Abstract
Extraskeletal Ewing’s sarcoma is a rare tumor, and the management of airway compromise in case of cervical Ewing’s sarcoma has not been established. This report describes the case of a patient with retrotracheal Ewing’s sarcoma and discusses a successful approach to airway management. A 12-year-old male presented with a 2-week history of sore throat and sleep-disordered breathing and 48 hours of stridor. Imaging confirmed a retrotracheal soft tissue mass with airway compromise. A planned and controlled approach to his airway management resulted in a secure airway prior to definitive treatment.
Keywords: Ewing’s sarcoma, airway management, trachea, malignancy
Introduction
Airway compromise secondary to Ewing’s sarcoma is rare. However, if not recognized early and treated promptly, it can be life threatening. This report describes the case of a patient with retrotracheal Ewing’s sarcoma and the successful management of the patient’s at-risk airway.
Case Presentation
A 12-year-old boy presented with a 2-week history of sleep disordered breathing and sore throat and a 48-h history of stridor. His prior medical history was unremarkable with a body mass index of Exact BMI is 32.01kg/m2. He did not have dysphagia, weight loss, night sweats, or voice changes.
On his initial examination, he exhibited biphasic stridor. His neck examination did not reveal any mass, adenopathy, or limitation of movement. Flexible fiber-optic nasendoscopy demonstrated a normal larynx with bilaterally mobile vocal cords. A lateral X-ray of the neck showed a large pre-vertebral low density mass that was uniform in appearance and which narrowed the trachea.
During admission, his breathing worsened, and he was unable to lie flat. He was brought to the operation theatre and was anesthetized in the semi-recumbent position after a period of pre-oxygenation. His larynx was sprayed with 1% lidocaine, and he remained on spontaneous ventilation without the use of muscle relaxants during anesthesia induction. A tracheotomy set was opened by a senior pediatric surgeon who was scrubbed and ready. Airway control was obtained with a rigid ventilating bronchoscope that was passed through the trachea to a position distal to the obstruction. A subsequent examination demonstrated a normal oropharynx, larynx, and subglottis. An extrinsic compression of the mid-trachea and trachealis muscle from posterior was seen with 90% lumen compression but without direct invasion of the trachea (Figure 1). This extended 3.5 cm along the trachea. The distal trachea and carina were normal. The ventilating bronchoscope was extracted, and the patient was intubated with a cuffed size 6.5 endotracheal tube and was transferred to the intensive care unit.
Figure 1.
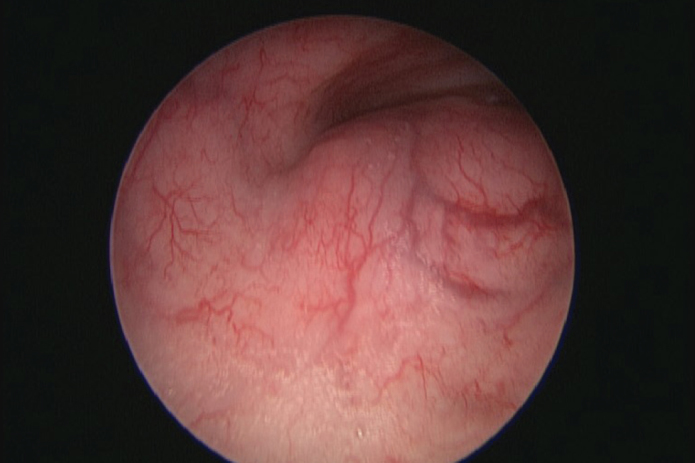
Tracheoscopic view of the mass
Computed tomography of the neck and chest showed a rounded, well-defined mass within the retrotracheal soft tissues, completely occluding the esophagus with a marked mass effect and narrowing the mid trachea (Figure 2–4). The mass was approximately 6.6×7.5×3.7 cm. There was no lymphadenopathy reported. On performing ultrasound, there were tiny flecks of calcification and evidence of vascularity. Ultrasound-guided biopsy showed a small round blue cell tumor. Immunohistochemistry showed strong membranous positivity with CD99 and some positivity with CD56 and synaptophysin. Myogenin and cytokeratin were negative. Genetic analysis showed an 11–22 translocation (Ewing’s Sarcoma breakpoint region one) gene rearrangement. These features were consistent with the diagnosis of Ewing’s sarcoma.
