Abstract
Objective
Pediatric patients with nasal obstruction due to adenoid vegetation (AV) can also encounter allergic rhinitis (AR) as a comorbidity. The aim of the study was to estimate the incidence of mite sensitization and its effect on adenoid size in children who underwent adenoidectomy.
Methods
This prospective randomized study conducted between August and September 2014 included 84 children. Skin Prick Test (SPT) for inhalant allergens was preoperatively applied to all children who underwent adenoidectomy for nasal obstruction. Children were divided into two study groups: AV only (Group I) (n=52) and AV with Dermatophagoides Pteronyssinus and/or D. farinae allergy (Group II) (n=32). Postoperative specimen volumes, visual analogue scale (VAS) scores, and adenoid volumes measured using flexible fiberoptic nasopharyngolaryngoscopy were compared between the two groups.
Results
Postoperative specimen volume measures were higher in Group II compared with those in Group I (p<0.05). Furthermore, in preoperative endoscopic examination, adenoid volume measures were higher in Group II compared with those in Group I (p<0.05). Pre and postoperative VAS scores in SPT+ group were higher in the Group II (p<0.05) than those in Group I.
Conclusion
We observed that children with AR tend to have an early onset of symptoms of adenoid hypertrophy. We believe that focusing on the management of role of allergy regarding these early symptoms will reduce the need for surgery in a large number of cases. We suggest that SPT must be performed in all children with AV and adenoid examination should not be neglected in children with AR.
Keywords: Adenoid vegetation, allergic rhinitis, adenoid volume
Introduction
Adenoid hypertrophy (AH) is the most common pathology that causes upper airway obstruction in childhood (1). Although there are several reasons of AH and its etiology has not been fully understood, it is a common opinion that chronic, severe, and recurrent inflammatory events growing around the adenoid tissue are important in this regard. Allergy is among the most frequently encountered inflammatory processes mentioned (2).
The adenoid is exposed to antigens through the airways and mediates the immunological protection of the upper respiratory and digestive tract (3). Meanwhile, the adenoid is constantly exposed to allergens in addition to viral and bacterial agents. Allergic diseases can initiate inflammatory processes that affect the adenoid tissue, and in this case, they can lead to the formation of an allergic adenoiditis condition that causes the adenoid tissue to contain numerous IgE-positive mast cells (2).
Allergic rhinitis (AR) is one of the most common chronic diseases, affecting 10–40% of the entire population. Epidemiological studies have suggested that the prevalence of this disease has increased (4, 5). AR typically arises after the second year of life, and at least two or more seasons of pollen exposure are necessary for sensitization in the pediatric AR group (6). The comorbid relationship between the adenoid and adenotonsillar hypertrophy of the upper airway allergy, which is termed as inflammation of the mucosal barrier of the upper airways due to IgE-mediated hypersensitivity, has been shown in many studies (6–8).
Allergy is thought to be a risk factor for AH. From another point of view, because the clinical symptoms of AR and AH are similar, only one of them may be diagnosed in a patient. In our study, we aimed to determine the incidence of AR and its effects on AH by making a comparison of adenoid volume measurements and preoperative and postoperative symptoms in patients who underwent adenoid surgery.
Methods
This randomized, double-blind, prospective study was conducted with the 19.08.2014-dated and 696-numbered approval of the Human Research Ethics Committee of Şişli Etfal Education and Research Hospital and with the consent of the parents of the individuals to be included in the study, and comprised 88 pediatric patients of the ages of 3–12 in whom adenoidectomy was performed in our clinic between August 2014 and September 2014. In all cases, the criteria for inclusion in the study were identified as having adenoid tissue causing varying degrees of nasal obstruction by closing the choanae, snoring, and chronic mouth breathing due to AH. The patients with situations (deviated septum, atresia, nasal polyposis) causing an anatomical nasal obstruction apart from AH and with a sensitivity for multiple allergens (food allergy, pollen, fungal spores, and others), except for mites, were not included in the study. Apart from these criteria, four individuals with cleft palate and bleeding diathesis were also excluded from the study, and the study was thus completed with a total of 84 pediatric patients.
The control group was composed of 52 patients who were determined not to have AR and who were shown to have AH through their clinical history and flexible fiberoptic nasopharyngolaryngoscopy (FNFL). The AR-positive group was comprised 32 patients with AH who were shown to have a sensitization of D. pteronyssinus mites (house dust mite 1, HDM1) and/or D. farinae mites (house dust mite 2, HDM2) through a skin prick test (SPT).
