Abstract
Obesity is a major public health problem affecting more than 12 million (~17%)U.S. children. The scientific community agrees that tackling this problem must begin in childhood to reduce risk of subsequent development of cardiovascular diseases and other chronic diseases. The Childhood Obesity Prevention and Treatment Research (COPTR) Consortium, initiated by the National Institutes of Health (NIH), is conducting intervention studies to prevent obesity in pre-schoolers and treat overweight or obese 7–13 year olds. Four randomized controlled trials plan to enroll a total of 1,700 children and adolescents (~ 50% female, 70% minorities), and are testing innovative multi-level and multi-component interventions in multiple settings involving primary care physicians, parks and recreational centers, family advocates, and schools. For all the studies, the primary outcome measure is body mass index; secondary outcomes, moderators and mediators of intervention include diet, physical activity, home and neighborhood influences, and psychosocial factors. COPTR is being conducted collaboratively among four participating field centers, a coordinating center, and NIH project offices.
Keywords: childhood obesity, prevention, treatment, multi-site trial, research consortium
INTRODUCTION
The current epidemic of childhood obesity requires urgent attention and action. Obesity rates have increased among children and adolescents over the past thirty years (1–3). Data from the 2009–2010 National Health and Nutrition Examination Survey (NHANES) indicate that about 32% of children and adolescents aged 2 to 19 years were overweight (≥ 85th percentile to <95th percentile of the body mass index [BMI]-for-age growth charts) or obese (≥95th percentile of the BMI-for-age growth charts) (1). While overall childhood obesity prevalence may have stabilized (1, 3), non-white children and adolescents continue to be disproportionately affected (1, 3). The prevalence of overweight is particularly high among Mexican American, Hispanic, and African American youth (1,6) and even higher among American Indian youth (7). The combined prevalence of overweight and obesity in 6–19 year-old non-Hispanic Blacks was 42%, compared to 29% in Caucasians (1). In the U.S., low socioeconomic status (SES) is a risk factor for obesity in non-white populations where the prevalence increases as household income decreases (9–10).
Obesity during childhood and adolescence has been associated with health complications such as hypertension, dyslipidemia, left ventricular hypertrophy, atherosclerosis, metabolic syndrome, type 2 diabetes, sleep disorders, orthopedic problems and non-alcoholic fatty liver disease (11–13) as well as psychological difficulties such as stigmatization, discrimination, depression and poor self-esteem (14–16). Childhood obesity also substantially increases the risk of being an obese adult (17). At the current rates of childhood obesity, 30 to 40% of today’s children may eventually develop type 2 diabetes, placing them at higher risk for cardiovascular diseases and other health complications, and potentially reducing their life expectancy (4).
The health consequences of childhood overweight and obesity add to the burden of health-care costs. The direct medical costs of childhood obesity have been estimated at $14.1 billion annually (18–19). Hospitalization costs for obesity-associated co-morbidities of pediatric patients were higher than those that were not obesity-associated (e.g., discharges for asthma with obesity as a secondary diagnosis cost $7,766 compared to those without obesity at $6,043; p<0.05), providing a financial imperative for obesity prevention initiatives (20–22).
The Childhood Obesity Prevention and Treatment Research (COPTR) Consortium is a three-phase multi-site research program created to address the growing epidemic of childhood obesity. Epidemiologic studies indicate that numerous factors shape daily diet and physical activity behaviors in children and adolescents, suggesting that successful prevention and treatment of childhood overweight and obesity must address many factors concurrently. Thus, the purpose of COPTR is to conduct rigorously designed trials that test innovative combinations of intervention components in multiple settings in which children live and play. Unlike previous intervention studies addressing childhood obesity, COPTR studies combine interventions at the individual, family, clinic and community levels requiring multidisciplinary collaborative teams, build on previous childhood obesity research and target a diverse population (N=1,700, ~ 50% female; ~30% whites, ~20% Hispanics and ~50% African Americans).
Background
Many studies set the stage for COPTR. These include NIH-initiated observational studies such as the National Growth and Health Study (23), intervention studies such as Pathways (24), the Girls Health Enrichment Multi-site Studies (GEMS) (25, 26) and the Trial of Activity for Adolescent Girls (TAAG) (27), as well as other national and international childhood obesity prevention and treatment research previously reported in the literature (e.g., systematic reviews). In addition, in August 2007, an NHLBI-convened workshop on childhood obesity [http://www.nhlbi.nih.gov/meetings/workshops/child-obesity/index.htm] provided recommendations, which led to the development of a research initiative on childhood obesity prevention and treatment. We present briefly the findings from three initial intervention studies and lessons that informed the development of the COPTR initiative.
