_P212180 | Unknown | −0.0413 | 0.0070 | −5.93 |
| A_23_P259357 | BC040191 | Solute carrier family 35, member E4 (SLC35E4) | −0.0063 | 0.0010 | −6.21 |
| A_23_P75097 | NM_024895 | PDZ domain containing 7 (PDZD7) | −0.0181 | 0.0009 | −20.05 |
AR is the log base 2 difference in the mean of the gene expression response to acetaminophen in the dosing period from the mean gene expression in the acclimation period. SE is the standard error and TS is the test statistic from the discontinuous piecewise regression.
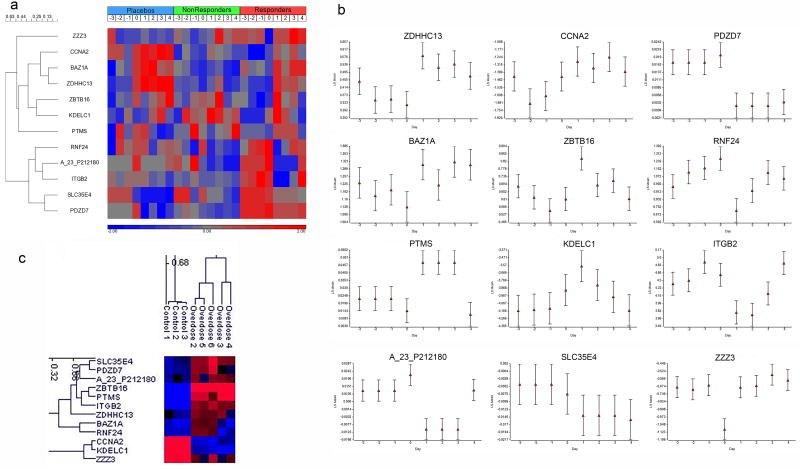
We then used principal component analysis (PCA) of the gene expression data to reduce the dimension of the 12 genes down to the top two principal components (PCs) that capture the majority of variability in the data. Plotting the data with these PCs allows for the projection of the samples in two dimensional space and interpretation of the proximity of them to one another. The 12 genes from the Responder class separate the acclimation period from the dosing period within each class (Figure 3b). Subjecting the expression data from the 12 genes to hierarchical clustering, groups the genes that responds similarly across the time points and reveals shared expression patterns amongst the samples. These 12 genes represented the classes relatively well as depicted by the gene expression patterns in the heat map (Figure 4a). Of the 12 early responsive genes specific to the Responder class, five decrease in expression 24 hrs after dosing and seven increase in expression (Table 2 and Figure 4b).
Figure 4.
Heat maps and clustering of responsive genes and samples. (a) Clustering of the 12 genes with change in mean expression following dosing. Cosine correlation dissimilarity with Ward clustering was used. The log base 2 gene expression ratio data (subject to reference) for subjects within each class at a given day were averaged and then each profile was standardized such that the distribution across the days has a mean equal to 0 and standard deviation of 1. The days relative to the initial dosing are denoted. The heat map color bar represents the relative gene expression: red is up-regulated, blue is down-regulated. (b) Plot of the gene expression data by day for the 12 early responsive genes. The x-axis is the day relative to the initial day of dosing. The y-axis is the least square (LS) mean of the log base 2 ratio data (subject to reference). The error bars represent the standard error of the mean. (c) Two-dimensional clustering of the 12 genes and subjects overdosed on acetaminophen or controls. Cosine correlation dissimilarity with average linkage clustering was used. Log base 2 ratio data (reference to subject) is represented by color: red is up-regulated, blue is down-regulated.
Early Responsive Genes Discern and Predict Exposure to Acetaminophen
To validate the 12 early responsive genes, we used the gene expression from them to cluster five acetaminophen overdose patients and controls from an independent gene expression data set according to exposure status. As shown in Figure 4c, the 12 genes cluster the overdosed patients together, yet apart from the controls essentially from three groups of moderately correlated genes (R > 0.6). All overdose patients presented with elevated ALT levels and four of the five with an estimated ingestion of acetaminophen greater than the maximum U.S. FDA approved daily dose of 4g (Table 3). However, only one had a blood serum acetaminophen level greater than 25 mcg/mL. In addition, using a SVM prediction model built from the 12 early responsive genes from the Placebo and Responder subjects in the present study at day 1 (24 hrs after receiving acetaminophen), prediction accuracy of the exposure status of the overdose patients and controls was 100% (Table 4).
Table 3.