A skin prick test was performed on the forearm interior face by using a testing panel (Stallergenes, Antony Cedex, France) containing the standard allergens. The sensitivity to allergens was evaluated with the allergens of HDM1, HDM2, weed mix (Golden Rod, Dandelion, Cocklebur, Ox-eye daisy), a mixture of woods: Betulaceae (alder, birch, hazel, hornbeam), Salicaceae (poplar, willow/poplar + willow), tree pollen mix (maple, horse chestnut, plane, Acacia, lime), 12 Herbal Blend (cocksfoot, ryegrass, timothy, meadow grass, sweet vernal grass, oat grass, wild oat, meadow fescue, bent grass, Yorkshire fog, Bermuda grass, bromus), cat dander, dog dander, and fungus (Alternaria alternata).
The FNFL method was used for the adenoid examination. The group of patients with AH was divided into four subgroups according to the degree of obstruction that the adenoid tissue formed in choana:
Group 1: 0–25% adenoid tissue
Group 2: 25–50% adenoid tissue
Group 3: 50–75% adenoid tissue
Group 4: 75–100% adenoid tissue.
Postoperative adenoid volume measurement was performed by placing the specimen in a 5 cc syringe. Adenoidectomy operation was performed in all patients.
The Simplified Visual Analog Scale (VAS) is a kind of survey that includes scores from 0 (no nasal congestion) to 6 (very severe congestion). In the cases where the child could not be cooperative in the survey, the parents’ help was received (open mouth sleeping, snoring). This survey was administered in patients preoperatively and postoperatively one month later.
Statistical Analysis
The mean, standard deviation, median, min–max, rate, and frequency values were used in the descriptive statistics of the data. The distribution of variables was checked with the Kolmogorov-Smirnov test. In the analysis of the quantitative data, the Mann-Whitney U test was used. The chi-square test was used in the analysis of the qualitative data. The Wilcoxon test was used to analyze the repeated measures. The SPSS 22.0 program (IBM Corp.; Armonk, New York, USA) was used in the analyses.
Results
The distribution of the gender and age of the patients with AR-positive and -negative results did not differ significantly (p>0.05) (Table 1).
Table 1.
Distribution of age and gender among the groups
| Skin prick test (+) | Skin prick test (−) | p | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Avg.±S.S./n% | Med (min–max) | Avg.±S.S./n% | Med (min–max) | |||
|
| ||||||
| Age | 7.1±2.5 | 6.5 (4.0–12.0) | 7.7±3.2 | 7.0 (3.0–15.0) | 0.503 | |
| Gender | Female | 14 | 43.8% | 20 | 38.5% | 0.632 |
| Male | 18 | 56% | 32 | 61.5% | ||
Mann-Whitney U test/Chi-square test
The preoperative and postoperative VAS values of AR (+) patients were shown to be significantly higher than those of AR (−) patients (p<0.05) (Table 2). In this group, the postoperative/preoperative VAS change was significantly high in AR (−) patients (p<0.05) (Table 3).
Table 2.
Comparison of the preoperative and postoperative VAS scores of the groups
| Skin prick test (+) | Skin prick test (−) | p | |||
|---|---|---|---|---|---|
|
| |||||
| Avg.±S.S./n% | Med (min–max) | Avg.±S.S./n% | Med (min–max) | ||
|
| |||||
| Specimen volume (cc) | 2.4±1.2 | 2.1 (0.5–4.0) | 1.6±1.2 | 1.0 (0.6–6.0) | 0.001 |
| Adenoid size % | 72%±14% | 75% 30%–90% | 57%±20% | 60% 20%–90% | 0.001 |
Mann-Whitney U test; VAS: visual analog scale
Table 3.