The Pathways study (24) was one of the first large, obesity prevention trials in youth. It tested the effect of a school-based, multi-component intervention to reduce the percentage of body fat in American Indian school children. Details of the intervention have been published by Caballero et al (24). There were no significant intervention effects on the primary outcome of percent body fat or the secondary outcome of BMI. However, significant reductions in total energy intake and percent energy from fat were observed in intervention schools as well as improvements in healthy food choice intentions and attitudes. This study demonstrated the feasibility of implementing a multi-component intervention in a large number of American Indian schools (N=41 schools with 1,704 children).
GEMS was a two-phase multi-site childhood obesity prevention study that was conducted to address the issue of obesity prevention in pre-adolescent and adolescent African American girls (25) in four different geographic locations across the U.S. Phase I evaluated four different intervention strategies. The two most promising strategies then proceeded to phase 2, a two-year clinical trial testing the interventions. The two studies targeted improved diet and increased physical activity to minimize excess weight gain (26, 27), but used different approaches. Details of the intervention have been reported by Robinson et al. (26) and Klesges et al. (27). Results indicated no significant intervention effects on BMI, the primary outcome, although improved cardiometabolic indicators (serum lipids and insulin) and depressive symptoms were observed (26) and subgroup analysis showed improved intervention effects on BMI in younger children compared to older children (27). The lack of substantial efficacy on overall BMI change in these well-executed but focused interventions suggests the need to address multiple levels of influence simultaneously, including the physical and social environments, and the need for more intensive behavior modification strategies and stronger physical activity components (26, 27).
TAAG was a school-based, community-linked intervention aimed at reducing the decline in physical activity often seen as girls enter adolescence (28). This two-year intensive intervention demonstrated no significant change in moderate to vigorous physical activity levels prior to a planned handoff of the program to local school champions in the third year. School champions were trained by study personnel and were allowed to adapt the program to local needs. Modest improvements in moderate-to-vigorous physical activity were seen after this transition (28), illustrating that local adaptation of a standard school- and community-linked intervention could potentially increase potency. Details of the TAAG interventions have been reported by Webber et al. (28).
A review of published literature also noted these shortcomings and gaps in our knowledge of childhood obesity. In a Cochrane review of 22 obesity prevention interventions, only two prevention trials resulted in significantly lowered BMI (29) and a second Cochrane review of 18 obesity treatment trials, the authors found very small studies with inadequate power to detect treatment effects, and lack of generalizability because the settings were mostly hospital-based (30). For both prevention and treatment trials, problems in research design and methods including use of inappropriate statistical methods, low participation and adherence rates, insufficient potency of interventions, limited use of theoretical frameworks to design the intervention, and short duration of studies (a majority less than 12 months) were identified. A more recent Cochrane review indicates promising effects of childhood obesity interventions on BMI and recommends that authors report process and implementation factors as well as potential harms and costs(31). These comprehensive reviews demonstrate the limitations of previous childhood obesity prevention and treatment studies.
Collectively, Pathways, GEMS, and TAAG suggest that multi-component or multi-level interventions may be more likely to succeed if they include behavioral goal setting and behavioral modification, changes in the environment, and simultaneously focus on both physical activity and dietary changes. In addition, previous study designs did not consider the impact of multiple environments (e.g., family, school, and neighborhood or community) that may influence the development of overweight and obesity nor did they target factors that may include the development of obesity in minority youth or food and environmental policies. Although there is strong justification from observational studies that multiple factors influence weight (e.g., individual behaviors; peer influences; home, family and school environments; and healthcare settings), sparse data exist on the effectiveness multi-component interventions targeted at multiple factors known to impact body weight. Additionally, novel approaches such as adaptive interventions have yet to be well-explored (32). Adaptive interventions tailor intervention components to each participant based on pre-specified decision rules built into the intervention protocol. To increase the evidence base for efficacious prevention and treatment methods, research is needed that is rigorous in methodology and builds on previous childhood obesity research to test promising intervention approaches. The COPTR trials attempt to fill this gap.
COPTR Consortium Overview
COPTR was initiated as the next generation of research studies to address these shortcomings and to take advantage of recent advances in our knowledge of environmental influences on energy balance, including physical environments and psychosocial environments such as social networks, and biological factors including genetics. COPTR studies build on previous research with emphasis on methodological rigor, multiple intervention components in multiple settings, and target children and youth of various racial/ethnic groups and socio-economic status.
