Abstract
Objective
Intranasal steroid sprays (INSS) are frequently prescribed for treating inferior turbinate hypertrophy (ITH). Complications due to the long-term application of INSS such as crusting, epistaxis, nasal mucosa dryness, and septal perforation may occur. Predicting patients who would benefit from INSS early might lower treatment costs and complication rates. We examined the predictive value of nasal decongestant response rates for the outcomes of INSS in ITH.
Methods
Fifty patients with bilateral ITH were included in two groups: patients benefiting from INSS and those not benefiting. Nasal airflow was assessed by peak nasal inspiratory flow (PNIF) measurement in all cases. Measurements were taken three times: before and after the application of nasal decongestant sprays and after the application of INSS.
Results
In both groups, the nasal air flow rates significantly increased after the application of nasal decongestant sprays; however, the nasal decongestant response rates were higher in the group with patients benefiting from INSS. There was a strong correlation between the nasal air flow rates measured after the application of nasal decongestant sprays and after the application of INSS. The cut-off value for the relationship between increased nasal air flow rates after the application of nasal decongestant sprays and outcomes of INSS was 23%.
Conclusion
Measurement of nasal airflow increase rate after the application of nasal decongestant sprays is a simple and easy method for the early prediction of the outcomes of INSS in ITH. A higher than 23% increase in nasal air flow rates after the application of nasal decongestant sprays indicates much better outcomes of INSS for patients.
Keywords: Nasal decongestants, nasal obstruction, inferior turbinate hypertrophy, intranasal steroid sprays
Introduction
Among the causes of nasal obstruction, the pathologies originating from the concha are quite common. The obstructive effect of the inferior concha may be due to the hypertrophy of the conchal soft tissue, the size of the conchal bone, or the deformities of the concha (1, 2). Conchal soft-tissue hypertrophies can be caused by effects such as allergic rhinitis, chronic or recurrent nasal infections, cigarette smoking, and polluted air; however, a definite factor cannot be identified in most patients and they are accepted as idiopathic (3).
The intranasal steroid spray (INSS) is a frequently used treatment option in the medical treatment of inferior concha hypertrophy (4, 5). Nasal steroids are used for the purpose of reducing the soft-tissue volume due to their non-specific anti-inflammatory properties. Although topical vasoconstrictor agents cause short-term reductions in the volume of the inferior concha, they are the preparations that are not suggested to be used more than five days due to their tolerance and rebound effects that they create together (6).
Various methods have been described in the surgical treatment of concha hypertrophies. The most commonly used methods are radiofrequency ablation, partial resection of the concha, conchoplasty, and concha lateralization (3, 7–10).
Although INSS are frequently preferred in concha hypertrophies, it is not always possible to provide recovery in all patients. Complications such as mucosal dryness, crusting, epistaxis, and rarely septum perforation are seen due to long-term use of steroid sprays (11). Predetermination of the patients who may respond positively to nasal steroid therapy will prevent complications and reduce treatment costs by eliminating unnecessary drug use. It will also allow patients to be promptly directed to other treatment options that they might benefit.
The relationship between the short-term decrease in the inferior concha volume with topical decongestant agents and the results obtained with long-term INSS therapy were investigated with regard to peak nasal inspiratory flow (PNIF) values in our study. It has been evaluated whether the response given to topical decongestant agents carries the threshold value characteristic that can provide a prediction for the INSS response.
Methods
The study was conducted prospectively between October 2016 and April 2017. Fifty patients who had a complaint of nasal obstruction more than 3 months, who were older than 18 years, and who had bilateral inferior concha hypertrophy were included in the study.
While the patients were evaluated with anamnesis, anterior rhinoscopy, and endoscopic nasal examination (after decongestion), paranasal sinus tomography examination was also performed in cases with additional complaints such as headache and postnasal discharge accompanied with nasal obstruction.
The patients in whom additional pathologies which could cause nasal obstruction, such as acute upper respiratory tract infections, nasal septum deviation, adenoid hypertrophy, allergic rhinitis, chronic sinusitis, and nasal cavity masses, were excluded. The patients who smoked and had previous nasal surgery; those who used oral contraceptives, topical or systemic steroids in the last year, and those who used medication due to additional health problems were not included in the study.