Overdose subjects and their clinical measures
| Patient Identifier | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| Gender | male | male | female | female | female |
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian | African American |
| Age (years) | 19 | 25 | 36 | 40 | 59 |
| Peak ALT (U/l) | 2630 | 6446 | 816 | 1582 | 435 |
| AST (U/l) | 726 | 1804 | 1123 | 121 | 552 |
| Total Billirubin (µmol/L) | 0.8 | 1.2 | 0.6 | 1.6 | 25.5 |
| White blood cell counts (103/µL) | 6.8 | 10.5 | 10.6 | 5.6 | 17 |
| INR | 1.3 | 2.1 | 1.8 | 1.3 | 1.8 |
| Acetaminophen level (mcg/mL) | <10 | <10 | 22/350 | <10 | <10 |
| Presented after ingestion | 3 days | 3 days | 12hrs | 4 days | 18hrs |
| Day blood drawn for study | 5 | 5 | 2 | 5 | 2 |
| Total estimated dose (g) | 10 | 15 | 125 | 10 | unknown |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR: international normalized ratio
Table 4.
Prediction of acetaminophen exposure
| Exposure | # Per Class | Proportion | # Correct | # Errors | % Correct |
|---|---|---|---|---|---|
| Overdose | 5 | 0.63 | 5 | 0 | 100 |
| Control | 3 | 0.37 | 3 | 0 | 100 |
| Total | 8 | 1 | 8 | 0 | 100 |
Molecular Interactions and Pathways Associated with the Acetaminophen Early Response
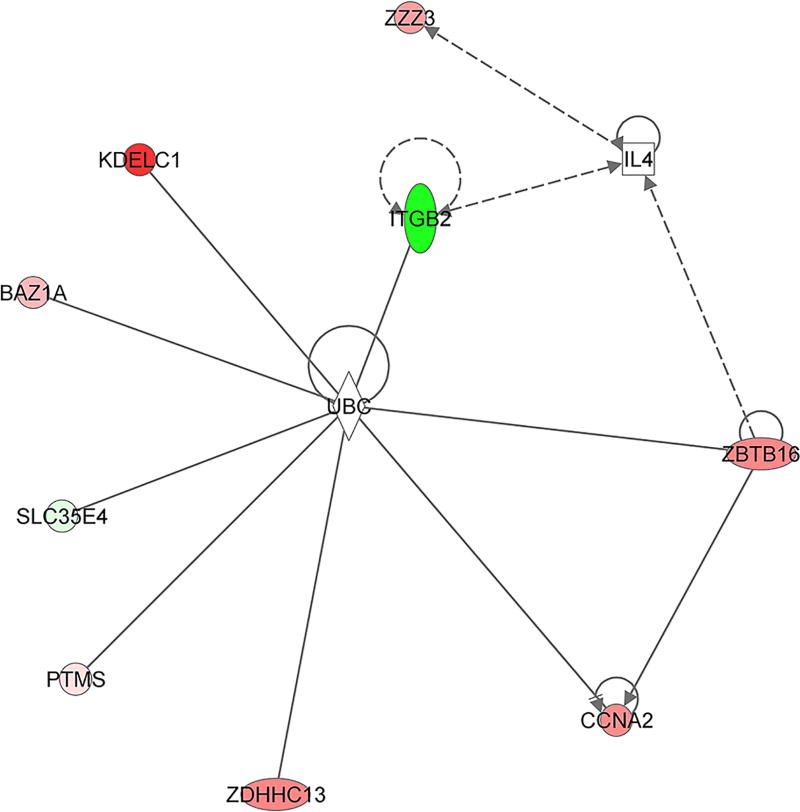
Using 9 of the 12 early response genes detected in the Responders that were mapped in the IPA canonical pathway database, we found a molecular interaction of interest with a score =26 (p-value = 1x10-26). As shown in Figure 5, several of these dysregulated early response genes are associated with ubiquitin C (UBC) as the central hub and three interact with interleukin 4 (IL4).
Figure 5.
Gene interaction network. Using 9 of the 12 early response genes detected in the responders that were mapped to pathways in the Ingenuity Pathway Analysis canonical database, molecular networks were generated. Colored nodes represent genes that are part of the 12 early response genes. Red represents increased expression, green, decreased expression. Shapes representations: Circles, protein-coding genes; diamond, enzyme; square, cytokine; horizontal ovals, transcription regulators; vertical oval, transmembrane receptor. A solid line represents a direct interaction whereas a dashed line represents an indirect interaction. A line with an arrow denotes activation whereas a line with an arrow and a pipe (|) denotes acts on and inhibits respectively. A line without an arrow or pipe (|) denotes a protein-protein interaction.