Consortium Structure
The four field centers that are conducting the COPTR trials are:
Vanderbilt University School of Medicine, Nashville (Principal Investigator: Shari L. Barkin, M.D., MSHS)
Case Western Reserve University, Cleveland (Principal Investigator: Leona Cuttler, M.D., Shirley Moore, Ph.D., and Elaine Borawski, Ph.D.)
Stanford University, Palo Alto, Calif. (Principal Investigator: Thomas N. Robinson, M.D., M.P.H.)
University of Minnesota, Twin Cities (Principal Investigators: Simone French, Ph.D., Nancy Sherwood, Ph.D.)
Details on the design of their studies are reported in this issue of the journal. The Research Coordinating Unit (RCU) is the University of North Carolina, Chapel Hill (Principal Investigator: June Stevens, Ph.D.).
The primary collaborative decision-making structure for the COPTR Consortium is the Steering Committee. This Committee meets monthly and consists of at least one Principal Investigator from each of the four field centers, the Principal Investigator of the RCU and the NHLBI Project Officer. The Steering Committee is charged with making decisions on policy that impact the overall consortium. Issues that relate to only one of the four centers are generally decided by the Principal Investigators of the affected center. Any member of the Steering Committee can bring an issue to that Committee for discussion. Discussions on site-specific issues are advisory only to the study center Principal Investigator (i.e., the Principal Investigator can elect to follow, or not to follow the decision of the Steering Committee).
There are five major subcommittees in COPTR: 1) Intervention; 2) Measurement; 3) Recruitment, Consent, Retention and Adverse Events; 4) Publications, Presentations, and Ancillary Studies; and 5) Early Stage Investigators. Subcommittees have at least one representative from each of the 6 “Constituents” (field centers, the RCU and the Project Office) and are advisory to the Steering Committee. Table 1 describes the functions of each subcommittee. Working Groups are established by the Subcommittees to perform tasks in focused areas. For example, the measurement subcommittee has 10 working groups: Biomedical Measures, Cost Effectiveness, Data Capture and Management, Design and Analysis, Diet and Physical Activity, Diet Derived Variables, Mediators and Moderators, Measurement Implementation, Physical Activity Derived Variables, and Primary Outcome and Body Composition.
Table 1.
COPTR Subcommittees
| Subcommittee | Examples of Subcommittee Functions |
|---|---|
|
| |
| Intervention | Reviews and enhances main trial intervention with lessons learned from phase 1 (e.g., pilot studies), and trouble shoots intervention challenges. |
| Reviews, enhances and monitors process evaluation measures and other assessments of intervention quality. | |
| Discusses procedures to enhance intervention retention along with the project coordinators working group | |
|
| |
| Measurement | Reviews and enhances study designs for the main trials to insure adequate statistical power to quantify precisely specified intervention effects for the primary outcomes. |
| Proposes new measures for the study as a whole. Reviews all measures and measurement tools and recommends common measures to be collected at multiple sites. | |
| Reviews and enhances data capture and analysis methods, plans and implements those methods when pertinent to common measures. | |
|
| |
| Recruitment, Consent, Retention and Adverse Events | Reviews subject eligibility criteria and recruitment plans at each Center and proposes strategies to promote recruitment and retention. |
| Works with the Coordinating Center to develop monitoring forms and monitors recruitment and retention and adverse events. | |
| Discusses methods to enhance recruitment and retention in sites that face challenges achieving their targeted enrollment or retention. | |
|
| |
| Publications, Presentations, and Ancillary Studies | Develops and recommends to the Steering Committee study policies regarding publications, ancillary studies and access to data from the COPTR studies. |
| Records and reports all manuscript proposals, abstract submissions and publications proposed by investigators. | |
| Reviews, advises and approves all proposed ancillary studies and ensure that they do not adversely affect the main COPTR trial. Submits proposed ancillary studies to the Steering Committee for approval. | |
|
| |
| Early Stage Investigator | Early Stage Investigators (ESIs) are new investigators who are within 10 years of having completed terminal research degree or medical residency and have not been awarded an NIH Research Project Grant. Each field site has at least one ESI who is included in the study to develop a cadre of investigators involved in childhood obesity research. Activities include monthly meetings; discussions on publications, presentations, and ancillary studies; and networking and sharing of research ideas. |
Role of the Research Coordinating Unit
The Research Coordinating Unit (RCU) at the University of North Carolina at Chapel Hill enhances the efforts of the field centers by collaborating as a scientific partner and coordinating the activities of the Consortium. The RCU establishes and monitors study timelines and creates reports that assist the work of the field centers. Communications are assisted through meetings by conference calls, in person and by a study website.
