Abstract
Objective
With regard to the correlation between T helper1/T helper2 (Th1/Th2) cell balance and 1α,25-dihydroxyvitamin D3, active metabolite of vitamin D, we studied Th1/Th2 cell balance by measuring levels of the cytokines interleukin (IL)-4, IL-10 and interferon-gamma (IFN-γ), which are important for immune response of patients with allergic rhinitis.
Methods
Thirty adult patients diagnosed with allergic rhinitis (study group) and 40 healthy volunteers (control group) are examined in the research. IFN-γ, IL-4, IL-10, and total immunoglobulin E (IgE) levels from serum samples and vitamin D3 levels from plasma were determined in all patients.
Results
In IgE, IL-4, IL-10, IFN-γ, and 1α,25-dihydroxyvitamin D3 levels (p<0.05), a statistically noticeable difference was observed between the study and control group. The 1α,25-dihydroxyvitamin D3 levels in both groups were compared and a statistically significant difference between the 1α,25-dihydroxyvitamin D3 levels in the study group and that in the control group (p<0.05) was observed. There was a positive correlation between IFN-γ and vitamin D levels (p<0.05) in the study group, whereas IgE, IL-4, and IL-10 levels showed a negative correlation with vitamin D3 levels (p<0.05).
Conclusion
In our study, 1α,25-dihydroxyvitamin D3 levels were associated with Th1/Th2 balance in allergic rhinitis, and a remarkable correlation was observed among vitamin D deficiency and allergy. These findings show that 1α,25-dihydroxyvitamin D3 may have a remarkable role in the severity and control of allergic disorders. In addition, further investigations are required to confirm how vitamin D should be used in allergic diseases. Furthermore, to reveal the exact mechanism of vitamin D on allergic diseases, further studies are required.
Keywords: Rhinitis, allergic, cholecalciferol, Th1 cells, Th2 cells, interleukin-4, interleukin-10, interferon-gamma
Introduction
Allergic rhinitis is the inflammation of the nasal membranes that is characterized by sneezing, nasal congestion, nasal itching, and rhinorrhea in any combination (1). Studies performed in Western Europe have shown that allergic rhinitis has a high prevalence and is frequently undiagnosed (2, 3).
As a steroid hormone, vitamin D3 is produced in skin and ingested with food and is sequentially metabolized by the liver and kidney to the biologically active form 1α,25-dihydroxyvitamin D3 (4). As a modulator of the immune system, it has been demonstrated that vitamin D has complex immunoregulatory effects on both cellular and humoral immune response (5). 1α,25-Dihydroxyvitamin D3 is liposoluble, and it has a nuclear receptor that plays an important role in the regulation of immune response. Most immune cells, including macrophages, T-cells, B-cells, and dendritic cells have a vitamin D receptor (VDR) (4).
The findings that confirm the immunoregulatory effects of vitamin D in allergic diseases are increasing (6, 7). The level of cytokines released by inflammatory cells is reduced by vitamin D; therefore, vitamin D may perform a therapeutic role as an anti-inflammatory agent in allergic disease.
In this study, we aimed to investigate T helper1/T helper2 (Th1/Th2) cell balance by measuring levels of the cytokines interleukin (IL)-4, IL-10, and interferon-gamma (IFN-γ), which play an important role in the immune response of patients with allergic rhinitis, and to determine the correlation between among the Th1/Th2 balance and 1α,25-dihydroxyvitamin D3, the active metabolite of vitamin D.
Methods
We conducted this study at the Ear Nose Throat Clinic at Fırat University, School of Medicine, Turkey, with the approval from the Fırat University Faculty of Medicine Ethical Committee for Investigations in Humans, between February 2010 and March 2011. All patients and volunteers participating in the study signed an informed consent form. The subjects enrolled in the study were 30 adult patients and 40 healthy volunteers. Furthermore, healthy volunteers including 18 men and 22 women in the control group were aged 18–40 years (mean: 31.2±7.13 years); 13 men and 17 women in the allergic rhinitis (study) group were similarly aged 18–40 years (mean: 28.7±6.89 years). Total serum immunoglobulin E (IgE) level examination, a skin prick test, and medical history and examination were performed in all subjects to exclude the presence of allergic rhinitis in the healthy volunteers to confirm the diagnosis in the allergic rhinitis group (8). The control group included 40 healthy volunteers aged 18–40 years, who have no professional and environmental allergen exposure; no allergic rhinitis that is determined by physical examinations, serum total IgE levels, and skin prick test; no allergy symptoms or atopy history, and normal body mass index consistent with their age.