The complaint of nasal obstruction was graded with the aid of visual analogue scale (VAS) at the time of admission and after the steroid treatment. VAS scores were classified into three grades as mild, moderate, and severe (1, 2, 3: mild; 4, 5, 6, 7: average; 8, 9, 10: severe). The patients who achieved one level of improvement after the treatment were considered to have benefited from the treatment. According to the results of these evaluations, the patients were divided into two groups as recovered and not recovered.
Nasal air flow rate was measured in all patients using the PNIF measuring device (PNIFmeter) (Clement Clarke International, Harlow, UK). All measurements were carried out in the same room and under similar ventilation conditions. Measurements were performed three times: as before topical nasal decongestant agent application, after topical nasal decongestant agent application and six weeks after topical nasal steroid therapy. The measurements were named as PNIF 1, 2, 3, respectively. Patients were allowed to rest for 15 minutes before all measurements. All PNIF measurements were repeated three times with 2-min intervals, and the arithmetic mean was considered as the test result.
Nasal decongestant agent application was performed using oxymetazoline hydrochloride nasal spray (Iliadin 0.05% 10 mL spray, MERCK Selbstmedikation GmbH, Darmstadt, Germany License and Palmer Pharmaceuticals Inc.) three times in both nasal cavities after the diagnosis.
Topical nasal steroid therapy was performed using mometasone furoate (Nazoster® 0.05% nasal spray, SantaFarma Pharmaceuticals Inc., İstanbul, Turkey) in both nasal cavities, twice for each, twice a day and for six weeks.
In our study, the average PNIF 1, 2, 3 values and the rates of change provided in PNIF 2, 3 measurements were compared. The correlation between PNIF 2 values obtained after oxymetazoline nasal spray and PNIF 3 values measured after INSS was investigated. It has been evaluated whether the rate of increase in the nasal airflow caused by nasal decongestion carries the threshold value characteristic that can give information about the possible INSS response.
Approval of the ethics committee regarding the work was received from the ethics committee of our hospital with the number of 400 and date of October 19, 2016. Verbal and written informed consent was received from all patients participating in the study in accordance with the Helsinki Declaration.
Statistical analysis
Mean, standard deviation, median, lowest, highest, frequency, and ratio values were used in the descriptive statistics of the data. Variable distributions were measured by the Kolmogorov-Smirnov test. Statistical significance was evaluated at p<0.05 level. Independent sample t-test, χ2 test, and one-way ANOVA were used for intra- and intergroup evaluations. While the relationship between responses to the topical decongestant agent and steroid therapy was assessed by the Pearson correlation test, the ROC curve was used to calculate the threshold value. Statistical package for the Social Sciences 22.0 program (IBM Corp.; Armonk, NY, USA) was used for all statistical calculations.
Results
Fifty patients with inferior concha hypertrophy, 32 males and 18 females, were included in our study. The mean age was found as 35.94 years.
In the VAS analysis, evaluations were made after intranasal steroid therapy; while recovery was obtained in 33 (66%) patients, no recovery was observed in 17 (34%) patients. Of the 33 patients who responded positively to topical nasal steroid therapy, 21 were male (63.6%) and 12 were female (36.4%), and the mean age was 35.88±9.77 years. Of the 17 patients in whom no recovery was obtained with nasal steroid therapy, 11 (64.7%) were male and 6 were female (35.3%), and the mean age was 36.06±9.71 years. There was no significant difference in terms of age and gender distribution among the groups (Table 1).
Table 1.
Distribution of patients who showed recovery and not after INSS therapy according to their ages and gender
| Presence of recovery | No recovery | p | |
|---|---|---|---|
| Age | |||
| M±SD | 35.88±9.77 | 36.06±9.71 | 0.951 |
| Med (min–max) | 37 (19–55) | 36 (20–57) | |
| Gender | 33 | 17 | |
| Male | 21 (63.6%) | 11 (64.7%) | 0.940 |
| Female | 12 (36.4%) | 6 (35.3%) |
INSS: intranasal steroid spray; M: mean; SD: standard deviation; med: median; min: minimum; max: maximum
While no significant difference was found between the mean PNIF 1 values measured before the treatment in patient groups in which recovery was and was not obtained with INSS therapy (p=0.393), the mean values of PNIF 3 and the increased rate of PNIF 3 were found to be significantly higher in the group in which recovery was obtained (p=0.001 and 0.024, respectively) (Table 2).