The RCU facilitates the selection of measurements and production of protocols and manual of procedures and conducts a central training for data collectors using a train-the-trainer model for common measures (Table 2), defined as data collected by two or more field centers across the consortium. A common protocol and procedures are used by all centers to collect common measures. The RCU monitors certification of data collectors and the quality of the common data collected, assembles a master data set, conducts analyses for publications that are study-wide specific, creates public use files, and archives data. The RCU Data Center provides a set of web-based tools for centers to upload completed common measures to the central repository. Data reports of both common and site specific variables are produced by the RCU for review by the Data Safety and Monitoring Board (DSMB). Documents supporting the study are held on a password-protected website (http://www.coptr.org/). This website will also have public access documents at the completion of the study.
Table 2.
Common Measures Across the Consortium
| Measure | Case | Stanford | Minnesota | Vanderbilt |
|---|---|---|---|---|
| ANTHROPOMETRICS | ||||
| Index Child | ||||
| Weight | X | X | X | X |
| Height | X | X | X | X |
| Waist circumference | X | X | X | X |
| Triceps skinfolds | X | X | X | X |
| Other Children | ||||
| Weight | X | X | ||
| Height | X | X | ||
| Waist circumference | X | |||
| Triceps skinfolds | X | |||
| Other Adults | ||||
| Weight | X | X | X | X |
| Height | X | X | X | X |
| Waist circumference | X | X | ||
| Triceps skinfolds | X | |||
| DIET | ||||
| 3 24hr dietary recalls | X | X | X | X |
| ACCELEROMETER | ||||
| Index child | X | X | X | X |
| Parent | X | X | ||
| QUESTIONNAIRES | ||||
| Household Configuration (gender, age, relationship to child) | X | X | X | |
| Child’s date of birth | X | X | X | X |
| Child Sex | X | X | X | X |
| Child Ethnicity | X | X | X | X |
| Child Race | X | X | X | X |
| Parent Ethnicity | X | X | X | X |
| Parent Race | X | X | X | X |
| Parent Country of Birth | X | X | X | |
| Child Country of Birth | X | X | X | |
| Years Parent Lived in USA | X | X | X | |
| Employment Status | X | X | X | X |
| Marital Status | X | X | X | X |
| Access to Car | X | X | X | |
| Frequency speak English at home | X | X | ||
| Other language speak at home | X | X | ||
| WIC | X | X | X | |
| Food Stamps/SNAP | X | X | X | X |
| Unemployment/Social Security/Disability | X | X | X | |
| Education | X | X | X | X |
| Education – other parent/adult | X | X | X | X |
| Child care in your own home | X | X | ||
| Child Care in someone else’s home | X | X | ||
| in childcare center/after school program | X | X | ||
| Household Income | X | X | X | X |
| Child Health Insurance | X | X | X | X |
| type of health insurance | X | X | X | |
| Free or Reduced Price Breakfast or Lunch | X | X | ||
| started menstrual period? | X | X | ||
| Age/date menstrual period 1st started | X | X | ||
| breastfeed for more than a month? | X | X | X | |
| 1st received a bottle of formula, cow’s milk, water, juice, tea, or cereal | X | X | X | |
| child weight at birth | X | X | X | |
| diabetes when pregnant | X | X | X | |
| hypertension when pregnant | X | X | X | |
| Food Security (6 questions) | X | X | X | X |
| # TVs | X | X | X | X |
| TV in child’s room | X | X | X | X |
| computer in your home | X | X | X | X |
| computer in child’s room | X | X | X | X |
| video game player in your home | X | X | X | X |
| video game player in child’s room | X | X | X | X |
| Internet access | X | X | X | |
| WEEK day hours watch TV? | X | X | ||
| WEEKEND hours watch TV? | X | X | ||
| Hours playing video games | X | X | ||
| family eat breakfast together? | X | X | ||
| family eat lunch together? | X | X | ||
| family eat dinner together? | X | X | ||
| classify your own weight? | X | X | X | X |
| classify child’s current weight? | X | X | X | X |
| BLOOD PRESSURE | ||||
| Blood pressure | X | X | ||
| Pulse | X | X | ||
| BIOMEDICAL | X | |||
| Insulin | X | X | ||
| Glucose | X | X | ||
| CRP | X | X | ||
| ALT | X | X | ||
| HbA1c | X | X | ||
| Total cholesterol | X | X | ||
| VLDL cholesterol | X | X | ||
| LDL cholesterol | X | X | ||
| HDL cholesterol | X | X | ||
| Triglycerides | X | X |
The RCU enriches the work of the Consortium by conducting targeted literature reviews on critical topics and facilitating consultations with experts from outside the study. In addition, epidemiologists and biostatisticians at the RCU provide expertise in study design and statistical analysis. Finally, in collaboration with the NHLBI project office, the RCU monitors evaluation of Consortium activities as a whole and will prepare public access data files of common measures at the completion of the study.
