Abstract
Thyroid nodules are extremely common and are detected in 3%–7% of the general population by palpation and in 70% of the population by ultrasonography (USG). Approximately 5%–15% of these nodules are malignant. Therefore, in nodule examination, our primary aim is to detect malignant nodules. Besides the medical history and the findings of the physical examination, USG and fine-needle aspiration biopsy (FNAB) are the most commonly used methods to examine these nodules. Ultrasound-guided FNAB and on-site assessment of FNA specimens are suggested to decrease false negative and non-diagnostic test results. FNAB results in the “atypia of undetermined significance” group is challenging in the follow-up or treatment of the nodule. In this group, to differentiate the malignant nodules, other developing methods, such as analyzing molecular genetic markers, protein markers, and elastography, are generally studied. However, these methods are not used in a routine nodule examination because of cost-benefit analysis.
Keywords: Thyroid nodule, fine-needle aspiration biopsy, ultrasonography, thyroid cancer
Introduction
Thyroid nodules are defined as the lesions that are localized in the thyroid gland and can be radiologically differentiated from surrounding thyroid parenchyma (1). They can be detected during physical examination or during other examinations performed for other diseases [such as ultrasonography (USG), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)]. The other type, which tends to be found coincidentally, is generally asymptomatic and is called “thyroid incidentaloma” (2).
Thyroid nodules are one of the most commonly seen clinical problems. It has been reported that palpable nodules are encountered approximately at the rate of 5% in women and 1% in men living in the regions where iodine intake is sufficient. In the autopsies of individuals without clinical thyroid disease, the frequency of nodules larger than 1 cm has been reported to be 50%. USG is more sensitive than palpation. With the use of USG devices with high resolution, the prevalence of nodules detected through USG in adult populations has increased to about 70% (1–3). In a review on thyroid incidentalomes, it has been reported that nodules are detected through USG at a rate of 67%, through CT and MRI at a rate of 16%, through carotid Doppler USG at a rate of 9.4%, and through PET or positron emission tomography/computed tomography (PET/CT) at a rate of 2–3% (4).
The incidence of thyroid nodules is four times higher among women than among men, and its prevalence increases depending on age and low iodine intake. Its frequent occurrence in women can probably be explained by the effects of estrogen and progesterone. The risk of cancer is higher in men. It has been reported that the exposure to ionizing radiation causes the development of thyroid nodules at a rate of 2% and this rate peaks in 15–20 years (2, 5).
Most thyroid nodules are asymptomatic. Only 5–15% of these nodules are malignant depending on a number of factors such as age, gender, exposure to radiation, and familial history. Hence, to avoid unnecessary operations and their complications, the important point in the evaluation is to detect the nodules that cause thyroid dysfunction, constitute the symptom of compression, and have a malignancy risk and to then treat them. USG and fine-needle aspiration biopsy (FNAB) are the two key techniques that help to decide whether surgical treatment for a thyroid nodule is required or not. Moreover, it has been reported that molecular genetic marker analyses can be used for promoting the accuracy of FNAB results (1–3).
Anamnesis and Physical Examination
Malignant conditions with a very aggressive course can be included in the etiology of thyroid nodules as well as the benign conditions (Table 1). Therefore, anamnesis and physical examination should particularly focus on the detection of features suggesting malignancy (2, 6). In general, patients apply with a palpable nodule in the neck or with a nodule incidentally detected during examinations performed for other disorders (3). In 50–60% of patients with one palpable nodule discovered during physical examination, more than one nodule is detected through USG. Only 5% of nodules found by USG are palpable (5). The possibility of malignancy is higher for a single dominant nodule or solitary nodule (2.7–30%) than for a single nodule in the multinodular gland (1.4–10%). However, the total malignancy risk in a gland including a single solitary nodule is approximately the same as that in a multinodular gland (as a result of the total of risks for each nodule) (3–5). In positron emission tomography imaging, the rate of incidentally detected nodules is 1–2%. However, the rate of malignancy risk for these nodules is 27%. Hence, immediate evaluation of these nodules is very important (2).
Table 1.
Causes of thyroid nodules
| Benign nodular goiter |
| Chronic lymphocytic thyroiditis |
| Cysts |
| Follicular adenoma |
| Subacute thyroiditis |
| Papillary carcinoma |
| Follicular carcinoma |
| Hurthle cell carcinoma |
| Medullary carcinoma |
| Anaplastic carcinoma |
| Primary thyroid lymphoma |
| Metastatic tumors |
The most important factors that increase the possibility of malignancy in anamnesis and physical examination are as follows:
a previous exposure to radiation in the head–neck region;
having undergone total body irradiation for bone marrow transplantation;
rapid growth in a nodule and complaints of dysphagia and dysphonia;
male gender;
age below 20 years and above 70 years;
familial history of medullary thyroid cancer or multiple endocrine neoplasia;
familial history of differentiated thyroid cancer;
history of Hodgkin and non-Hodgkin lymphoma;
presence of a nodule larger than 4 cm (19.3% malignancy risk);
hard and fixed nodule with palpation;
coexisting cervical lymphadenopathy; and
In thyroid diseases, familial history must be investigated. Familial medullary thyroid cancer (originating from C cells producing calcitonin) and familial non-medullary thyroid cancer (originating from follicular cells) are rare but important familial thyroid syndromes. The presence of papillary thyroid cancer history in parents and in a sibling increases the risk of developing papillary thyroid cancer in a patient by a factor of three and six fold, respectively. Familial medullary thyroid cancer can occur as a part of multiple endocrine neoplasia (MEN) II-A (pheochromocytoma, medullary thyroid cancer, and primary hyperparathyroidism) and II-B (pheochromocytoma, medullary thyroid cancer, marfanoid appearance, and mucosal and neurofibromatosis in the digestive system). Familial thyroid cancers, which originate from follicular cells, can be seen as isolated or can coexist with some syndromes such as Cowden syndrome (autosomal dominant inherited; hamartomatous neoplasms in the skin, oral mucosa, gastrointestinal system, central nervous system and genitourinary system, breast, and thyroid cancers), Carney complex (autosomal dominant; cardiac and cutaneous myxomas, skin pigmentation, and endocrine and malignancies with other origins), Werney syndrome (characterized by premature aging), and familial polyposis (2).