Table 2.
Mean PNIF values and change rates in the groups showing recovery and no recovery
| Presence of recovery M±SD |
No recovery M±SD |
p | |
|---|---|---|---|
| PNIF 1 | 66.21±13.64 | 62.94±10.61 | 0.393 |
| PNIF 2 | 87.03±15.95 | 76.41±11.27 | 0.018 |
| PNIF 3 | 97.36±16.07 | 70.00±11.22 | 0.001 |
| PNIF 2 chn % | 31.45±7.77 | 20.70±5.24 | 0.001 |
| PNIF 3 chn % | 63.52±6.44 | 9.53±2.80 | 0.024 |
PNIF: peak nasal inspiratory flow; chn: change; M: mean; SD: standard deviation
The mean PNIF 2 value and change rate determined after nasal decongestant in the groups in which recovery was and was not obtained with INSS therapy were significantly higher in the group that showed recovery (p=0.018 and 0.001, respectively) (Table 2).
Mean PNIF 2 was significantly higher than PNIF 1, and mean PNIF 3 was significantly higher than PNIF 2 and 1 (p<0.001) in patients who were treated with INSS therapy (Figure 1).
Figure 1.
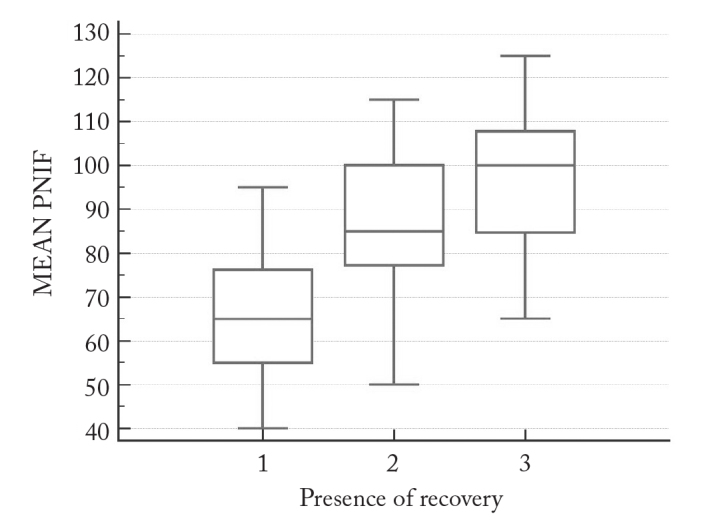
PNIF 1, 2, and 3 measurement values in the group showing recovery
In the patient group which did not show any recovery after intranasal steroid therapy, mean PNIF 2 value was significantly higher than PNIF 1 (p=0.004), but there was no significant difference between PNIF 1 and PNIF 3 values and PNIF 2 and 3 values (Figure 2).
Figure 2.
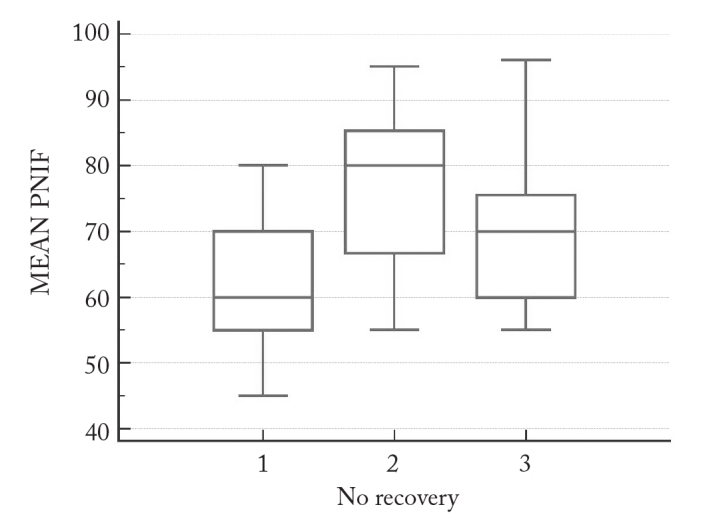
PNIF 1, 2, and 3 measurement values in the group showing no recovery
Two groups of patients responding differently to INSS therapy seem to respond similarly to nasal decongestant agent administration. After nasal decongestant, significant increases in nasal air flow were obtained in both groups (Figures 1 and 2).