Discussion
Previously, the Centers for Disease Control and Prevention and the FDA indicated that about 55 000 to 80 000 people in the U.S. overdose from acetaminophen each year of which at least 500 die particularly from liver failure 2–6. The U.S. FDA has issued a warning for manufactures of acetaminophen to indicate the potential risk of sudden liver failure and more recently the manufacture of Tylenol®, an over-the-counter product, has included a warning on the label that the drug content contains acetaminophen. We sought to use blood as a surrogate tissue in order to identify gene expression profiles derived from blood cells as early indicators of an acetaminophen response in other tissues.
Based on microarray gene expression data we identified 54 genes, 12 which are unique to the Responder group, that were significantly differentially expressed as early as 24 hrs subsequent to dosing with acetaminophen. Using the 12 early responsive genes, we could differentiate the samples of subjects within the acclimation period from those in the dosing period. In addition, they allowed us to separately cluster Responder, Non-responder and Placebo subjects distinctly from each other and also allowed for the separate clustering of subjects who overdosed on acetaminophen from controls. Furthermore, against an independent human blood gene expression data set, the early responsive genes allowed us to predict the acetaminophen overdosed exposure samples and control samples with 100% accuracy.
Of the 12 early responder genes, several have been reported to be associated with the immune system or cell division and some code for zinc finger binding proteins or complexes in the endoplasmic reticulum. In published studies, five of these genes have been shown to be differentially expressed in rodents following acetaminophen exposure 19–22. Early consequences of acetaminophen intoxication in rats revealed structural changes of the liver cells and dilatation of the endoplasmic reticulum 23 as well as damage to mitochondria 20. KDELC1 encodes a protein that is localized to the lumen of the endoplasmic reticulum 24 whereas SLC35E encodes a multiple spanning domain protein which transports nucleotide-sugar (a substrate for glycosyltransferases) from the cytosol to the lumen of the Golgi apparatus and endoplasmic reticulum 25. Interestingly, zinc finger and BTB domain containing 16 (ZBTB16) is associated with increase of the mitochondria membrane potential 26 and integrin-beta 2 (ITGB2), which encodes CD18, has been shown to be associated with increased liver damage 27.
Exposure to a toxic dose of acetaminophen leads to an inflammatory/immune response that consists of a release of cytokines/chemokines as well as an innate immune cell infiltration in the liver 28–31 in addition to hepatocyte damage. Our IPA analysis of the 12 early response “predictor” genes revealed potential molecular interactions between 9 of these genes with ubiquitin C (UBC), a stress response gene 32. This potential interaction may be suggestive of an early cellular repair response to acetaminophen exposure. Toxic stress can cause protein misfolding and the accumulation of misfolded proteins in the endoplasmic reticulum leading to ubiquitination of these macromolecules 33–35. UBC is involved with ubiquitination and is associated with protein degradation, DNA repair and cell cycle regulation 32. CCNA2, which produces cyclin-A2, regulates cell cycle progression, cell division and cell proliferation. The induction of this gene in the Responders to acetaminophen may be associated with a signal to proliferate in response to acetaminophen-induced cell death 34. Finally, ITGB2 plays a role in the immune response and it has been shown that neutrophil chemotaxis correlates significantly with liver necrosis from acetaminophen toxicity 19.
Our findings support the hypothesis that blood gene expression profiles can serve as candidates for early biomarkers of an acetaminophen-induced response. Due to the small sample size of the test data set, the predictive value of the 12 early responder genes should be interpreted with caution. Further studies to evaluate the performance of the predictive genes are needed as resources and clinical samples become available.
Other mechanistic biomarkers for acetaminophen toxicity have been investigated 36–41. Genes and their expression as early biomarkers of an adverse acetaminophen response have some advantages in that they are mostly well-annotated, they are protein-coding and harbor the genetic code of variants, and they have relationships with each other in a systems/gene network manner. Furthermore, gene expression assays are typically more robust and reproducible than proteomic and metabolomics assays albeit they can be more laborious and costly to implement. We anticipate that as with FDA-approved microarray-based clinical screens for determination of how a person metabolizes medicines or the assessment of risk that a breast tumor will metastasize, a diagnostic gene expression assay using genomic biomarkers for early detection of acetaminophen exposure is quite plausible for translation to clinical practice.