In the study of Raza et al. (8), it was reported that the positive predictive value for the detection of a single or multiple lymphadenopathies larger than 1 cm or vocal cord paralysis in the cervical 2nd-5th regions was 100% with regard to demonstrating a nodule as malignant. The evaluation of a patient’s voice alone is not enough for the detection of vocal cord paralysis. Hanna et al. (9) reported that the positive predictive value was 55% for determining vocal cord paralysis when only the voice was evaluated. Therefore, in patients with a thyroid nodule, an initial assessment of their vocal cord movements with a complete head-neck examination is highly important.
Diagnostic Tests
There are many examination methods that can be used in the evaluation of a patient applying with thyroid nodule. The measurement of thyroid-stimulating hormone, USG, and FNAB when necessary are the most important and basic techniques used for this purpose. In addition, other diagnostic techniques, such as scintigraphy, genetic tests (BRAF, RAS, etc.), immunohistochemical tests (Galactin-2, etc.), elastography, MRI, CT, and PET imaging are used (2).
Blood Analyses
For evaluating nodule functions in a patient with a thyroid nodule, the level of thyroid-stimulating hormone (TSH) is assessed at the beginning. If the TSH level is found to be low, total or free thyroxine (T4) and total triiodothyronine (T3) levels are measured for better evaluating hyperthyroidism. This condition is observed approximately in 10% of patients with a solitary thyroid nodule and it suggests an adenoma displaying benign hyperfunction (3). In patients with a low level of thyroid-stimulating hormone, thyroid scintigraphy must be performed with technetium 99-m pertechnetate or iodine-123 for evaluating the function of nodules. Because a nodule displaying hyperfunction (i.e., a hot nodule) rarely contains malignancy, cytological evaluation is not required (1, 2, 6). When a cold or warm nodule is detected in scintigraphy, USG is performed and FNAB is recommended in the presence of suspicious findings (1, 5). It has been reported that high levels of serum TSH (including levels near the upper limit) are associated with an increased malignancy risk in thyroid nodules (1). If the serum TSH level is normal or high in a patient with a nodule and USG reveals suspicious findings, FNAB is recommended (1, 5). The routine measurement of calcitonin is controversial in thyroid nodules but it must be required for patients with a history of familial medullary thyroid carcinoma, MEN II-A or B, phaeochromocytoma, or hyperparathyroidism. The level of serum calcitonin is not sufficient alone for the differentiation of benign and malignant (3, 5). However, if the serum calcitonin level (in case of not performing stimulation) is over 100 pg/mL, there is a likelihood of medullary cancer (1).
Thyroglobuline and thyroid antibodies are not used for the evaluation of nodules. The thyroglobuline level is used for the monitorization of papillary thyroid cancers (1–3, 5).
Thyroid USG
Thyroid USG is an important imaging technique that is commonly used in the detection and evaluation of thyroid nodules. It is a non-invasive, easily accessible, and cheap method that reveals the diameter, number, and localization of nodule, solid/cystic differentiation, accompanying lymphadenopathies in the head-neck region, thyroid volume, and changes in thyroid parenchyma. Its disadvantages include its dependence on the device that is used and on the practitioner, it not being able to image retrosternal goiters, and the absence of a definite criterion for malignancy. Today, even 2–3 mm nodules can be detected through USG devices. What is important here is the determination of which nodule must clinically be evaluated with further examinations (2, 3, 5, 6).
It has been reported that some features found through USG in thyroid nodules increase the possibility of malignancy. Compared to normal thyroid parenchyma, some features, such as a hypoechogenic nodule, increased intra-nodular blood supply, irregular margin, invasion to the surrounding tissue, presence of microcalcification, thick irregular halo or absence of halo, a nodule’s length being greater than its width, and the presence of lymphadenopathy in the neck, have been defined as the risk factors for malignancy (1–3, 5, 6, 10, 11). However, the sensitivity and specificity of ultrasonographic findings, apart from for suspected cervical lymphadenopathy, are insufficient for defining all malignant nodules (Table 2) (1, 8). Nevertheless, the combination of some features has a high predictive value for malignancy. Further cytological examination is needed for nodules having a hypoechoic appearance with one or other suspicious findings (1, 2). In a study, it was reported that the coexistence of age at 39 years or above, an irregular margin, microcalcification, and the nodule being larger than 2 cm could predict malignancy at an accuracy rate of 81.7% (11). Moreover, it was specified that the specificity of a spongiform appearance for demonstrating a benign nodule was 99.7% and the negative predictive value for malignancy was 98.5%. The risk of malignancy is very low for pure thyroid cysts (less than 2% of all nodules) (1, 2). In the presence of suspicious USG findings, the likelihood of malignancy is similar for a nodule smaller than 1 cm as for a larger nodule. The detection of spread outside the capsule, which causes the involvement of the thyroid capsule, perithyroid muscles, and recurrent laryngeal nerve, is a strong indicator of malignancy (2).
Table 2.
Sensitivity and specificity of suspicious ultrasonography findings in nodulesa
| Suspicious ultrasonography findings | Mean sensitivity % | Mean specificity % |
|---|---|---|
|
| ||
| Microcalcification | 52 | 83 |
| Lack of halo | 66 | 54 |
| Irregular margin | 55 | 79 |
| Hypoechogenity | 81 | 53 |
| Increased intra-nodule blood supply | 67 | 81 |
(3)
In papillary thyroid cancer, USG generally reveals some findings, such as whether it is solid or mostly solid, hypoechoic, infiltrative, and if there is increased intra-nodular blood supply. Furthermore, the presence of microcalcification is highly specific for papillary thyroid cancer. On the contrary, isoechoic/hyperechoic, and thick and irregular halo findings not accompanied by microcalcification are found in follicular cancer.
In addition, USG is useful for the selection of the appropriate nodule for multinodular goiter biopsy and for the determination of the most suitable place for FNAB in the nodule.