However, the mean increase rate of PNIF determined after nasal decongestant agent application was found to be significantly higher in patients who were treated with INSS therapy than the patient group that did not respond to the treatment (p=0.001) (Table 2).
The correlation between the nasal air flow rates (PNIF 2) measured after nasal decongestion agent application and the flow rates measured after nasal steroid therapy (PNIF 3) was evaluated by Pearson correlation test and a strong correlation was found (R=0.8083). As the nasal airflow rate increased after decongestion, it was observed that the rates measured after nasal steroid treatment increased (Figure 3).
Figure 3.
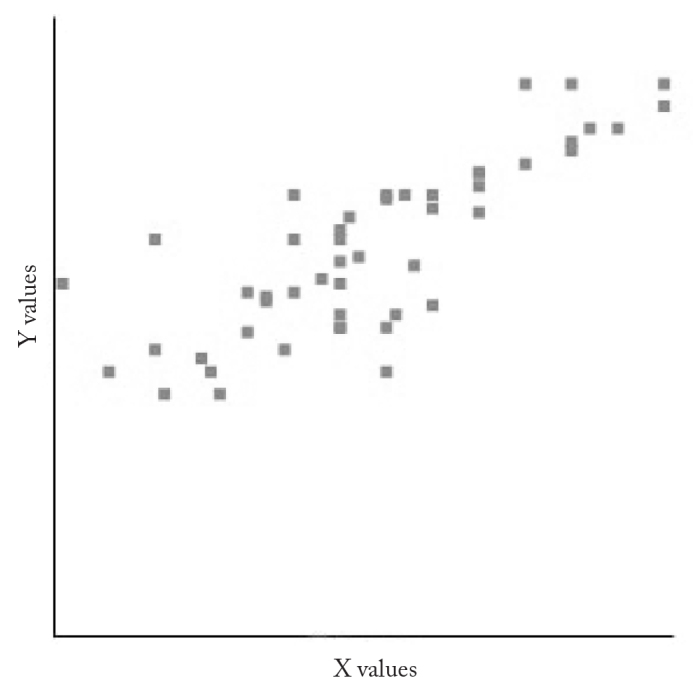
The relationship between nasal air flow rates after nasal decongestion application (PNIF 2) and after nasal steroid therapy (PNIF 3)
X values: PNIF 2; Y values: PNIF 3
The ROC analysis was used to assess whether or not the nasal airflow increase rates after decongestant agent application carried the threshold value characteristics that could provide a prediction about the response to the nasal steroid therapy. In this evaluation, the threshold value was found to be 23% (sensitivity, 84.8%; specificity, 82.4%; area under the ROC curve, 0.883). It was found that the rate of positive response to INSS treatment was found to be higher in cases with a 23% increase in PNIF values measured after nasal decongestant (Figure 4).
Figure 4.
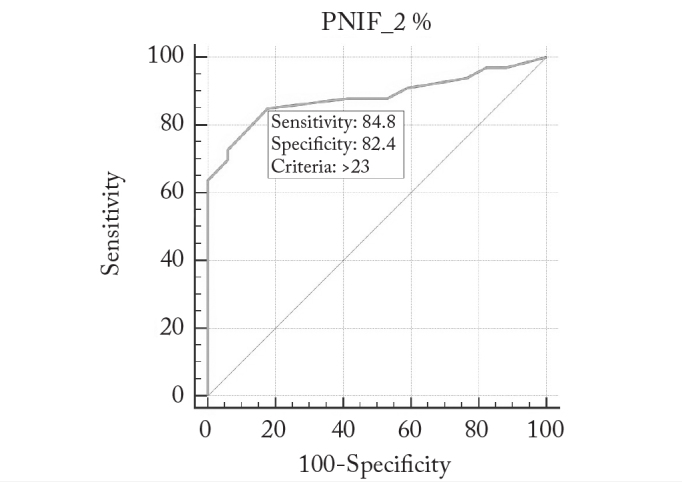
Determination of threshold values in the PNIF change rates detected after the administration of nasal decongestant (ROC analysis)
Discussion
Complaints of nasal obstruction are usually assessed subjectively in routine ENT examinations. However, some methods that allow the measurement of nasal airflow have also been described. Rhinomanometry, acoustic rhinometry, odiosoft rhino, and PNIF measurements are among these methods (12–15). It is also stated that ultrasound elastography can be used to evaluate the stromal structure of inferior concha (16). PNIF application is a preferred method for objectively evaluating nasal obstruction due to the fact that it is low cost and easy to apply when compared to the other tests. Several studies in the literature have found it to be as reliable as the other methods (13, 14, 17, 18).