Postoperative/preoperative VAS changes
| Skin prick test (+) | Skin prick test (−) | p | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Avg.±S.S./n% | Med (min–max) | Avg.±S.S./n% | Med (min–max) | |||
|
| ||||||
| VAS | Preop | 33±0.6 | 3.0 (2.0–4.0) | 2.0±0.2 | 2.0 (2.0–3.0) | 0.000 |
| Postop | 5.1±0.8 | 5.0 (4.0–6.0) | 2.0±0.3 | 2.0 (1.0–3.0) | 0.000 | |
| Change | 1.8±0.8 | 2.0 (0.0–3.0) | −0.1±0.3 | 0.0 (−1.0–0.0) | 0.000 | |
|
| ||||||
| Change p | 0.000 | 0.0051 | ||||
Mann-Whitney u test/Wilcoxon test; VAS: visual analog scale
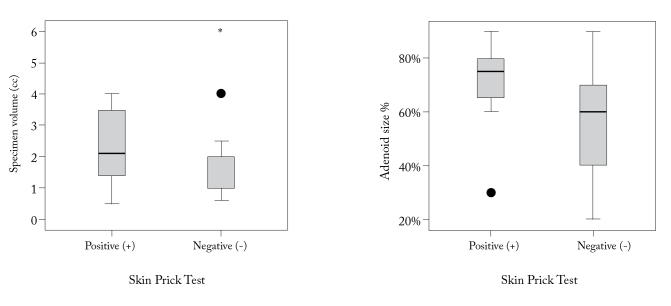
The values of specimen volume and the adenoid size % in preoperative endoscopic examination were shown to be significantly higher in AR (+) patients than in AR (−) patients (p<0.05) (Figure 1).
Figure 1.
The value of specimen volume and adenoid size % in preoperative endoscopic examination Skin Prick Test
Discussion
Allergy is thought to be a risk factor for the formation of AH. A positive relationship between a positive skin prick test and adenotonsillar hypertrophy and 70% sensitivity to aeroallergens was determined in a study conducted with 117 pediatric patients with chronic adenotonsillitis (9).
The most common inhalant allergens are the mite allergy in Istanbul. In a study conducted by Küçükosmanoğlu et al. (10), HDM1 (D. pteronyssinus) (96.7%) was found to be the most common inhalant allergen in Istanbul, and the sensitivity of HDM2 (D. farinae) (89.3%) was detected. In a study conducted with 45 pediatric patients by Kutlu et al. (11), HDM1 and HDM2 (50–60%) were found to be the most common inhalant allergens in Istanbul, following which the tree and grass pollen (about 40%) and other aeroallergens (fungi, animal dander, and others) were observed at a decreasing frequency. Based on these data, we investigated the frequency of mite allergy in patients on whom we planned to perform adenoidectomy. We found AR due to HDM1 and HDM2 allergies in 32 (38%) of 84 patients in our study group.
Ganzer and Bachert (12) demonstrated that the production of IgE is not only limited to the nasal mucosa but also exists in the lymphatic tissue in Waldeyer’s ring.
Modrzynski et al. (13) showed in nasal endoscopy and acoustic rhinometry that the pharyngeal tissue temporarily becomes hypertrophied in spring. They also investigated the effect of atopy on AH, and stated that AR is a risk factor for AD in children. The incidence of AH was investigated in another study by Modrzynski et al. (14), where it was concluded that the risk of AH is greater in children with AR who have HDM allergy.
In another study, in patients who had undergone adenoidectomy, the average value of total IgE in adenoid tissue homogenates of the atopic group was found to be higher than non-atopic ones, and the average value of HDM1-specific IgE and IgA antibodies was found to be significantly higher than non-atopic ones (15).
In the literature, there are many studies examining the relationship between AR and AH in biochemical, histological, cytological, and immunohistochemical terms (16, 17). There are also studies showing the effectiveness of anti-allergic drugs in AH (18).
In a study that Demirhan et al. (19) conducted, a significant improvement in nasal obstruction symptoms was seen in children with AH in the results of an 8-week treatment following intranasal fluticasone propionate treatment, while a significant decline was detected in the rate of adenoid/choana in the same study. The first study on the effective use of intranasal steroid in AH was presented by Demain and Goetz (20). In another study, in the treatment of nasal spray containing fluticasone propionate, an improvement in obstructive sleep apnea syndrome (OSAS), which is associated with AR, and a decrease in the treatment-associated apneas and hypopneas were observed (21). Criscuoli et al. (22) detected a 45% clinical improvement in children with adenotonsillar hypertrophy after 2 weeks of intranasal beclomethasone treatment and stated that surgery was still necessary in 13 (54%) of 24 patients who had responded to the first treatment in their 100-week long-term study. Usta et al. (23) reported that after mometasone furoate treatment, surgical treatment was needed only in 2 of 39 patients in whom adenoidectomy indication was determined. Similarly, Demain and Goetz (20) reported a reduction in nasal congestion and a decrement in adenoid volume after the treatment of nasal steroids. Cengel and Akyol (24) performed a similar study with intranasal mometasone furoate and achieved a 50% reduction in the starting size of the adenoid.