Elastography
Elastography is a promising method that has recently been developed for the determination of the malignancy potential of thyroid nodules (Figure 1, 2: US elastographic examinations were performed using Hitachi EUB 7500 elastography-Hitachi Medical Corporation 4-14-1; Soto-Kanda, Chiyoda-ku, Tokyo, Japan). Elastography is a technique that is used for assessing the hardness of tissue, which is an indicator of malignancy (12, 13). In the color scale used in elastography, the color red shows the highest elasticity, while blue shows the hardest components. However, the color scale is a qualitative method; therefore, the “strain index” is now used as a quantitative parameter (13). While there are some studies reporting that the specificity (96–100%) and sensitivity (82–97%) of elastography are high in the evaluation of thyroid nodules independently of the size and location of nodules, there are other studies reporting a sensitivity between 15.7% and 98% and a specificity between 58.2% and 100%. This technique can be especially beneficial for follicular lesions and for cases in which the FNAB results are ambiguous. The value of elastography decreases in nodules with calcified periphery, cystic lesions, and multinodular goiters in which nodules merge. Its disadvantages include problems in the standardization of the technique, the possibility of being affected by carotid pulsation, variable pressure applied by the practitioner, and insufficient pressure on nodules with a diameter above 3 cm. It has been reported that the dependence on a practitioner is not valid for shear wave elastography. Although further prospective studies are needed for elastography, it is a promising diagnostic technique for the selection of the nodule to expose to FNAB (2, 5, 6, 12, 13).
Figure 1.
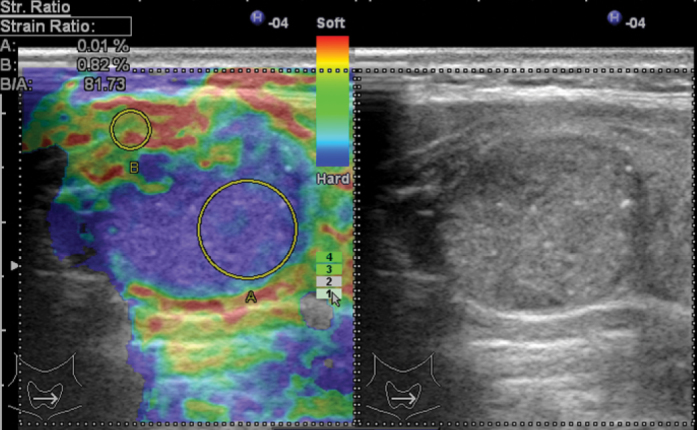
Malignant nodule sample; Gray scale: iso-hypoechoic, including microcalcifications, Transverse/Longitudinal Axis ratio >0.5.
Elastography: Especially posterior region of nodule is observed as dark blue. Score 4. (Image Archive of Yıldırım Beyazıt University Faculty of Medicine, Department of Endocrinology and Metabolic Diseases).
Figure 2.
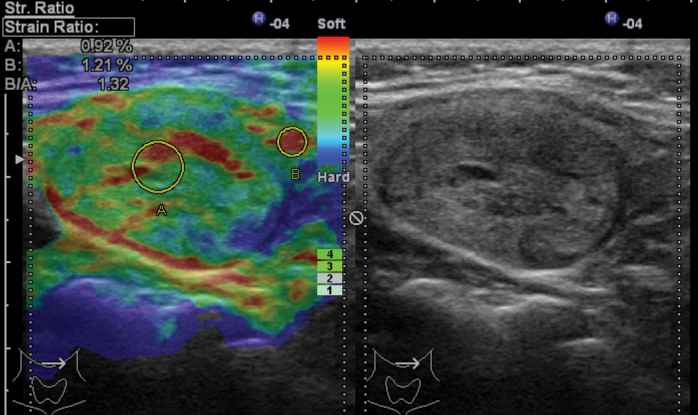
Benign nodule sample; Gray scale: isoechoic, regular margins, hypoechoic and straight halo, Transverse /Longitudinal Axis Ratio <0.5.
Elastography: Nodule is yellow-green in general. Score: 1–2. (Image Archive of Yıldırım Beyazıt University Faculty of Medicine, Department of Endocrinology and Metabolic Diseases).
Thyroid Scintigraphy
Thyroid scintigraphy is a method used for imaging the thyroid gland and thyroid tissue functioning in any region of the body. In thyroid scintigraphy, while technetium-99 m and iodine 123 are routinely used, iodine-131 is used in some special situations. Scintigraphy is used for the evaluation of nodule function rather than the size of nodule. There is no other imaging technique that gives information about the nodule function in thyroid nodules, except scintigraphy. Today, the indication for the use of scintigraphy in the evaluation of thyroid nodules is patients with a serum TSH level below normal values. Furthermore, it can be used for the detection of hypoactive nodules before FNAB in multinodular goiters. Thyroid nodules are called hot, cold, and warm nodules according to their scintigraphic appearances (3, 5, 6).
Hot (hyperactive) nodules display more intense activity uptake compared to the surrounding tissue. They constitute 5% of all nodules. Hot nodules occur as toxic adenoma and toxic multinodular goiters. Moreover, they can lead to hyperthyroidism. Their malignancy rates are below 1% (3, 5, 6).
Cold (hypoactive) nodules are the ones that do not display activity uptake compared to the surrounding tissue. They constitute 80–85% of all thyroid nodules. Approximately 10% of cold nodules are malignant. Thyroid adenomas, colloid and degenerative nodules, cystic and necrotic nodules, inflammatory changes, and thyroid cancers have the characteristics of cold nodules (3, 5).
Warm (normoactive) nodules show activity uptake at the same rate with non-nodule tissue. They constitute 10% of thyroid nodules. Warm nodules are considered as cold nodules with regard to malignancy risk (5).