Consortium Design Elements
COPTR consists of three phases conducted over 7 years. Phase 1 focuses on protocol development, planning, formative research and pilot testing for up to 2 years. Phase 2 is four to five years and includes three years of intervention, process and outcome measurements, and follow-up. Phase 3 is up to one year and focuses primarily on data analyses and dissemination of study results. Bi-annual scientific and administrative reviews and evaluation are conducted by an NHLBI-appointed Data and Safety Monitoring Board. Details of the study designs for each field center are reported in this journal.
Briefly, three of the studies (Minnesota, Vanderbilt, Case Western Reserve) employ an individually randomized group-treatment (IRGT) design (33). In this design, individuals are randomized to study conditions but interact in small groups post-randomization. Such interaction creates the expectation for positive correlation among observations on participants from the same small group and must be considered in the design and analytic plan (33). The fourth study (Stanford) employs a randomized clinical trial design in which individuals are randomized to study conditions. In this case, there is an expectation for unpredictable and inconsistent pattern of interaction post-randomization and the study investigators judged such interaction to be ignorable. Table 3 presents a summary of the study design at each field center and details are available in the separate papers for each study presented elsewhere in this issue.
Table 3.
Summary of key design elements in the COPTR Trials
| Minnesota | Vanderbilt | Stanford | Case Western Reserve | |
|---|---|---|---|---|
| Design | 2 arm IRGT (I vs. C) with small groups in I only | 2 arm IRGT (I vs. C) with small groups in I (weight) and C (literacy) | 2 arm RCT (I vs. C) with mix of individual, family and non-fixed group interventions in I (weight) and C (active placebo) | 3 arm IRGT (I #1 andI #2 vs. C) with small groups in two arms (I #1 and I #2) crossed with 2 arm NRGT (I vs. C) |
| Intervention channels | Intervention aimed at parents via family connector home visitation programs and parenting classes at community centers to improve and reinforce healthy dietary patterns, and promote physical activity. | Intervention aimed at parents and children in classes at community centers. It includes social media and phone coaching to improve dietary patterns, and use of the built environment to enhance physical activity of parent and child. | Intervention aimed at youth and parents in home-based intervention to reduce screen time, increase physical activity and alter dietary practices; community-based after school team sports, and primary care counseling. | Intervention aimed at youth and parents in small group sessions and at youth in school-based activities through a series of goal setting, skills building, changes in the family environment and daily routines to improve diet and physical activity patterns. |
| Eligibility | BMI>= 50th percentile | 50th percentile<=BMI<95th percentile (may consider≥ 45th percentile and<99th percentile) | BMI >= 85th percentile for age and sex | BMI >= 85th percentile for age and sex |
| Randomization | A priori stratification by age (2,3,4) and sex (girl, boy) | A priori stratification by language preference (English, Spanish) | A priori stratification on BMI percentile category (overweight, obese) | A priori stratification by weight status, blood pressure status, and gender |
| Age group | 2–4 yrs at baseline | 3–5 yrs at baseline | 7–11 yrs at baseline | 10–13 yrs at baseline |
| Analysis | Mixed model | Mixed non-linear model | Two-stages: individual slopes in first stage, ANCOVA in second stage | Two stages; individual slopes in first stage, ANCOVA in second stage |
| Retention | Expect to retain 75%–85% at 24 and 36 months. | Expect to retain 80% at 36 months | Expect to retain > 85% at 36 months | Expect to retain 70% at 36 months |
| Missing data | Assumed MAR based on experience from earlier studies with similar outcome (weight) showing no differential loss to follow-up associated with baseline measures | Assumed MAR or MCAR. Will consider NMAR in secondary analyses. | Assume MAR after conditioning on baseline BMI and other baseline values. Will consider multiple MAR models and multiple NMAR models in secondary analyses. | Assumed MAR. Will consider NMAR in secondary analyses. |
| Imputation | Missing data will be considered in secondary analyses | Missing data will be considered in secondary analyses | Missing data will be considered in primary analysis | Missing data will be considered in secondary analyses. |
| Effect size expected | 0.3 sd units for means at 24 or 36 months | 0.4sd units for quadratic term | 0.4 sd units for slopes | 0.4 sd units for slopes |
C is control; I is intervention; IRGT is individually randomized group-treatment trial; MAR is missing at random; MCAR is missing completely at random, NMAR is not missing at random; NRGT is a non-randomized group trial; RCT is randomized controlled trial. Details are available in the separate papers for each study presented elsewhere in this issue.