Supplementary Material
Acknowledgments
This research was supported [in part] by the Intramural Research Program of the National Institute of Environmental Health Sciences, NIH, and National Institute of Environmental Health Sciences grant P30ES10126, NIH grants R37 GM38149 and K23 RR21857-01, and The University of North Carolina General Clinical Research Center grant M000046.
Footnotes
The raw and preprocessed data for the clinical study are publicly available in the Gene Expression Omnibus (GEO) database under accession number: GSE70784. The raw and preprocessed data for the overdose patient data are publicly available in the GEO database under accession number: GSE70786.
Conflict of Interest
P.B.W. has served as a consultant for McNeil Corporation, a major manufacturer of acetaminophen-containing products. All other authors declare that they have no competing interests.
References
- 1.Ferner RE, Dear JW, Bateman DN. Management of paracetamol poisoning. BMJ. 2011;342:d2218. doi: 10.1136/bmj.d2218. [DOI] [PubMed] [Google Scholar]
- 2.Manthripragada AD, Zhou EH, Budnitz DS, Lovegrove MC, Willy ME. Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol Drug Saf. 2011;20(8):819–826. doi: 10.1002/pds.2090. [DOI] [PubMed] [Google Scholar]
- 3.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 4.Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15(6):398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- 5.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL. 2008 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 26th Annual Report. Clin Toxicol (Phila) 2009;47(10):911–1084. doi: 10.3109/15563650903438566. [DOI] [PubMed] [Google Scholar]
- 7.Mitka M. FDA asks physicians to stop prescribing high-dose acetaminophen products. JAMA. 2014;311(6):563. doi: 10.1001/jama.2014.716. [DOI] [PubMed] [Google Scholar]
- 8.Kuehn BM. FDA focuses on drugs and liver damage: labeling and other changes for acetaminophen. JAMA. 2009;302(4):369–371. doi: 10.1001/jama.2009.1019. [DOI] [PubMed] [Google Scholar]
- 9.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296(1):87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 10.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359(3):285–292. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou JW, Paules RS, Bushel PR. Systematic variation normalization in microarray data to get gene expression comparison unbiased. J Bioinform Comput Biol. 2005;3(2):225–241. doi: 10.1142/s0219720005001028. [DOI] [PubMed] [Google Scholar]
- 12.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 13.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. 4. xv. Irwin; Chicago: 1996. p. 720. [Google Scholar]
- 14.Storey JD. A direct approach to false discovery rates. Journal of the Royal Statistical Society Series B-Statistical Methodology. 2002;64:479–498. [Google Scholar]
- 15.Storey JD. The positive false discovery rate: A Bayesian interpretation and the q-value. Annals of Statistics. 2003;31(6):2013–2035. [Google Scholar]
- 16.Hughes TR, Marton MJ, Jones AR, Roberts CJ, Stoughton R, Armour CD, et al. Functional discovery via a compendium of expression profiles. Cell. 2000;102(1):109–126. doi: 10.1016/s0092-8674(00)00015-5. [DOI] [PubMed] [Google Scholar]
- 17.Hastie T, Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning : data mining, inference, and prediction. xvi. Springer; New York: 2001. p. 533. [Google Scholar]
- 18.Omer A, Singh P, Yadav NK, Singh RK. An overview of data mining algorithms in drug induced toxicity prediction. Mini Rev Med Chem. 2014;14(4):345–354. doi: 10.2174/1389557514666140219110244. [DOI] [PubMed] [Google Scholar]
- 19.Beyer RP, Fry RC, Lasarev MR, McConnachie LA, Meira LB, Palmer VS, et al. Multicenter study of acetaminophen hepatotoxicity reveals the importance of biological endpoints in genomic analyses. Toxicol Sci. 2007;99(1):326–337. doi: 10.1093/toxsci/kfm150. [DOI] [PubMed] [Google Scholar]
- 20.Heinloth AN, Boorman GA, Foley JF, Flagler ND, Paules RS. Gene expression analysis offers unique advantages to histopathology in liver biopsy evaluations. Toxicol Pathol. 2007;35(2):276–283. doi: 10.1080/01926230601178207. [DOI] [PubMed] [Google Scholar]
- 21.Baken KA, Pennings JL, Jonker MJ, Schaap MM, de Vries A, van Steeg H, et al. Overlapping gene expression profiles of model compounds provide opportunities for immunotoxicity screening. Toxicol Appl Pharmacol. 2008;226(1):46–59. doi: 10.1016/j.taap.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Bushel PR. PhD dissertation. 2005. Clustering of Mixed Data Types with Application to Toxicogenomics. [Google Scholar]