Computed Tomography and Magnetic Resonance Imaging
In the initial evaluation of thyroid nodules, both techniques are rarely used. Computed tomography (CT) provides three-dimensional (3D) imaging of the thyroid gland. Besides that, the sensitivity of both techniques in the assessment of goiters that can compress on surrounding structures extending to the substernal region has been reported to be 100%. In addition, CT can be utilized for evaluating the invasion of thyroid cancer to the surrounding structures. It is suggested that the use of a contrast agent including iodine in computed tomography can trigger hyperthyroidism in patients living in places having iodine deficiency and it can prevent radioactive iodine therapy for 1–2 month. Gadolinium used in magnetic resonance imaging does not affect the uptake of radioisotopes by the thyroid tissue (3, 5, 6).
Positron Emission Tomography-Computed Tomography
Positron emission tomography-computed tomography is commonly used for staging in oncology, evaluation of the response to treatment, and the detection of recurrences. No significant difference has been found between benign and malignant nodules in terms of “maximum uptake value” (SUV max). Nevertheless, there are some studies reporting that it can reduce the need for diagnostic lobectomy in lesions with unclear FNAB results because the negative predictive value of PET is high (95–100%). During PET performed due to other reasons, focal and diffuse activity uptake of the thyroid gland has been found at the rate ranging from 1% to 3 %. In the case of focal uptake, the risk of malignancy is approximately 30%, and diffuse uptake is associated with thyroiditis and Graves’ disease (3, 5). Therefore, although it is not used in the initial assessment of a nodule, if a nodule displaying uptake is incidentally detected through PET, it must be examined carefully in terms of malignancy and USG and FNAB must then be performed (3, 5, 14).
FNAB
FNAB is the most important diagnostic method with USG for the evaluation of thyroid nodules. The mean sensitivity, specificity, positive predictive value, false negative rate, and false positive rate have been reported as being 83%, 92%, 75%, 5%, and 5%, respectively (6). Because the false negative rate and non-diagnostic cytology rate are lower, FNAB is recommended to be performed with USG guidance instead of with palpation. USG-guided biopsy is a better approach, particularly when a nodule is impalpable, deeply located, or if it is mainly cystic (1, 2).
The decision of FNAB must be made by considering the patient’s history, USG findings, and the size of the nodule (Table 3). In the presence of one or more suspicious USG finding in a nodule smaller than 1 cm, extracapsular growth, abnormal cervical lymph node, and a history of a feature suggesting a high risk, FNAB must be performed. Moreover, a nodule size of 1 cm can be used as a criterion for FNAB if only one suspicious finding is available in the USG of solid nodules (such as microcalcification and hypoechogenity). If the size of the nodule is larger than 1.5 cm for mixed (cystic-solid) nodules, biopsy is recommended to be performed from the solid component. Because the risk of malignancy is low, spongioform lesions can be followed or biopsy can be performed if the nodule is larger than 2 cm (2, 5, 6). According to the Bethesda system, the results of FNAB are divided into six categories: benign, non-diagnostic, atypia of undetermined significance, neoplasm (Hurthle cells or follicular neoplasms), suspicious for malignancy, and malignant (15).
Table 3.
FNAB recommendations according to ultrasonography and clinical findings (American Thyroid Association)
| USG and clinical findings | Nodule size recommended for FNAB | Strength of recommendation |
|---|---|---|
|
| ||
| High risk in history | ||
| Existent suspicious USG findings | >5 mm | Strongly recommended (A) |
| Nonexistent suspicious USG findings | >5 mm | Controversial (I) |
| Abnormal cervical lymph node | All nodules | Strongly recommended (A) |
| Microcalcification in nodule | ≥1 cm | Recommended (B) |
| Solid nodule | ||
| Hypoechoic | >1 cm | Recommended (B) |
| Iso- or hyperechoic | ≥1–1.5 cm | Recommended (C) |
| Mixed cystic-solid nodule | ||
| Existent suspicious USG findings | ≥1.5–2 cm | Recommended (B) |
| Nonexistent suspicious USG findings | ≥2 cm | Recommended (C) |
| Spongioform nodule | ≥2 cm | Recommended (C) |
| Pure cystic nodule | No FNAB indication (unless it is for therapeutic purpose) | Not recommended (E) |
FNAB: fine-needle aspiration biopsy; USG: ultrasonography
The most common benign lesions are colloid nodules, macrofollicular adenoma, and lymphocytic thyroiditis. The most commonly seen malignant lesions include papillary thyroid cancer, follicular thyroid cancer, medullary thyroid cancer, anaplastic thyroid cancer, and high-grade metastatic neoplasms. Suspicious lesions due to the lack of descriptive diagnostic criteria can be papillary thyroid cancer, follicular neoplasm, Hurthle cell neoplasm, lymphoma or a follicular variant of papillary thyroid carcinoma (2).
As the size of the nodule increases, the rate of sampling error also increases in FNAB. While the false negative rate is 17% in solid thyroid nodules at the size of 3 cm or above, the false negative rate for cystic nodules at the size of 3 cm or above is 30%. Therefore, diagnostic lobectomy is recommended for any nodule at the size of 3 cm and above. In recent studies, it has been reported that the limit for diagnostic lobectomy may be 4 cm because the diagnostic value of USG-guided FNAB has increased (3). For a 4-cm nodule, the false negative rate and malignancy rate of FNAB have been found as 12.7% and 19%, respectively. It is controversial to recommend surgery just by considering the size of the nodule. The risk factors of the patient must be taken into consideration for this decision. The age of the patient must definitely be considered. For instance, because multiple FNAB procedures will be required in the monitorization of a nodule in a young patient, diagnostic lobectomy is recommended for this patient (3).
The measurement of thyroglobuline levels in FNAB wash-out samples elevates the value of FNAB in the investigation of lymph node metastases of differentiated thyroid carcinomas. A thyroglobulin level above 10 ng/mL has been reported to suggest lymph node metastasis. On the other hand, its disadvantages are its being a non-standardized technique, the non-clarity of which level exactly shows malignancy, and continuing arguments on the accuracy of results in patients not having undergone thyroidectomy (16).