The exclusion criteria for subjects in this study are as follows:
patients who have used antihistamines during the previous 15 days and steroids during the previous month;
diseases with the potential to affect the serum 1α,25-dihydroxyvitamin D3 levels (osteoporosis and rickets);
upper respiratory tract infection, except for allergic rhinitis;
patients who use drugs with the potential to affect the serum 1α,25-dihydroxyvitamin D3 levels (cholestyramine, phenobarbital, and phenytoin);
drug-induced rhinitis, nonallergic eosinophilic rhinitis (NARES syndrome), and other acute airway diseases;
immune system disorders that have the potential to change cytokine levels;
patients with nasal polyposis or isolated polyps and asthma; asthma diagnosis of the patients is excluded using the diagnostic criteria of GINA 2002 guidelines using medical history, physical examination, and pulmonary function tests (9).
Demographical features such as age and sex of all subjects were registered. Blood draws were performed during spring and summer.
The 1α,25-dihydroxyvitamin D3, IL-4, IL-10, IgE and IFN-γ levels were compared between the study and control groups. The degree and direction of reciprocal correlations among IgE, IL-4, IL-10, IFN-γ, and vitamin D levels were calculated in the allergic rhinitis group.
The IL-4, IL-10, and IFN-γ levels were measured using enzyme- linked immunosorbent assay (ELISA), using commercial human IL-4, IL-10 and IFN-γ kits (Boster Immunoleader; Wuhan, China). 1α,25-Dihydroxyvitamin D3 levels were measured using high-pressure liquid chromatography (HPLC) (HPLC equipment by WICOM; Heppenheim, Germany). Skin prick tests were performed in multitest fashion with sterile, disposable plastic applicators. Total serum immunoglobulin levels were analyzed using an N Latex IgE mono kit (Siemens Healthcare Diagnostics Inc.; Newark, NJ, USA) with an automated nephelometer (Dade Behring GmbH; Marburg, Germany) according to the manufacturers’ instructions.
Statistical Analysis
SPSS for Windows v11.5 software package (SPSS Inc.; Chicago, IL, USA) is used for the assessment of statistical results. A one-way analysis of variance multiple comparisons test was used for comparing the groups, with post hoc analysis using Tukey’s honest significant difference (HSD) test. Statistical significance was set at p≤0.05.
Results
In the control group, mean IgE, IL-4, IL-10, IFN-γ, and vitamin D levels were measured. (IgE: 35.1±23.1 IU/mL; IFN-γ: 32.1±20.9 pg/mL; IL-4: 3±1.9 pg/mL; IL-10: 41.4±21.8 pg/mL; and 1α,25-dihydroxyvitamin D3: 28±13.2 μg/mL). In the study group, IgE, IL-4, IL-10, IFN-γ, and vitamin D mean levels were measured. (IgE: 199.79±158 IU/mL; IFN-γ: 15.45±9.5 pg/mL; IL-4: 6.3±2.9 pg/mL; IL-10: 69±29.1 pg/mL; and 1α,25-dihydroxyvitamin D3: 10.8±8.4 μg/mL).
IgE, IL-4, and IL-10 levels in the study group were found to be higher, whereas IFN-γ and 1α,25-dihydroxyvitamin D3 levels were lower, than those in the control group (p<0.05; Table 1).
Table 1.