Allergic inflammation of the nasal cavity may exacerbate the existing OSAS condition by narrowing the airway. Adenotonsillectomy is the most effective method in treating OSAS; however, anti-inflammatory treatments such as leukotriene receptor antagonists or nasal steroids may be useful in the residual OSAS after adenotonsillectomy (25, 26).
Nasal obstruction is a very common complaint in children, but it is more difficult to detect the symptoms in pediatric patients than in adult patients. It has been argued in many studies that VAS is a simple and objective method for determining the AR seriousness in school-age children (27–29). In this study, we used the Simplified Visual Analog Scale (VAS), which scores the severity of nasal obstruction from 0 to 6. Symptoms such as a runny nose and itching nose that are questioned in classic VAS were not included in this scoring. In many studies, VAS has been reported to be clinically appropriate to measure nasal congestion and as a reliable method in the absence of rhinomanometry or when rhinomanometry cannot be used (30, 31). The postoperative VAS value was greater in the AR positive group than in the control group despite the adenoidectomy; we thought this was related to ongoing allergic inflammation.
In assessing the size of adenoid tissue, we used the FNFL method to see the adenoid tissue itself, to determine the upper airway gap, and to exclude reasons such as nasal septum deviation, nasal polyps, and choanal atresia that could cause nasal congestion. Our study supports that the preoperative adenoid volume is greater in allergic patients than in nonallergic ones.
Conclusion
Based on our study, we can say that AR increases the predisposition of children to AH. The same effects can also be created by a number of other inflammatory processes. The frequency of atopic diseases affecting children has increased in recent decades, and this increase may have contributed to the increase in the frequency of AH occurrence induced by allergies. Therefore, the presence of AR should be investigated in all children with AH, and at least a simple diagnostic procedure for allergies, such as a skin prick test, should be applied and the treatment should be started. In addition, adenoid examination should not be neglected and should be performed preferably with flexible endoscopes in children with AR.
Footnotes
This study was presented in 2013 World Allergy and Asthma Congress in Milan, Italy.
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Şişli Etfal Training and Research Hospital. /19.08.2014/340.
Informed Consent: Written informed consent was obtained from the parents of the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.B., A.Y.K.; Design - G.B., S.K.D., A.Y.K.; Supervision - G.B., A.Y.K., B.U.C.; Resources - S.K.D.; Materials - S.K.D.; Data Collection and/or Processing - S.K.D.; Analysis and/or Interpretation - G.B., A.Y.K.; Literature Search - G.B, A.Y.K.; Writing Manuscript - G.B.; Critical Review - B.U.C.; Other - S.K.D.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Bernstein JM, Scheeren R, Schoenfeld E, Albini B. The distribution of immunocompetent cells in the compartments of the palatine tonsils in bacterial and viral infections of the upper respiratory tract. Acta Otolaryngol Suppl. 1998;454:153–62. doi: 10.3109/00016488809125019. [DOI] [PubMed] [Google Scholar]
- 2.Marseglia GL, Caimmi D, Pagella F, Matti E, Labò E, Licari A, et al. Adenoids during childhood: the facts. Int J Immunopathol Pharmacol. 2011;24:1–5. doi: 10.1177/03946320110240S401. [DOI] [PubMed] [Google Scholar]
- 3.Wiatrak BJ, Woolley AL. Farenjit ve adenotonsiller hastalık. In: Koç C, editor. Cummings Otolaringoloji Baş ve Boyun Cerrahisi. 4 Baskı. Güneş Tıp Kitabevleri; Ankara: 2007. p. 4137. [Google Scholar]