Molecular Markers in FNAB
Although it is early as yet in its application in clinical practice, the use of genetic markers together with cytology can be beneficial in the differentiation of benign and malignant thyroid nodules. At present, there are two commercial panels used for performing molecular tests in FNAB samples. One of them evaluates the levels of mRNA for 142 genes. When this panel was used for samples of undetermined significance in FNAB, the negative predictive value has been reported as being 96% (2). Hence, it can be useful for the prevention of unnecessary surgical interventions in patients with benign lesions. Another commercial panel evaluates seven molecular markers (BRAF, KRAS, HRAS, NRAS, RET/PTC1, RET/PTC3, PPAX8/PPARγ) that are commonly encountered in thyroid cancers.
Genetic mutations are responsible for the development of differentiated thyroid carcinomas. While BRAF mutations are seen in papillary thyroid cancer more commonly, RAS mutations are mostly observed in the follicular variant of papillary thyroid cancer, follicular hyperplasia, follicular adenoma, and follicular carcinoma. Although RET proto-oncogen is classically associated with medullary thyroid cancer and MEN syndromes, it also has a relationship with papillary thyroid cancer. The BRAF V600E mutation is associated with a more aggressive form of papillary carcinoma. Despite the fact that the studies on RET/PTC and PAX-8-PPARγ mutations are promising, the number of present studies is insufficient (3, 19).
Frozen Section Examination
There are different opinions regarding frozen section examination. In order to eliminate the need of complementary thyroidectomy with a second surgery, diagnostic lobectomy is frequently performed along with intraoperative frozen section examination in patients with uncertain FNAB results. Intraoperative frozen section analysis was reported to decrease the number of complementary thyroidectomies and to reduce the cost when used, especially in follicular neoplasms (20). Cheng et al. (21) reported that frozen section examination was useful in patients having suspected FNAB results or follicular lesions, especially in making the diagnosis of papillary cancer and making the decision of total thyroidectomy in one stage. It has been reported that frozen section examination is useful especially in patients whose FNAB results are considered as insufficient material or benign, but who clinically have risk factors (22).
However, Richards et al. (23) performed FNAB in 155 patients, frozen section examination in 140 patients, and both analyses in 103 patients; in their results, they reported that FNAB sensitivity was 50% and specificity was 99%; frozen section examination sensitivity was 50% and specificity was 100%, and frozen section examination changed the decision on the width of thyroidectomy in one of 103 patients. They reported that it did not provide a significant advantage in terms of patient results and, due to the prolongation of operation time and the necessity for pathologists in frozen section examination, the costs increased. Therefore, the use of frozen section examination varies among individuals and institutions.
Clinical and Research Impact
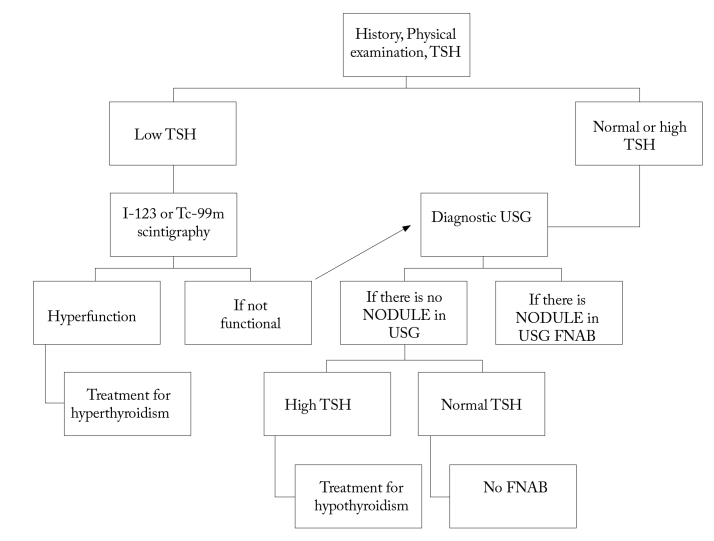
First, USG and TSH measurement should be performed in the assessment of nodules (Figure 3, 4). USG should be performed in all patients known or suspected to have nodules. USG examination gives information about the size, appearance, and number of nodules. The risk of malignancy in multinodular goiters is the same as the one in a solitary nodule. However, the possibility of malignancy per nodule decreases as the number of nodules increases. In the selection of the nodule on which FNAB will be performed in the presence of two or more nodules larger than 1 cm, the nodules with suspicious findings seen on ultrasound should be taken into account. It is also reported that the largest nodule may be selected for biopsy (1–3, 6).
Figure 3.
Algorithm for approach to thyroid nodules-1 (American Thyroid Association).
TSH: thyroid-stimulating hormone; USG: ultrasonography; FNAB: fine-needle aspiration biopsy
Figure 4.
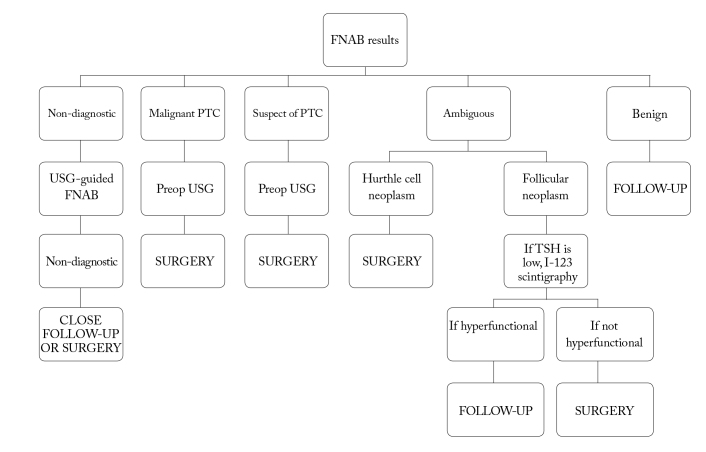
Algorithm for approach to thyroid nodules-2 (American Thyroid Association).
TSH: thyroid-stimulating hormone; USG: ultrasonography; FNAB: fine-needle aspiration biopsy; PTC: papillary thyroid carcinoma
Thyroid incidentalomas detected in computed tomography or MRI should initially be evaluated through USG and further scrutiny should be decided according to the detected results. However, thyroid nodules detected through 18-FDG PET should be evaluated with ultrasound because they have a high likelihood of malignancy and FNAB should be performed (2, 6).