Comparison of IgE, IL-4, IL-10, IFN-γ, and vitamin D levels between the study and control groups
| Study group | Control group | p | |
|---|---|---|---|
|
| |||
| IgE | 199.81±158.02 | 44.82±38.86 | p<0.05 |
| IL-4 | 6.34±2.99 | 3.25±1.94 | p<0.05 |
| IL-10 | 69.06±29.11 | 42.18±21.18 | p<0.05 |
| IFN-γ | 15.45±9.5 | 30.81±20.44 | p<0.05 |
| Vitamin D | 10.89±8.42 | 26.51±13.59 | p<0.05 |
IgE: immunoglobulin E; IL: interleukin; IFN-γ: interferon gamma
With regard to the correlation between the levels of IgE, IL-4, IL-10, and IFN-γ levels and 1α,25-dihydroxyvitamin D3, it is observed that there is a significant correlation among the increase in IgE, IL-4, and IL-10 levels, and the decrease in IFN-γ and 1α,25-dihydroxyvitamin D3 levels (p<0.05; Figure 1–4).
Figure 1.
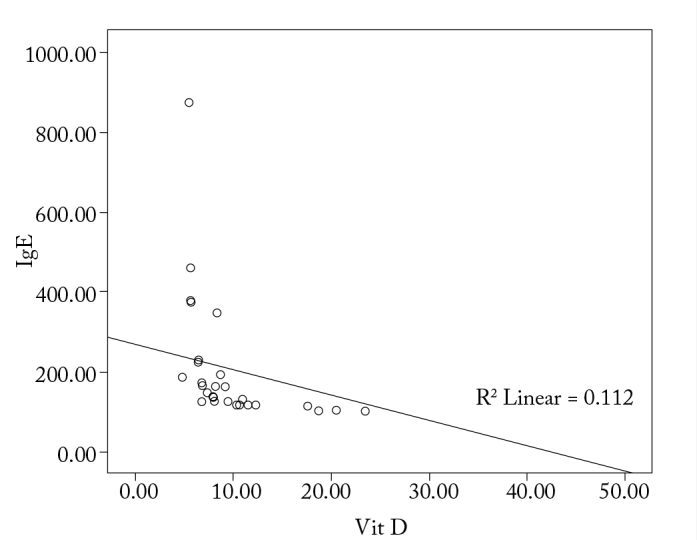
Correlation between vitamin D and IgE in the study group
Figure 2.
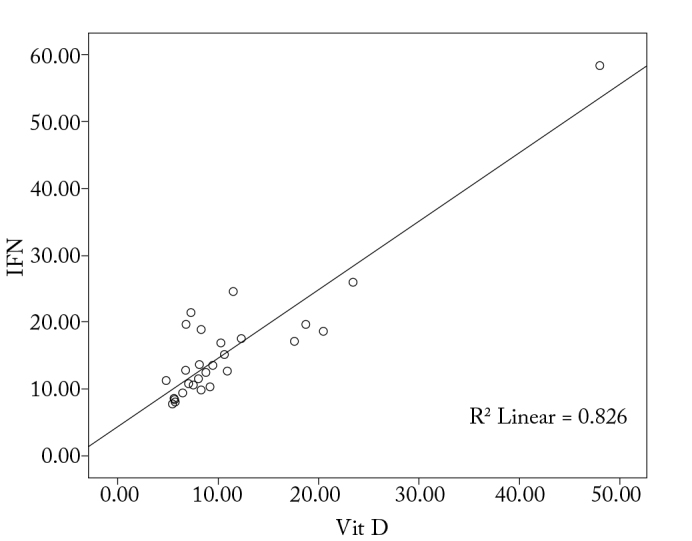
Correlation between vitamin D and IFN-γ in the study group
Figure 3.
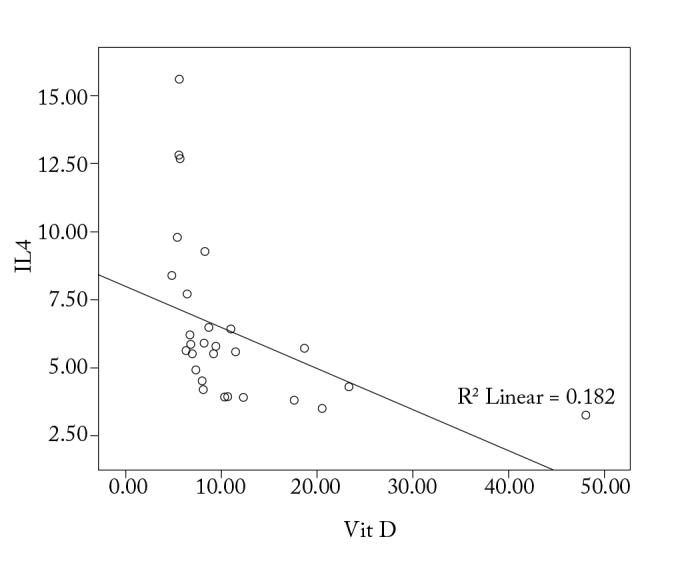
Correlation between vitamin D and IL-4 in the study group
Figure 4.