Figure 2.
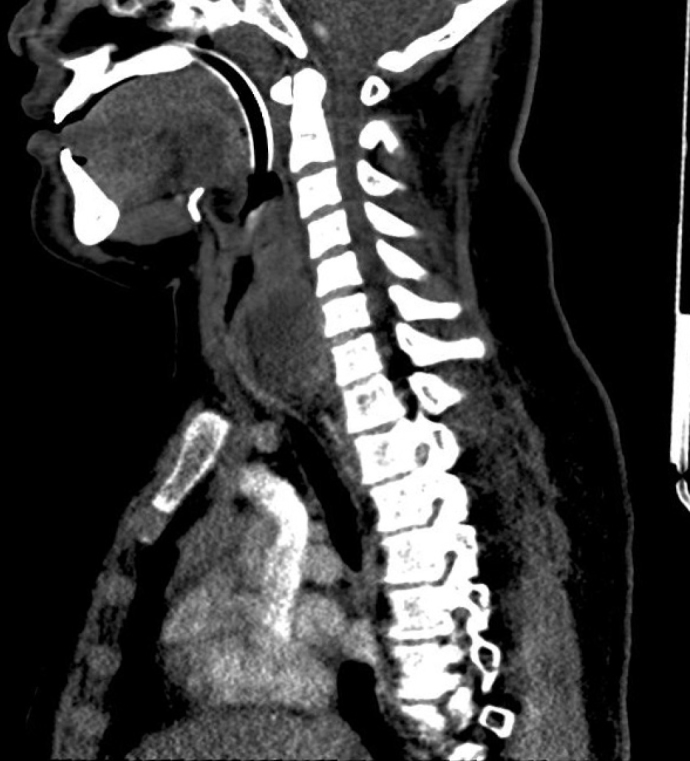
Parasagittal computed tomographic (CT) views of the lesion
Figure 3.
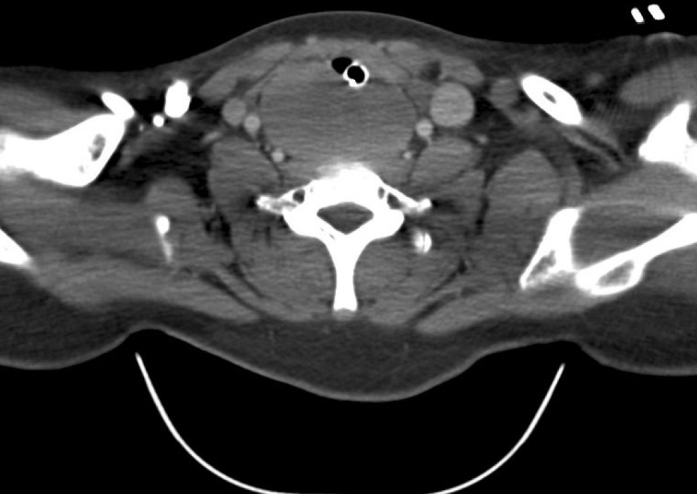
Axial CT view of the lesion
Figure 4.