Nodules smaller than 1 cm in a patient with a normal level of thyroid-stimulating hormone can be followed through ultrasound annually. If a growth or change in appearance occurs in the nodule, it should be evaluated with FNAB. FNAB should be performed in patients having a nodule smaller than 1 cm, radiation exposure in the story, thyroid cancer in the family history, or suspicious ultrasound findings. In order to assess the nodular function, I-123 or Tc-99m scintigraphy should be performed in patients with low values of thyroid-stimulating hormone or at the lower limit of normal values. FNAB is not recommended in hot nodules because the likelihood of malignancy is very low (less than 1%) (1–3). If hot nodules are detected in scintigraphy, radioactive iodine therapy (RAI) or surgical treatment is recommended. The first choice is surgery in toxic adenomas with a diameter larger than 3 cm and in young patients (5). FNAB is suggested in cold nodules as the likelihood of malignancy is between 5 and 15% (2). In patients in whom a cold nodule was detected in scintigraphy, USG accompanying FNAB sensitivity was reported as 73.9%, specificity as 99.2%, the positive predictive value as 89.5%, and the negative predictive value as 97.7% (24) in terms of malignancies. Scintigraphy generally provides limited information to evaluate the nodular function in lesions smaller than 1 cm. If the thyroid-stimulating hormone level is found to be high (including those at the upper limit of the reference range), this is associated with an increased risk of malignancy in nodules (1, 2, 6).
The results of cytological examinations of most nodules (70%) are found to be benign. Indeed, the likelihood of malignancy is less than 1% in benign nodules. Therefore, no specific initiatives are required in most benign thyroid nodules. Lobectomy or total thyroidectomy should be performed in benign nodules larger than 4 cm or in nodules leading to local pressure findings such as dysphagia, shortness of breath, hoarseness, or pain. Benign thyroid nodules require long-term follow-up due to the false negative rate of approximately 5% in FNAB. A follow-up with regular serial ultrasound in 6–18 month intervals is recommended to detect significant changes in the size and appearance of the nodules. The nodules with no significant growth in serial USG follow-up can be followed every 3–5 years through ultrasound (1–3, 5, 15). There is no consensus about in which cases FNAB should be repeated in terms of the quantity of growth in a nodule and the size of the nodules. However, most of specialists suggest that a growth of 50% in the size or an increase of 20% in at least two diameters in the solid part of solid nodules and mixed nodules should be considered as criteria. Although growth in nodules is an indication for FNAB, it is not pathognomonic for malignancy. Because the false negative rate is lower, the repeat of FNAB is recommended to be performed accompanied by USG (1, 2, 15).
The FNABs biopsy results that do not meet the criteria (at least six follicular cell groups, each containing 10–15 cells in the result of at least two aspirations performed in one nodule) and that were previously defined for cytological examination are defined as inadequate (non-diagnostic). This constitutes approximately 20% of the fine-needle aspiration biopsies (1, 2). The experience of the person making the radiological and pathological evaluation, the nature of the nodule (solid, cystic, sclerotic, hypervascular, or necrotic) and the number of aspirations affect the result of the insufficient material. If the result of FNAB is non-diagnostic and the suspicion of malignancy is not high clinically, repeat FNAB is recommended to be performed 3 months later (6, 15). Performing ultrasound -guided second FNAB in a nodule whose first FNAB result was inadequate increases the probability of making diagnosis in 75% of solid nodules and 50% of cystic nodules. Therefore, in these nodules that require ultrasound-guided biopsy, performing pathological examinations in place significantly reduces the likelihood of non-diagnostic cytology. However, non-diagnostic cytology in 7% of cases can actually be identified (1, 5, 15). There are studies reporting that malignancy can definitely be eliminated if the PET involvement is negative in non-diagnostic cytology (14). While close observation or surgery is recommended, if repeated FNAB results return as non-diagnostic in partial cystic nodules, surgery is recommended if the result is non-diagnostic when FNAB is repeated in a solid nodule (1, 2).
When a suspicion of malignancy is detected in the result of FNAB, the risk of malignancy has been reported to be 50–75%. This constitutes 3–9% of all FNABs. Follicular adenoma, follicular thyroid carcinoma, or the papillary thyroid carcinoma follicular variants are in this group. At the least, lobectomy is required to detect capsular and vascular invasion. Therefore, if there is malignancy or a suspicion of malignancy in the FNAB results, surgery is recommended (1–3, 5, 19).
When the result of FNAB is atypia of an undetermined significance, the risk of malignancy is 5–10%. This is a heterogeneous group, where the distinction between benign and follicular neoplasm cannot be made. It is a category that is insufficient to be defined as cellular atypia follicular neoplasm or suspected malignancy; yet, it cannot be exactly defined as benign, either. Considering the clinical and radiological findings, FNAB repeat can be suggested in this group. Although the situations such as male gender, a nodule larger than 4 cm, presence of atypia, and advanced age are findings that suggest malignancy, the predictive value for malignancy has still been reported as low (1, 3, 15). It was reported that the use of molecular markers (genetic markers, such as BRAF, RAS, RET/PTC, and protein markers, like Galectin-3) would be beneficial in nodules in which the FNAB result is a lesion of uncertain significance (2, 17, 18). In a study conducted in Korea, it was reported that the specificity according to only FNAB increased from 36% to 95% when FNAB cytology and BRAF mutation were evaluated together. In addition, it was reported that the likelihood of malignancy increased if any gene mutation was detected in the group of patients (cytology of undetermined significance), whereas the risk of malignancy decreased if gene mutation was not detected (2, 17). It has been reported that the existence of BRAF mutations or RET/PTC or PAX-8-PPARγ rearrangements in the samples of FNAB of an undetermined significance has a specificity of 100% for thyroid cancer and, similarly, when RAS mutation is seen in any FNAB sample, the risk of malignancy is 83–87% of (3). Similarly, in the study of Armstrong et al. (25), they reported PAX8/PPARγ to be 100% predictive for differentiated thyroid carcinomas. Galectin-3 can be more beneficial for the diagnosis of papillary thyroid cancer than for follicular thyroid cancer. Moreover, it has been specified that the TSH receptor mRNA level can help to differentiate malignancies in the group of undetermined significance. If the thyroid-stimulating hormone receptor mRNA level is above 1 ng/μg, the positive predictive value for malignancy has been reported to be over 90%. Although these examinations are available in reference centers, they are not routinely used in clinical practice yet. In spite of the fact that the routine use of molecular tests in all FNABs seems impossible due to their current costs, FNAB results can be used in situations with undetermined significance (2, 3, 6, 19). In this group, PET imaging has been used for the differentiation of benign and malignant nodules, although the results vary among studies, but for malignancy, sensitivity has been reported to be relatively high and specificity has been reported to be low. However, the use of PET imaging for this purpose is limited (1, 2). As a result, if molecular tests can be performed when the FNAB reveals a follicular lesion of undetermined significance, then FNAB can be repeated for those with a negative BRAF or RAS mutation, while total thyroidectomy can be carried out for those with a positive BRAF or RAS mutation (3, 19).