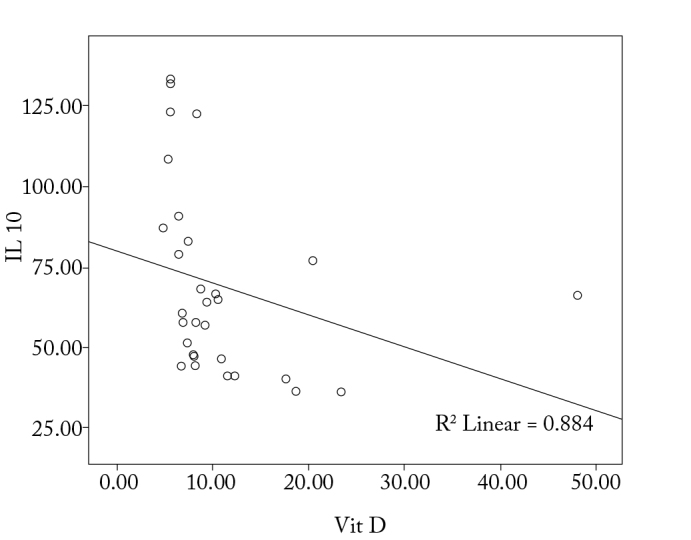
Correlation between vitamin D and IL-10 in the study group
Discussion
The significance of vitamin D, 1α,25-dihydroxyvitamin D3, and VDR has already been mentioned (5). Vitamin D derivatives exert their biological action through their specific nuclear receptor (VDR), present in several immune competent cell types including T and B cells, macrophages, and dendritic cells; they are considered to be significant in asthma and allergic rhinitis (4, 5). The fact that vitamin D appears to reduce inflammatory cytokine release has resulted in speculation regarding a possible therapeutic use in allergic diseases (6, 7).
Different studies indicate a variable effect of 1α,25-dihydroxyvitamin D3 on cytokine output by Th2 cells (10–18). Various studies show a continuous Th2 response in the presence of 1α,25-dihydroxyvitamin D3, although some other, and mostly more recent, studies indicate that Th2 response may be inhibited by 1α,25-dihydroxyvitamin D3 (15, 16). In this study, an inverse correlation of 1α,25-dihydroxyvitamin D3 levels was found in patients with allergic rhinitis with the levels of IL-4 and IL-10, which are Th2 cytokines (p<0.05).
Studies in animal models have established that vitamin D has a positive effect in autoimmune disease, exerted through a decrease in IFN-γ production (11, 16). Vitamin D reduces IFN-γ synthesis in human cell studies (16, 17).
IFN-γ levels were positively correlated to the 1α,25-dihydroxyvitamin D3 levels in the allergic rhinitis group (p<0.05). A statistically significant difference in IFN-γ levels was detected between the study and control groups (p<0.05).
Boonstra et al. (11) reported that vitamin D increased the synthesis of IL-4, IL-5, and IL-10 in a CD4+ T-cell culture. However, Staeva-Vieira and Freedman (15) found that the addition of vitamin D to their CD4+ T-cell culture during the first phase of T-cell differentiation inhibited IL-4 synthesis. Naive T-cells were used in both cases; the cause of this difference in the response to vitamin D is unexplained as yet. It may result from differences in the phases of the CD4+ T-cell differentiation process and their activation status (15). Furthermore, it could be associated with the difference in the vitamin D doses administered in the two studies (17). Studies investigating the circulating Th2 cytokines in peripheral blood following vitamin D or calcitriol administration have shown no evidence of increase in these cytokines (19).