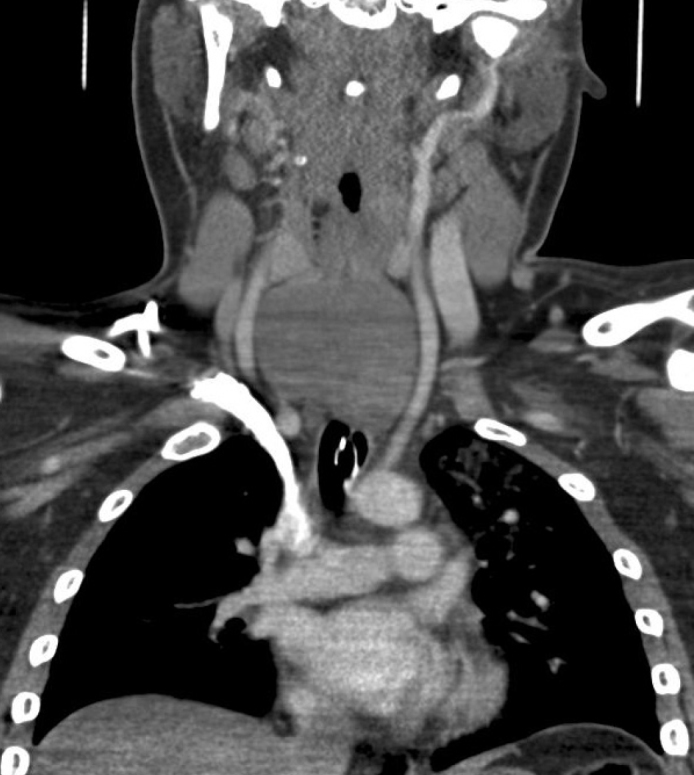
Coronal CT view of the lesion
He subsequently underwent tracheotomy with careful consideration of the placement of the tracheotomy to avoid potential erosive complications of the tumor and interference of the tracheotomy tube with radiotherapy.
Given the localization of the tumor, he underwent chemoradiotherapy rather than surgery. This took the form of 14 cycles of vincristine, doxorubicin, cytoxan, ifosfamide, and etoposide combined with 55.8 Gy of radiotherapy in 31 fractions. He required antibiotics for three episodes of neutropenic sepsis.
Although clinically disease-free after 18 months, tracheotomy decannulation was delayed due to radiotherapy side effects (causing an edematous larynx and mucositis) as well as suprastomal tracheal collapse.
Informed consent was obtained from the patient and his guardian for publishing this report.
Discussion
Ewing’s sarcoma was first described by James Ewing in 1921. The Ewing’s sarcoma family of tumors is a group that shares the same EWS/Ets oncogenic translocations. This family includes Ewing’s sarcoma, peripheral neuroepithelioma, peripheral primitive neuroectodermal tumor, and Askin tumor. The most common bony sites for involvement are the long bones, pelvis, chest wall, and spine (1).
Extraskeletal Ewing’s sarcoma (EES) is a rare tumor and is usually found in the pelvis, trunk, and extremities. Most head and neck cases have been described in the nasal passages or sinuses. There were four laryngeal cases, one tracheal case, and one esophageal case that we identified in our literature search (2–5).
Tefft et al. (6) first described extraskeletal soft tissue masses in four patients in 1969; these were identified as paravertebral soft tissue sarcomas having a histology similar to that of skeletal Ewing’s sarcoma. Histological features are small round blue cells with hyperchromatic nuclei, but other malignancies can also have this appearance. Cells typically express high levels of cell-surface glycoprotein, which can be identified by immunohistochemical studies of CD99 (7). The diagnosis is confirmed by bimolecular genetic testing to identify the characteristic translocation abnormalities. The most common translocation is gene EWSR1 on chromosome 22q1. This can be identified using fluorescent in situ hybridization or reverse transcription polymerase chain reaction (8).
Soft tissue (extraskeletal/extraosseous/primitive neuroectodermal) tumors are rare (9). These are more commonly reported to involve the nasal or oral cavities. Sinuses and soft tissues of the neck occur in the head and neck region. We found four case reports on laryngeal tumors (2–5) where their management has been described.
It is important to be aware of acute and long-term indications and implications in patients with oncological masses that compromise the airway and management options. The two laryngeal cases that were non-operatively managed did not require tracheotomies; a 74-year-old man requiring acute tracheotomy went on to undergo laryngectomy, similar to a 9 month baby (3). Our case is unique because we have not found any patient with a retrotracheal sarcoma. This has important clinical implications for maintaining a safe airway. Our patient developed an unstable airway shortly after presentation and required intubation and then tracheotomy for airway control.
The prognosis of EES is determined by stage and surgical margins if surgery is the first line of treatment. The 5-year overall survival is superior for localized EES than for localized skeletal tumors (69.7% vs 62.6%) (10).