- 4.McColley SA, Carroll JL, Curtis S, Loughlin GM, Sampson HA. High prevalence of allergic sensitization in children with habitual snoring and obstructive sleep apnea. Chest. 1997;111:170–3. doi: 10.1378/chest.111.1.170. http://dx.doi.org/10.1378/chest.111.1.170. [DOI] [PubMed] [Google Scholar]
- 5.Lee JT, Lam ZC, Lee WT, Kuo LC, Jayant V, Singh G, et al. Familial risk of allergic rhinitis and atopic dermatitis among Chinese families in Singapore. Ann Acad Med Singapore. 2004;33:71–4. [PubMed] [Google Scholar]
- 6.Sih T, Mion O. Allergic rhinitis in the child and associated comorbidities. Pediatr Allergy Immunol. 2010;21(1 Pt 2):e107–13. doi: 10.1111/j.1399-3038.2009.00933.x. http://dx.doi.org/10.1111/j.1399-3038.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- 7.Kjellman NI, Synnerstad B, Hansson LO. Atopic allergy and immunoglobulins in children with adenoids and recurrent otitis media. Acta Paediatr Scand. 1976;65:593–600. doi: 10.1111/j.1651-2227.1976.tb04938.x. http://dx.doi.org/10.1111/j.1651-2227.1976.tb04938.x. [DOI] [PubMed] [Google Scholar]
- 8.Modrzynski M, Zawisza E. An analysis of the incidence of adenoid hypertrophy in allergic children. Int J Pediatr Otorhinolaryngol. 2007;71:713–9. doi: 10.1016/j.ijporl.2006.12.018. http://dx.doi.org/10.1016/j.ijporl.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghi-Shabestari M, Jabbari Moghaddam Y, Ghaharri H. Is there any correlation between allergy and adenotonsillar tissue hypertrophy? Int J Pediatr Otorhinolaryngol. 2011;75:589–91. doi: 10.1016/j.ijporl.2011.01.026. http://dx.doi.org/10.1016/j.ijporl.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Küçükosmanoğlu E, Tanıdır C, Demir F, Coşkun Ş, Hafızoğlu T, Şeşeoğulları Y, et al. İstanbul’da çocuklarda solunum alerjenleri duyarlılığı. Gaziantep Tıp Dergisi. 2009;15:10–3. [Google Scholar]
- 11.Kutlu A, Karabacak E, Aydın E, Ozturk S, Taskapan O, Aydinoz S, et al. Relationship between skin prick and atopic patch test reactivity to aeroallergens and disease severity in children with atopic dermatitis. Allergol Immunopathol (Madr) 2013;41:369–73. doi: 10.1016/j.aller.2013.02.007. http://dx.doi.org/10.1016/j.aller.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Ganzer U, Bachert C. Localization of IgE synthesis in immediate-type allergy of upper respiratory tract. ORL J Otorhinolaryngol Relat Spec. 1988;50:257–64. doi: 10.1159/000276000. http://dx.doi.org/10.1159/000276000. [DOI] [PubMed] [Google Scholar]
- 13.Modrzynski M, Mierzwinski J, Zawisza E, Piziewicz A. Acoustic rhinometry in the assessment of adenoid hypertrophy in allergic children. Med Sci Monit. 2004;10:CR431–8. [PubMed] [Google Scholar]
- 14.Modrzynski M, Zawisza E. An analysis of the incidence of adenoid hypertrophy in allergic children. Int J Pediatr Otorhinolaryngol. 2007;71:713–9. doi: 10.1016/j.ijporl.2006.12.018. http://dx.doi.org/10.1016/j.ijporl.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Shin SY, Choi SJ, Hur GY, Lee KH, Kim SW, Cho JS, et al. Local production of total IgE and specific antibodies to the house dust mite in adenoid tissue. Pediatr Allergy Immunol. 2009;20:134–41. doi: 10.1111/j.1399-3038.2008.00756.x. http://dx.doi.org/10.1111/j.1399-3038.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- 16.Hellings PW, Fokkens WJ. Allergic rhinitis and its impact on otorhinolaryngology. Allergy. 2006;61:656–64. doi: 10.1111/j.1398-9995.2006.01109.x. http://dx.doi.org/10.1111/j.1398-9995.2006.01109.x. [DOI] [PubMed] [Google Scholar]
- 17.Modrzynski M, Mazurek H, Zawisza E. Allergic tonsillitis: myth or reality. Postepy Hig Med Dosw. 2005;59:450–6. [PubMed] [Google Scholar]