Common and Site-Specific Measurements
The COPTR investigators established some common measures (Table 2) and common data collection methodologies to facilitate the potential for combining data across sites to support additional analysis. For issues involving common variables, the decisions of the Steering Committee are binding, not advisory.
BMI is the primary outcome variable at all the COPTR sites. Other common variables include waist circumference, triceps skinfold, calculated percent body fat, diet (by three 24-hour recalls), physical activity (by 7 days of accelerometry using Actigraph GT3X+ or GT3X monitors), and questionnaires that assess demographics, food security, TV and media use and food norms. Blood specimens are collected at the two sites intervening on school-aged children (Stanford and Case Western Reserve) and analyzed by a common laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratory, University of Washington). Measurements to be obtained from these samples include fasting blood glucose, hemogloblin A1c (HbA1c), total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, high-sensitivity C-reactive protein (hs-CRP), insulin and alanine aminotransferase (ALT). Blood pressure is also obtained at these two sites (Stanford and Case Western Reserve) using automated blood pressure devices and similar protocols.
Site-specific measurements are those collected at only one of the four COPTR sites (see details in site-specific design papers). These measurements include process evaluation assessments that are tailored to the intervention delivered at that site, as well as assessments of mediators and moderators that are hypothesized to be important to a specific intervention. Intervention participation rates are collected at all four sites for key activities.
Consortium goals and public health significance
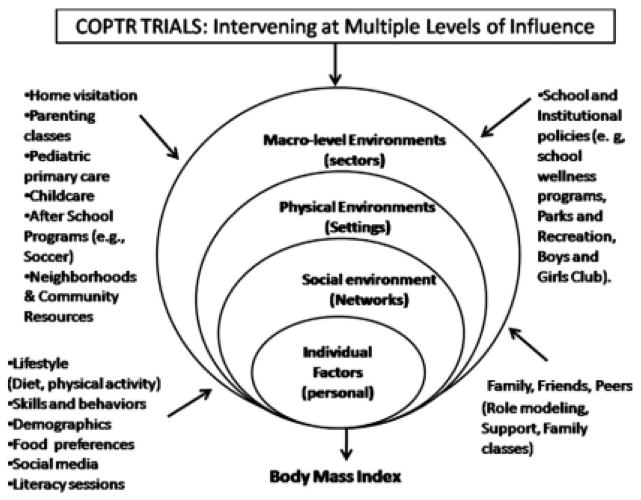
There is a paucity of childhood obesity prevention and treatment studies that evaluate interventions addressing multiple levels in multiple settings and use multiple intervention components. Communities nationwide face serious challenges in facilitating improvements in overweight and obesity in youth. A major research objective of COPTR is to enhance and optimize interventions that are practical with respect to implementation in real-world settings, such as in schools, community centers, and parks and recreation programs, but also consistent with past research that suggests the importance of using multi-level, multi-component interventions that directly promote healthy weight behaviors. COPTR distinguishes from past studies by intervening on multiple levels of influence– child, parent, family, home, health care provider, schools, child recreational facilities–, with multiple components of intervention. The Consortium structure facilities a) methodological rigor in study design, analysis, and quality; b) use of common measures, constructs and a common database for multisite analysis; c) ancillary studies across multiple sites; d) the involvement of early stage investigators in implementing the study; and e) the inclusion of a diverse and large sample (N=1,700), all of which are unlikely to be feasible in individual studies. Despite the differences in study design and intervention approaches, the studies have the potential to add to our knowledge on the impact on childhood and adolescent BMI of interventions that are multi-level, multi-component and conducted in multi-settings. There is also the potential to produce intervention resources and strategies that– if found effective– may be rapidly disseminated to communities nationwide because the interventions use and build on existing structures that are available and utilized by families and health professionals. Finding ways to effectively prevent and treat childhood obesity is a top public health priority.