Conclusion
A structured and planned approach to the management of a compromised airway is critical for good outcomes. The early recognition of the potential for airway compromise is paramount. A multidisciplinary team consisting of experienced anesthetists, pediatric otorhinolaryngology surgeons, intensive care physicians as well as support staff (nurses and anesthetic technicians) needs to be constituted when treating patients such as ours. Familiarity with airway devices, techniques of airway control, and the use of advanced airway equipment are essential. Good communication and an open-minded collaborative approach between the staff with different specialities, each with their specific skills and competencies, is a prerequisite for good outcomes.
Finally, it is important for head and neck surgeons to be aware of short and long-term chemoradiotherapy effects that will affect the management of similar airway tumors. In our case, significant laryngeal edema prevented tracheotomy decannulation.
Footnotes
Informed Consent: Written informed consent was obtained from the parents of patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.V.D.M., M.G.; Design - G.V.D.M, H.L., M.M., C.B.; Supervision - M.M., C.B.; Resource - G.V.D.M., C.B.; Materials - G.V.D.M, H.L., C.B.; Data Collection and/or Processing - G.V.D.M., H.L.; Analysis and/or Interpretation - G.V.D.M., M.G., C.B.; Literature Search - H.L.; Writing - G.V.D.M., H.L., M.G.; Critical Reviews - M.M., C.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This study was supported by the departmental resources.
References
- 1.Li S, Siegal GP. Small cell tumors of bone. Adv Anat Pathol. 2010;17:1–11. doi: 10.1097/PAP.0b013e3181bb6b9c. [DOI] [PubMed] [Google Scholar]
- 2.Yang YS, Hong KH. Extraskeletal Ewing’s sarcoma of the larynx. J Laryngol Otol. 2004;118:62–4. doi: 10.1258/002221504322731682. https://doi.org/10.1258/002221504322731682. [DOI] [PubMed] [Google Scholar]
- 3.Jones JE, McGill T. Peripheral primitive neuroectomdermal tumours of the head and neck. Arch Otolaryngol Head and Neck Surg. 1995;121:1392–5. doi: 10.1001/archotol.1995.01890120050009. https://doi.org/10.1001/archotol.1995.01890120050009. [DOI] [PubMed] [Google Scholar]
- 4.Lynch MC, Baker A, Drabick JJ, Williams N, Goldberg D. Extraskeletal Ewing’s sarcoma arising in the larynx. Head and Neck Pathol. 2014;8:225–8. doi: 10.1007/s12105-013-0492-6. https://doi.org/10.1007/s12105-013-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wygoda A, Rutkowski T, Poniekiewska D, Hejduk B, Skladowski K. Ewings sarcoma of the larynx, effective treatment with organ preservation. Strahlenther Onkol. 2013;189:586–9. doi: 10.1007/s00066-013-0356-8. https://doi.org/10.1007/s00066-013-0356-8. [DOI] [PubMed] [Google Scholar]
- 6.Tefft M, Vawter GF, Mitus A. Paravertebral ‘round cell’ tumors in children. Radiology. 1969;92:1501–9. doi: 10.1148/92.7.1501. https://doi.org/10.1148/92.7.1501. [DOI] [PubMed] [Google Scholar]
- 7.Fellinger EJ, Garin-Chesa P, Triche TJ, Huvos AG, Rettig WJ. Immunohistochemical analysis of Ewing’s sarcoma cell surface antigen p30/32MIC2. Am J Pathol. 1991;139:317–25. [PMC free article] [PubMed] [Google Scholar]
- 8.Applebaum MA, Worch J, Matthay KK, Goldsby R, Neuhaus J, West DC, et al. Clinical features and outcomes in patients with extraskeletal Ewing sarcoma. Cancer. 2011;117:3027–32. doi: 10.1002/cncr.25840. https://doi.org/10.1002/cncr.25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, et al. The Ewing family of tumours - a subgroup of small round cell tumours defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–9. doi: 10.1056/NEJM199408043310503. https://doi.org/10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- 10.Simada H, Newton WA, JR, Soule EH, Qualman SJ, Aoyama C, Maurer HM. Pathologic features of extraosseous Ewing’s sarcoma: a report from the intergroup rhabdomyosarcoma study. Hum Pathol. 1988;19:442–53. doi: 10.1016/s0046-8177(88)80495-7. https://doi.org/10.1016/S0046-8177(88)80495-7. [DOI] [PubMed] [Google Scholar]