A negative correlation was observed in our study between IL-10 and 1α,25-dihydroxyvitamin D3 levels in patients with allergic rhinitis (p<0.05). A published report has shown that vitamin D, with or without a glucocorticoid, increased IL-10 synthesis by T-cells (20). The mentioned study investigated the action of vitamin D on Treg cells expressing the TLR9 gene. This effect could not be shown in Treg cells without TLR9 gene expression, Th1 cells, or Th2 cells (20).
Domdey et al. (21) showed an elevation of IL-10 levels in atopic patients compared with nonatopic individuals. A statistically significant difference in IL-10 levels was detected among the study and control groups in our study (p<0.05).
In a study, Hyppönen et al. (22) reported a nonlinear association between 1α,25-dihydroxyvitamin D3 and IgE levels. The study indicated that 1α,25-dihydroxyvitamin D3 levels may be low with regard to very low and very high IgE levels. In this study, threshold levels of the effects of 1α,25-dihydroxyvitamin D3 has been mentioned. In our study, we demonstrated that in the study group, there is negative correlation between 1α,25-dihydroxyvitamin D3 and IgE levels (p<0.05).
Several publications document elevated IgE levels in allergic rhinitis (10, 13, 14, 23). This study reported a similar, and statistically significant, difference in the IgE levels between the study and control groups (p<0.05).
Hidaka and Tatsumi (24) reported high IL-4 levels in patients with allergic rhinitis. On comparing IL-4 levels between the allergic rhinitis and control groups in our study, a statistically significant difference was observed (p<0.05).
The studies regarding allergic rhinitis have indicated that Th1/Th2 balance had deteriorated because of Th2 increase (10, 25, 26). In our patients with allergic rhinitis, a strong positive correlation was observed between IgE levels and both IL-4 and IL-10 levels (p<0.05), and a negative correlation was determined between IFN-γ and IgE levels (p<0.05). A moderate negative correlation was determined among IFN-γ levels and both IL-4 and IL-10 levels in the study group (p<0.05). A positive, moderately strong correlation was determined between IL-4 and IL-10 levels (p<0.05). Therefore, in our study, we determined that in the study group, Th1/Th2 balance is impaired with the increase of Th2.
Studies investigating the correlation between 1α,25-dihydroxyvitamin D3 and allergic rhinitis are available in the literature (10, 23–25). Sharief et al. (27) studied the correlation between vitamin D deficiency and allergy in children and teenagers by evaluating the vitamin blood levels and food and peripheral allergens in US. The study demonstrated that there is a correlation between vitamin D deficiency and allergy in these populations; however, this correlation has not been shown in adults. Ehlayel et al. (28) demonstrated that vitamin D deficiency has been observed more often in children who have acute urticaria, asthma, atopic dermatitis, allergic rhinitis, or food allergy. Wjst and Hyppönen (29) demonstrated that allergic rhinitis has been observed more often in a vitamin D deficient population.
In our study, on comparing the groups for their 1α,25-dihydroxyvitamin D3 levels, a statistically significant difference was observed among the allergic rhinitis and control groups (p<0.05).
In our study, 1α,25-dihydroxyvitamin D3 levels were associated with Th1/Th2 balance in allergic rhinitis, and a remarkable interrelation was observed among 1α,25-dihydroxyvitamin D3 deficiency and allergy. These findings show that 1α,25-dihydroxyvitamin D3 may have a remarkable role in the severity and control of allergic disorders. In addition, further investigations are required to confirm how vitamin D should be used in allergic diseases. Moreover, to reveal the exact mechanism of vitamin D on allergic diseases, further studies are required.
Conclusion
Levels of 1α,25-dihydroxyvitamin D3, a vitamin D metabolite, in patients with allergic rhinitis are found to be low.
When the study and control groups were compared, IgE, IL-4, and IL-10 levels in the study group were higher, whereas IFN-γ and 1α,25-dihydroxyvitamin D3 levels were lower, than those in the control group.