- 18.Georgalas C, Thomas K, Owens C, Abramovich S, Lack G. Medical treatment for rhinosinusitis associated with adenoidal hypertrophy in children: an evaluation of clinical response and changes on magnetic resonance imaging. Ann Otol Rhinol Laryngol. 2005;8:638–44. doi: 10.1177/000348940511400810. http://dx.doi.org/10.1177/000348940511400810. [DOI] [PubMed] [Google Scholar]
- 19.Demirhan H, Aksoy F, Özturan O, Yıldırım S, Veyseller B. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773–6. doi: 10.1016/j.ijporl.2010.03.051. http://dx.doi.org/10.1016/j.ijporl.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Demain JG, Goetz DW. Pediatric adenoidal hypertrophy and nasal airway obstruction: reduction with aqueous nasal beclomethasone. Pediatrics. 1995;95:355–64. [PubMed] [Google Scholar]
- 21.Bender BG, Milgrom H. Comparison of the effects of fluticasone propionate aqueous nasal spray and loratadine on daytime alertness and performance in children with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92:344–9. doi: 10.1016/S1081-1206(10)61573-6. http://dx.doi.org/10.1016/S1081-1206(10)61573-6. [DOI] [PubMed] [Google Scholar]
- 22.Criscuoli G, D’Amora S, Ripa G, Cinquegrana G, Mansi N, Impagliazzo N, et al. Frequency of surgery among children who have adenotonsillar hypertrophy and improve after treatment with nasal beclomethasone. Pediatrics. 2003;111:236–8. doi: 10.1542/peds.111.3.e236. http://dx.doi.org/10.1542/peds.111.3.e236. [DOI] [PubMed] [Google Scholar]
- 23.Usta BE, Arslan Z, Özmen S, Atmaca S, Aslan B. Adenoid hipertrofili çocuklarda nazal momatezon furoatın etkisi. Astım Allerji İmmünoloji. 2003;1:17–24. [Google Scholar]
- 24.Cengel S, Akyol MU. The role of topical nasal steroids in the treatment of children with otitis media with effusion and/or adenoid hypertrophy. Int J Pediatr Otorhinolaryngol. 2006;70:639–45. doi: 10.1016/j.ijporl.2005.08.013. http://dx.doi.org/10.1016/j.ijporl.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Brouillette RT, Manoukian JJ, Ducharme FM, Oudjhane K, Earle LG, Ladan S, et al. Efficacy of fluticasone nasal spray for pediatric obstructive apnea. J Pediatr. 2001;138:838–44. doi: 10.1067/mpd.2001.114474. http://dx.doi.org/10.1067/mpd.2001.114474. [DOI] [PubMed] [Google Scholar]
- 26.Kheirandish-Gozal L, Gozal D. Genotype-phenotype interactions in pediatric obstructive sleep apnea. Respir Physiol Neurobiol. 2013;189:338–43. doi: 10.1016/j.resp.2013.03.016. http://dx.doi.org/10.1016/j.resp.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akasawa A, Adachi Y, Yoshida K, Furukawa M, Odajima H. Visual analog scale showed a good correlative with allergic rhinitis and its impact on asthma (ARIA) classification in school children. J Allergy Clin Imunol. 2013 Feb; [Google Scholar]
- 28.Demoly P, Bousquet PJ, Mesbah K, Bousquet J, Devillier P. Visual analogue scale in patients treated for allergic rhinitis: an observational prospective study in primary care: ashma and rhinitis. Clin Exp Allergy. 2013;43:881–8. doi: 10.1111/cea.12121. http://dx.doi.org/10.1111/cea.12121. [DOI] [PubMed] [Google Scholar]
- 29.Bousquet PJ, Combescure C, Klossek JM, Daurès JP, Bousquet J. Change in visual analog scale score in a pragmatic randomized cluster trial of allergic rhinitis. J Allergy Clin Immunol. 2009;123:1349–54. doi: 10.1016/j.jaci.2009.02.033. http://dx.doi.org/10.1016/j.jaci.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Ciprandi G, Mora F, Cassano M, Gallina AM, Mora R. Visual analog scale (VAS) and nasal obstruction in persistent allergic rhinitis. Otolaryngol Head Neck Surg. 2009;141:527–9. doi: 10.1016/j.otohns.2009.06.083. http://dx.doi.org/10.1016/j.otohns.2009.06.083. [DOI] [PubMed] [Google Scholar]
- 31.Ciprandi G, Tosca MA, Signori A, Cirillo I. Visual analogue scale assessment of nasal obstruction might define patients candidates to spirometry. Rhinology. 2011;49:292–6. doi: 10.4193/Rhino10.303. [DOI] [PubMed] [Google Scholar]