In patients whose FNAB results reveal neoplasm, the malignancy risk is between 15% and 30%. Follicular cell neoplasm and Hurthle cell neoplasm are included in this group (1, 15). When follicular neoplasm is detected in FNAB, scintigraphy is recommended if the TSH level is low or normal. If the nodule is not functional, lobectomy or total thyroidectomy is performed. If the nodule is functional, the patient is followed up (1, 2, 5, 6). There are some studies reporting that total thyroidectomy can be performed in the presence of a BRAF or RAS mutation in follicular neoplasm. In the absence of a BRAF or RAS mutation, lobectomy will be more appropriate (3). However, in Hurthle cell neoplasm, scintigraphy is not necessary and lobectomy or total thyroidectomy is recommended, depending on the size of lesion and the existence of risk factors (1, 15).
Total cystic lesions are generally benign and further investigation is not needed unless they contain a solid component. Aspiration in thyroid cysts is one of the treatment alternatives and the recurrence rate varies between 10% and 80%, depending on the number of aspirations and the volume of the cyst (26). It has been reported that percutaneous ethanol injection can be an alternative to surgery in nodules in which the cystic component is dominant and the result of FNAB is benign (27). It has been specified that complaints due to compression and cosmetic problems disappear at the rate of 74–80% after percutaneous ethanol injection (28). Percutaneous ethanol injection is a safe process but it can cause some rare complications, such as recurrent laryngeal nerve paralysis, as well as common side effects, such as local pain, dysphonia, and a feeling of dizziness (26–28).
The use of USG-guided percutaneous laser ablation in selected cases has been reported as an invasive approach. It has been suggested that it can effectively be used for relieving cosmetic and compression symptoms in patients who reject surgery or who are inappropriate for surgery, without causing any change in thyroid functions (29, 30).
The evaluation of a nodule in a pregnant woman is similar to that in other patients except it is contraindicated for scintigraphy. It has been reported that postponing the treatment of patients to undergo FNAB during pregnancy and whose FNAB results reveal differentiated thyroid cancer until after delivery will not affect the outcomes (1, 6).
The diagnosis and treatment of single or multiple nodules in children is the same as those in adults. However, hot nodules have a higher malignancy risk in children than in adults (1, 6).
Conclusion
With developments in imaging techniques and the more frequent use of these imaging techniques at present, thyroid nodules have become one of the most commonly encountered health concerns. Considering that most of these nodules are benign, intense research on the detection of malignant nodules is ongoing for preventing patients undergoing unnecessary surgeries. However, anamnesis, physical examination, measurement of the TSH level, USG, and FNAB are basic steps in the evaluation of nodules at present. In particular, how to approach cases in which FNAB reveals atypia of an undetermined significance seems to be the subject that is mostly open to investigation. Although improvements in elastography and molecular genetic markers are promising, they are not routinely used yet.
Acknowledgements
The author would like to thank to Prof. Dr. Reyhan Ersoy from the Department of Endocrinology and Metabolic Diseases of our hospital for their help on USG images.
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. http://dx.doi.org/10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96:329–49. doi: 10.1016/j.mcna.2012.02.002. http://dx.doi.org/10.1016/j.mcna.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am. 2010;43:229–38. doi: 10.1016/j.otc.2010.01.002. http://dx.doi.org/10.1016/j.otc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin J, McHenry CR. Thyroid incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012;26:83–96. doi: 10.1016/j.beem.2011.06.004. http://dx.doi.org/10.1016/j.beem.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Özarmağan S, Erbil Y, Ünalp H, editors. Tiroid ve Paratiroid Cerrahisi Atlası. İstanbul: Ekspres Basımevi; 2010. [Google Scholar]
- 6.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010;16:1–43. doi: 10.4158/10024.GL. http://dx.doi.org/10.4158/EP.16.3.468. [DOI] [PubMed] [Google Scholar]
- 7.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socíe G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. http://dx.doi.org/10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 8.Raza SN, Shah MD, Palme CE, Hall FT, Eski S, Freeman JL. Risk factors for well-differentiated thyroid carcinoma in patients with thyroid nodular disease. Otolaryngol Head Neck Surg. 2008;139:21–6. doi: 10.1016/j.otohns.2007.10.021. http://dx.doi.org/10.1016/j.otohns.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Hanna BC, Brooker DS. A preliminary study of simple voice assessment in a routine clinical setting to predict vocal cord paralysis after thyroid or parathyroid surgery. Clin Otolaryngol. 2008;33:63–6. doi: 10.1111/j.1749-4486.2007.01592.x. http://dx.doi.org/10.1111/j.1749-4486.