With regard to the correlation between IgE, IL-4, IL-10, and IFN-γ levels and 1α,25-dihydroxyvitamin D3 levels, a significant correlation was observed between the increase in IgE, IL-4, and IL-10 levels and the decrease in IFN-γ and 1α,25-dihydroxyvitamin D3 levels.
This study is noteworthy for studies that will highlight the correlation among the vitamin D metabolites and allergic diseases.
We believe that our study will contribute to other studies with larger series concerning the changes in the allergic rhinitis treatment protocols and the correlation among the vitamin deficiency and the severity of allergic diseases.
Footnotes
This study was presented as a verbal report in 33rd Turkish National Otorhinolaryngology and Head and Neck Surgery Congress, 26–30 October 2011, Antalya, Turkey.
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Fırat University School of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.K., Ş.Y.; Design - Ş.Ö., N.İ.; Supervision - E.K., T.K.; Resources - Ş.Ö., N.İ., H.G.; Materials - T.K., H.G.; Data Collection and/or Processing - Ş.Ö., Ş.Y.; Analysis and/or Interpretation - E.K., Ş.Y.; Literature Search - N.İ., Ş.Ö.; Writing Manuscript - Ş.Ö., E.K.; Other - T.K., H.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.König K, Klemens C, Eder K, San Nicoló M, Becker S, Kramer MF, et al. Cytokine profiles in nasal fluid of patients with seasonal or persistent allergic rhinitis. Allergy Asthma Clin Immunol. 2015;1:26. doi: 10.1186/s13223-015-0093-x. http://dx.doi.org/10.1186/s13223-015-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24:758–64. doi: 10.1183/09031936.04.00013904. http://dx.doi.org/10.1183/09031936.04.00013904. [DOI] [PubMed] [Google Scholar]
- 3.Bauchau V, Durham SR. Epidemiological characterization of the intermittent and persistent types of allergic rhinitis. Allergy. 2005;60:350–3. doi: 10.1111/j.1398-9995.2005.00751.x. http://dx.doi.org/10.1111/j.1398-9995.2005.00751.x. [DOI] [PubMed] [Google Scholar]
- 4.Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30:397–409. doi: 10.1016/j.iac.2010.05.005. http://dx.doi.org/10.1016/j.iac.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D. Modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96. doi: 10.1016/j.coph.2010.04.001. http://dx.doi.org/10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Sandhu MS, Casale TB. The role of vitamin D in asthma. Ann Allergy Asthma Immunol. 2010;105:191–9. doi: 10.1016/j.anai.2010.01.013. http://dx.doi.org/10.1016/j.anai.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippilä C, Ahonen S, Nevalainen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39:875–82. doi: 10.1111/j.1365-2222.2009.03234.x. http://dx.doi.org/10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2) LEN and AllerGen) Allergy. 2008;(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. http://dx.doi.org/10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 9.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, Fitz-Gerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;3:143–78. doi: 10.1183/09031936.00138707. http://dx.doi.org/10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 10.Ozkara S, Keles E, Ilhan N, Gungor H, Kaygusuz I, Alpay HC. The relationship between Th1/Th2 balance and 1α,25-dihydroxyvitamin D3 in patients with nasal polyposis. Eur Arch Otorhinolaryngol. 2012;269:2519–24. doi: 10.1007/s00405-012-1967-x. http://dx.doi.org/10.1007/s00405-012-1967-x. [DOI] [PubMed] [Google Scholar]
- 11.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1α,25-dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. http://dx.doi.org/10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 12.Goetz DW. Vitamin D treatment of idiopathic itch, rash, and urticaria/angioedema. Allergy Asthma Proc. 2010;31:158–60. doi: 10.2500/aap.2010.31.3322. http://dx.doi.org/10.2500/aap.2010.31.3322. [DOI] [PubMed] [Google Scholar]