- 23.Poulsen HE, Petersen P, Vilstrup H. Quantitative liver function and morphology after paracetamol administration to rats. Eur J Clin Invest. 1981;11(3):161–164. doi: 10.1111/j.1365-2362.1981.tb01835.x. [DOI] [PubMed] [Google Scholar]
- 24.Lewis MJ, Pelham HR. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 1992;68(2):353–364. doi: 10.1016/0092-8674(92)90476-s. [DOI] [PubMed] [Google Scholar]
- 25.Handford M, Rodriguez-Furlan C, Orellana A. Nucleotide-sugar transporters: structure, function and roles in vivo. Braz J Med Biol Res. 2006;39(9):1149–1158. doi: 10.1590/s0100-879x2006000900002. [DOI] [PubMed] [Google Scholar]
- 26.Parrado A, Robledo M, Moya-Quiles MR, Marin LA, Chomienne C, Padua RA, et al. The promyelocytic leukemia zinc finger protein down-regulates apoptosis and expression of the proapoptotic BID protein in lymphocytes. Proc Natl Acad Sci U S A. 2004;101(7):1898–1903. doi: 10.1073/pnas.0308358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyonaga T, Hino O, Sugai S, Wakasugi S, Abe K, Shichiri M, et al. Chronic active hepatitis in transgenic mice expressing interferon-gamma in the liver. Proc Natl Acad Sci U S A. 1994;91(2):614–618. doi: 10.1073/pnas.91.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krenkel O, Mossanen JC, Tacke F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg Nutr. 2014;3(6):331–343. doi: 10.3978/j.issn.2304-3881.2014.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2(4):493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- 30.Masson MJ, Peterson RA, Chung CJ, Graf ML, Carpenter LD, Ambroso JL, et al. Lymphocyte loss and immunosuppression following acetaminophen-induced hepatotoxicity in mice as a potential mechanism of tolerance. Chem Res Toxicol. 2007;20(1):20–26. doi: 10.1021/tx060190c. [DOI] [PubMed] [Google Scholar]
- 31.Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32(1):8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radici L, Bianchi M, Crinelli R, Magnani M. Ubiquitin C gene: Structure, function, and transcriptional regulation. Advances in Bioscience and Biotechnology. 2013;4:1057–1062. [Google Scholar]
- 33.Nagy G, Kardon T, Wunderlich L, Szarka A, Kiss A, Schaff Z, et al. Acetaminophen induces ER dependent signaling in mouse liver. Arch Biochem Biophys. 2007;459(2):273–279. doi: 10.1016/j.abb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 34.McQueen CA. Comprehensive Toxicology. Elsevier Science & Technology Books; San Diego, Saint Louis: 2010. [Google Scholar]
- 35.Yoshida H. ER stress and diseases. FEBS J. 2007;274(3):630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106(11):4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward J, Kanchagar C, Veksler-Lublinsky I, Lee RC, McGill MR, Jaeschke H, et al. Circulating microRNA profiles in human patients with acetaminophen hepatotoxicity or ischemic hepatitis. Proc Natl Acad Sci U S A. 2014;111(33):12169–12174. doi: 10.1073/pnas.1412608111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar BS, Chung BC, Kwon OS, Jung BH. Discovery of common urinary biomarkers for hepatotoxicity induced by carbon tetrachloride, acetaminophen and methotrexate by mass spectrometry-based metabolomics. J Appl Toxicol. 2012;32(7):505–520. doi: 10.1002/jat.1746. [DOI] [PubMed] [Google Scholar]
- 39.van Swelm RP, Laarakkers CM, van der Kuur EC, Morava-Kozicz E, Wevers RA, Augustijn KD, et al. Identification of novel translational urinary biomarkers for acetaminophen-induced acute liver injury using proteomic profiling in mice. PLoS One. 2012;7(11):e49524. doi: 10.1371/journal.pone.0049524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prot JM, Briffaut AS, Letourneur F, Chafey P, Merlier F, Grandvalet Y, et al. Integrated proteomic and transcriptomic investigation of the acetaminophen toxicity in liver microfluidic biochip. PLoS One. 2011;6(8):e21268. doi: 10.1371/journal.pone.0021268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merrick BA, Bruno ME, Madenspacher JH, Wetmore BA, Foley J, Pieper R, et al. Alterations in the rat serum proteome during liver injury from acetaminophen exposure. J Pharmacol Exp Ther. 2006;318(2):792–802. doi: 10.1124/jpet.106.102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.