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 10.Maia FF, Zantut-Wittmann DE. Thyroid nodule management: clinical, ultrasound and cytopathological parameters for predicting malignancy. Clinics (Sao Paulo) 2012;67:945–54. doi: 10.6061/clinics/2012(08)15. http://dx.doi.org/10.6061/clinics/2012(08)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maia FF, Matos PS, Silva BP, Pallone AT, Pavin EJ, Vassallo J, et al. Role of ultrasound, clinical and scintigraphyc parameters to predict malignancy in thyroid nodule. Head Neck Oncol. 2011;3:17. doi: 10.1186/1758-3284-3-17. http://dx.doi.org/10.1186/1758-3284-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantisani V, Lodise P, Grazhdani H, Mancuso E, Maggini E, Di Rocco G, et al. Ultrasound elastography in the evaluation of thyroid pathology. Current status. Eur J Radiol. 2014;83:420–8. doi: 10.1016/j.ejrad.2013.05.008. http://dx.doi.org/10.1016/j.ejrad.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Çakır B, Ersoy R. Tiroid nodüllerinin ayırıcı tanısında elastosonografi. Endokrinolojide Diyalog. 2012;9:71–6. [Google Scholar]
- 14.Treglia G, Muoio B, Giovanella L, Salvatori M. The role of positron emission tomography and positron emission tomography/computed tomography in thyroid tumours: an overview. Eur Arch Otorhinolaryngol. 2013;270:1783–7. doi: 10.1007/s00405-012-2205-2. http://dx.doi.org/10.1007/s00405-012-2205-2. [DOI] [PubMed] [Google Scholar]
- 15.Baloch ZW, LiVolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425–37. doi: 10.1002/dc.20830. http://dx.doi.org/10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 16.Torres MR, Nóbrega Neto SH, Rosas RJ, Martins AL, Ramos AL, da Cruz TR. Thyroglobulin in the washout fluid of lymph-node biopsy: what is its role in the follow-up of differentiated thyroid carcinoma? Thyroid. 2014;24:7–18. doi: 10.1089/thy.2013.0244. http://dx.doi.org/10.1089/thy.2013.0244. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao SJ, Nikiforov YE. Molecular approaches to thyroid cancer diagnosis. Endocr Relat Cancer. 2014;21:301–13. doi: 10.1530/ERC-14-0166. http://dx.doi.org/10.1530/erc-14-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yip L. Molecular diagnostic testing and the indeterminate thyroid nodule. Curr Opin Oncol. 2014;26:8–13. doi: 10.1097/CCO.0000000000000023. http://dx.doi.org/10.1097/CCO.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 19.Mehta V, Nikiforov YE, Ferris RL. Use of molecular biomarkers in FNA specimens to personalize treatment for thyroid surgery. Head Neck. 2013;35:1499–506. doi: 10.1002/hed.23140. [DOI] [PubMed] [Google Scholar]
- 20.Miller MC, Rubin CJ, Cunnane M, Bibbo M, Miller JL, Keane WM, et al. Intraoperative pathologic examination: cost effectiveness and clinical value in patients with cytologic diagnosis of cellular follicular thyroid lesion. Thyroid. 2007;17:557–65. doi: 10.1089/thy.2006.0166. http://dx.doi.org/10.1089/thy.2006.0166. [DOI] [PubMed] [Google Scholar]
- 21.Cheng MS, Morgan JL, Serpell JW. Does frozen section have a role in the intraoperative management of thyroid nodules? ANZ J Surg. 2002;72:570–2. doi: 10.1046/j.1445-2197.2002.02474.x. http://dx.doi.org/10.1046/j.1445-2197.2002.02474.x. [DOI] [PubMed] [Google Scholar]
- 22.Saydam L, Kalcioğlu MT, Kizilay A, Bozkurt MK. The evaluation of thyroid nodules: is routine use of frozen-section examination necessary following preoperative fine-needle aspiration biopsy? Kulak Burun Bogaz Ihtis Derg. 2003;11:80–4. [PubMed] [Google Scholar]
- 23.Richards ML, Chisholm R, Bruder JM, Strodel WE. Is thyroid frozen section too much for too little? Am J Surg. 2002;184:510–4. doi: 10.1016/s0002-9610(02)01074-7. http://dx.doi.org/10.1016/S0002-9610(02)01074-7. [DOI] [PubMed] [Google Scholar]
- 24.Rossing M, Nygaard B, Nielsen FC, Bennedbaek FN. High prevalence of papillary thyroid microcarcinoma in Danish patients: a prospective study of 854 consecutive patients with a cold thyroid nodule undergoing fine-needle aspiration. Eur Thyroid J. 2012;1:110–7. doi: 10.1159/000338921. http://dx.doi.org/10.1159/000338921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong MJ, Yang H, Yip L, Ohori NP, McCoy KL, Stang MT, et al. PAX8/PPARγ rearrangement in thyroid nodules predicts follicular-pattern carcinomas, in particular the encapsulated follicular variant of papillary carcinoma. Thyroid. 2014;24:1369–74. doi: 10.1089/thy.2014.0067. http://dx.doi.org/10.1089/thy.2014.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennedbaek FN, Hegedüs L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003;88:5773–7. doi: 10.1210/jc.2003-031000. http://dx.doi.org/10.1210/jc.2003-031000. [DOI] [PubMed] [Google Scholar]
- 27.Çakır B, Üçler R, Arpacı D, Balkan F, Dirikoç A, Ersoy R. Kistik tiroid nodüllerinde perkutan etanol enjeksiyonu ile tedavi. Endokrinolojide Diyalog. 2011;8:162–65. [Google Scholar]
- 28.Valcavi R, Frasoldati A. Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocr Pract. 2004;10:269–75. doi: 10.4158/EP.10.3.269. http://dx.doi.org/10.4158/EP.10.3.269. [DOI] [PubMed] [Google Scholar]
- 29.Achille G, Zizzi S, Di Stasio E, Grammatica A, Grammatica L. Ultrasound-guided percutaneous laser ablation (LA) in treating symptomatic solid benign thyroid nodules: Our experience in 45 patients. Head Neck. 2014 Dec 18; doi: 10.1002/hed.23957. [Epub ahead of print] http://dx.doi.org/10.1002/hed.23957. [DOI] [PubMed] [Google Scholar]
- 30.Papini E, Rago T, Gambelunghe G, Valcavi R, Bizzarri G, Vitti P, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99:3653–9. doi: 10.1210/jc.2014-1826. http://dx.doi.org/10.1210/jc.2014-1826. [DOI] [PubMed] [Google Scholar]