Fig. 1.
COPTR intervening on multiple influences on child and adolescent obesity.
Acknowledgments
This research is being supported by grant numbers: U01 HL103561, U01 HL103620, U01 HL103622, U01 HL103629, and U01 HD068890 from the National Heart, Lung, and Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Development, and the NIH Office of Behavioral and Social Sciences Research.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index. United States 1960–2002. Adv Data. 2004;(347):1–17. [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2012;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 4.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 5.Harris KM, Gordon-Larsen P, Chantala K, Udry JR. Longitudinal trends in race/ethnic disparities in leading health indicators from adolescence to young adulthood. Arch Pediatr Adolesc Med. 2006;160(1):74–81. doi: 10.1001/archpedi.160.1.74. [DOI] [PubMed] [Google Scholar]
- 6.Anderson SE, Whitaker RC. Prevalence of obesity among US preschool children in different racial and ethnic groups. Arch Pediatr Adolesc Med. 2009;163(4):344–8. doi: 10.1001/archpediatrics.2009.18. [DOI] [PubMed] [Google Scholar]
- 7.Schell LM, Gallo MV. Overweight and obesity among North American Indian infants, children, and youth. Am J Hum Biol. 2012;24(3):302–13. doi: 10.1002/ajhb.22257. Epub 2012 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults. 1999–2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 9.Eagle TF, Sheetz A, Gurm R, Woodward AC, Kline-Rogers E, Leibowitz R, Durussel-Weston J, Palma-Davis L, Aaronson S, Fitzgerald CM, Mitchell LR, Rogers B, Bruenger P, Skala KA, Goldberg C, Jackson EA, Erickson SR, Eagle KA. Understanding childhood obesity in America: linkages between household income, community resources, and children’s behaviors. Am Heart J. 2012;163(5):836–43. doi: 10.1016/j.ahj.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Tandon PS, Zhou C, Sallis JF, Cain KL, Frank LD, Saelens BE. Home environment relationships with children’s physical activity, sedentary time, and screen time by socioeconomic status. Int J Behav Nutr Phys Act. 2012 Jul 26;9:88. doi: 10.1186/1479-5868-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, Robinson TN, Scott BJ, St Jeor S, Williams CL. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111(15):1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 12.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys 1963–2002. Circulation. 2007;116:1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 13.Lorch SM, Sharkey A. Myocardial velocity, strain, and strain rate abnormalities in healthy obese children. J Cardiometabolic Syndrome. 2007;2(1):30–34. doi: 10.1111/j.1559-4564.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- 14.Whitlock EP, Williams SB, Gold R, Smith PR, Shipman SA. Screening and interventions for childhood overweight: a summary of evidence for the US Preventive Services Task Force. Pediatrics. 2005;116(1):e125–144. doi: 10.1542/peds.2005-0242. [DOI] [PubMed] [Google Scholar]
- 15.Dietz W. Health consequences of obesity in youth: Childhood predictors of adult disease. Pediatrics. 1998;101:518–525. [PubMed] [Google Scholar]
- 16.Swartz MB, Puhl R. Childhood obesity: a societal problem to solve. Obesity Reviews. 2003;4(1):57–71. doi: 10.1046/j.1467-789x.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 17.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 18.Trasande L, Chatterjee S. The impact of obesity on health service utilization and costs in childhood. Obesity. 2009;17(9):1749–1754. doi: 10.1038/oby.2009.67. [DOI] [PubMed] [Google Scholar]
- 19.Cawley J. The economics of childhood obesity. Health Affairs. 2010;29(3):364–371. doi: 10.1377/hlthaff.2009.0721. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein EA, Fiebelkorn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obesity Research. 2004;12:18–24. doi: 10.1038/oby.2004.4. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, Dietz WH. Economic Burden of Obesity in Youth Aged 7 to 17 years: 1979–1999. Pediatrics. 2002;109(5):E81–1. doi: 10.1542/peds.109.5.e81. [DOI] [PubMed] [Google Scholar]