- 13.Wisniewski JA, Borish L. Novel Cytokines and cytokine-producing T cell in allergic disorders. Allergy Asthma Proc. 2011;32:83–94. doi: 10.2500/aap.2011.32.3428. http://dx.doi.org/10.2500/aap.2011.32.3428. [DOI] [PubMed] [Google Scholar]
- 14.Broide DH. Allergic rhinitis: Pathophysiology. Allergy Astma Proc. 2010;31:370–4. doi: 10.2500/aap.2010.31.3388. http://dx.doi.org/10.2500/aap.2010.31.3388. [DOI] [PubMed] [Google Scholar]
- 15.Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168:1181–9. doi: 10.4049/jimmunol.168.3.1181. http://dx.doi.org/10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 16.Mattner F, Smiroldo S, Galbiati F. Inhibition of Th1 development and treatment of chronicrelapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3) Eur J Immunol. 2000;30:498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–32. doi: 10.1002/jcb.10580. http://dx.doi.org/10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 18.Sloka S, Silva C, Wang J, Yong VW. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflammation. 2011;24:8–56. doi: 10.1186/1742-2094-8-56. http://dx.doi.org/10.1186/1742-2094-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Ambrosio D. Increased IgE but reduced Th2-type inflammation in vitamin D receptor-deficient mice. J Immunol. 2005;174:4451–5. doi: 10.4049/jimmunol.174.8.4451. http://dx.doi.org/10.1186/1742-2094-8-56. [DOI] [PubMed] [Google Scholar]
- 20.Urry Z, Xystrakis E, Richards DF. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1α,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest. 2009;119:387–98. doi: 10.1172/JCI32354. http://dx.doi.org/10.4049/jimmunol.174.8.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domdey A, Liu A, Millner A, Lund K, Jacobi H, Malling HJ, et al. The T cell response to major grass allergens is regulated and includes IL-10 production in atopic but not in non-atopic subjects. Int Arch Allergy Immunol. 2010;152:243–54. doi: 10.1159/000283033. http://dx.doi.org/10.1159/000283033. [DOI] [PubMed] [Google Scholar]
- 22.Hyppönen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE - a significant but nonlinear relationship. Allergy. 2009;64:613–20. doi: 10.1111/j.1398-9995.2008.01865.x. http://dx.doi.org/10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez-Segura A, Brieva JA, Rodríguez C. T lymphocytes that infiltrate nasal polyps have a specialized phenotype and produce a mixed TH1/TH2 pattern of cytokines. J Allergy Clin Immunol. 1998;102:953–60. doi: 10.1016/s0091-6749(98)70333-1. http://dx.doi.org/10.1016/S0091-6749(98)70333-1. [DOI] [PubMed] [Google Scholar]
- 24.Hidaka Y, Tatsumi K. Secretion of interleukin 4 and immunoglobulin G from peripheral blood mononuclear cells in allergic rhinitis. J Investig Allergol Clin Immunol. 2007;17:413–4. [PubMed] [Google Scholar]
- 25.Wosinska-Becler K, Plewako H, Håkansson L, Rak S. Cytokine production in peripheral blood cells during and outside the pollen season in birch-allergic patients and non-allergic controls. Clin Exp Allergy. 2004;34:123–30. doi: 10.1111/j.1365-2222.2004.01850.x. http://dx.doi.org/10.1111/j.1365-2222.2004.01850.x. [DOI] [PubMed] [Google Scholar]
- 26.Benson M, Strannegård IL, Wennergren G, Strannegård O. Low levels of interferon-gamma in nasal fluid accompany raised levels of T-helper 2 cytokines in children with ongoing allergic rhinitis. Pediatr Allergy Immunol. 2000;11:20–8. doi: 10.1034/j.1399-3038.2000.00051.x. http://dx.doi.org/10.1034/j.1399-3038.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127:1195–1202. doi: 10.1016/j.jaci.2011.01.017. http://dx.doi.org/10.1016/j.jaci.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehlayel MS, Bener A, Sabbah A. Is high prevalence of vitamin D deficiency evidence for asthma and allergy risks? Eur Ann Allergy Clin Immunol. 2011;43:81–8. [PubMed] [Google Scholar]
- 29.Wjst M, Hyppönen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007;62:1085–6. doi: 10.1111/j.1398-9995.2007.01437.x. http://dx.doi.org/10.1111/j.1398-9995.2007.01437.x. [DOI] [PubMed] [Google Scholar]