- 22.Woolford SJ, Gebremarian A, Clark SJ, Davis MM. Incremental hospital charges associated with obesity as a secondary diagnosis in children. Obesity. 2007;15(7):1895–901. doi: 10.1038/oby.2007.224. [DOI] [PubMed] [Google Scholar]
- 23.Kimm SYS, Barton BA, Obarzanek E, McMahon RP, Sabry ZI, Waclawiw MA, Schreiber GB, Morrison JA, Similo S, Daniels SR. Racial divergence in adiposity during adolescence: The NHLBI Growth and Health Study. Pediatrics. 2011;107:34. doi: 10.1542/peds,107.3.e34. [DOI] [PubMed] [Google Scholar]
- 24.Caballero B, Clay T, Davis SM, Ethelbah B, Rock BH, Lohman T, Norman J, Story M, Stone EJ, Stephenson L, Stevens J Pathways Study Research Group. Pathways: a school-based, randomized controlled trial for the prevention of obesity in American Indian schoolchildren. Am J Clin Nutr. 2003;78(5):1030–8. doi: 10.1093/ajcn/78.5.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obarzanek E, Pratt CA. Girls health Enrichment Multi-site Studies (GEMS): new approaches to obesity prevention among young African-American girls. Ethn Dis. 2003;13(1 Suppl 1):S1–5. [PubMed] [Google Scholar]
- 26.Robinson TN, Kraemer HC, Matheson DM, Obarzanek E, Wilson DM, Haskell WL, Pruitt LA, Thompson NS, Haydel KF, Fujimoto M, Varady A, McCarthy S, Watanabe C, Killen JD. Stanford GEMS phase 2 obesity prevention trial for low-income African-American girls: design and sample baseline characteristics. Contemp Clin Trials. 2008;29(1):56–69. doi: 10.1016/j.cct.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klesges RC, Obarzanek E, Kumanyika S, Murray DM, Klesges LM, Relyea GE, Stockton MB, Lanctot JQ, Beech BM, McClanahan BS, Sherrill-Mittleman D, Slawson DL. The Memphis Girls’ health Enrichment Multi-site Studies (GEMS): an evaluation of the efficacy of a 2-year obesity prevention program in African American girls. Arch Pediatr Adolesc Med. 2010;164(11):1007–14. doi: 10.1001/archpediatrics.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webber LS, Catellier DJ, Lytle LA, Murray DM, Pratt CA, Young DR, Elder JP, Lohman TG, Stevens J, Jobe JB, Pate RR TAAG Collaborative Research Group. Promoting physical activity in middle school girls: Trial of Activity for Adolescent Girls. Am J Prev Med. 2008;34(3):173–84. doi: 10.1016/j.amepre.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summerbell CD, Waters E, Edmunds L, Kelly SAM, Brown T, Campbell KJ. Interventions for preventing obesity in children. Cochrane Database of Systematic Reviews. 2005;(3) doi: 10.1002/14651858.CD001871.pub2. Art. No.: CD001871. [DOI] [PubMed] [Google Scholar]
- 30.Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews. 2003;(3) doi: 10.1002/14651858.CD001872. Art. No.: CD001872. [DOI] [PubMed] [Google Scholar]
- 31.Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, Armstrong R, Prosser L, Summerbell CD. Interventions for preventing obesity in children. Cochrane Database of Systematic Reviews. 2011;(12) doi: 10.1002/14651858.CD001871.pub3. Art. No.: CD001871. [DOI] [PubMed] [Google Scholar]
- 32.Collins LM, Murphy SA, Bierman KL. A Conceptual Framework for Adaptive Preventive Interventions. Prevention Science. 2004;5(3):185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pals SP, Murray DM, Alfano CM, Shadish WR, Hannan PJ, Baker WL. Individually randomized group treatment trials: a critical appraisal of frequently used design and analytic approaches. Am J Public Health. 2008;98(8):1418–1424. doi: 10.2105/AJPH.2007.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belle SH, Czaja SJ, Schulz R, Zhang S, Burgio LD, Gitlin LN, Jones R, Mendelsohn AB, Ory MG. Using a New Taxonomy to Combine the Uncombinable: Integrating Results Across Diverse Interventions. Psychology and Aging. 2003;18(3):396–405. doi: 10.1037/0882-7974.18.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.1Weiner BJ, Lewis MA, Clauser SB, Stitzenberg KB. In Search of Synergy: Strategies for Combining Interventions at Multiple Levels. J Natl Cancer Inst Monogr. 2012;44:34–41. doi: 10.1093/jncimonographs/lgs001. [DOI] [PMC free article] [PubMed] [Google Scholar]