The inferior concha is histologically composed of three layers as the medial and lateral mucosal folds and the bone layer between them (1, 19, 20). The medial layer is thicker than the lateral layer. The thickness depends on the richer content of lamina propria in this region, and the vascular network structure in this area is more advanced. Various irritant factors and infections cause an inflammatory response in the lamina propria region and consequently cause an increase in concha volume. The resulting inflammatory response emerge with mast cells, basophils, leucocytes, and mediators released from them and cause short- and long-term changes in the concha structure leading to soft-tissue hypertrophy (19, 20).
Medical treatment is the first choice in the majority of inferior concha hypertrophies. Depending on the etiology, topical or systemic nasal decongestants, antihistamines, leukotriene receptor antagonists, systemic steroids, and INSS may be preferred (21–28). Considering the rebound and tolerance properties and side effect profiles of other options, INSSs are the most commonly preferred agents in isolated cases of concha hypertrophy (4–6). It is believed that steroids have an effect due to the fact that they reduce the volume of the concha with the non-specific anti-inflammatory properties (4–6).
The inferior concha mucosa is the respiratory epithelium which is composed of pseudostratified columnar ciliated epithelium. The submucosa has a complex arterial network structure, arteriovenous anastomoses, venous sinusoids, and muscular nerves. The vascular characteristics of the submucosa provide the inferior concha with the feature of an erectile tissue, and the contracting or dilating venous sinusoids and arteriovenous anastomoses affect the nasal passage airflow (1, 19, 20). Nasal decongestant s agents reduce concha volume and cause an increase in nasal airflow by causing vasoconstriction in these vascular elements (18).
We think that the structural characteristics of concha hypertrophy are influential in the different responses that emerge. The nasal congestion resulting from the inferior concha may be due to the increase of the soft-tissue burden, or it may be due to the thickness of the inferior conchal bone layer and the angular characteristics of the concha placement (1).
The effects of new-generation INSSs on the hypothalamo-pituitary arc are considered minimal, and long-term use of INSS is found to be safe (11). However, long-term use of INSS can lead to local complications such as mucosal dryness, crusting, epistaxis, and septal perforation (11). In addition, long-term use of medications in patients who will not benefit from treatment disrupts patient comfort and creates additional costs.
Significant increases were found in PNIF measurements made after nasal decongestant agent applications in both groups of patients who responded and did not respond to topical nasal steroid therapy. However, in the group of patients who responded positively to the intranasal steroid therapy, the mean values of PNIF and increases in PNIF after nasal decongestant were significantly higher than the group of patients who did not respond to the nasal steroid treatment. In the analysis that was performed, the rate of positive response to intranasal steroid therapy was found to be significantly higher in the patients who had inferior concha hypertrophy and in whom an increase of more than 23% was found in PNIF measurements after nasal decongestant agent applications.
Because the anamnesis and examination findings were sufficient in the differential diagnosis of inferior concha hypertrophy, imaging methods were not used. We think that extreme conchal soft-tissue burden, conchal bone lamellar hypertrophies, and inferior concha topographic anomalies may have been effective in different responses to the treatment of INSS in our study. The limitations of our study are that these features of the inferior concha were not evaluated by imaging techniques and the patients with allergic rhinitis were excluded only by means of the methods of history and examination.
Conclusion
Our study has revealed that the determination of the change values in PNIF measurement values after nasal decongestion agent applications can be used to determine the cases who have a 23% threshold value and who have high chance to give positive response to INSS treatment, and it has revealed that it is a non-invasive, effective, reliable, and inexpensive method.
Footnotes
Ethics Committee Approve: Ethics committee approval was received for this study from the ethics committee of Haseki Training and Research Hospital (19.10.2016/2016-400).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Conflict of Interest: No conflict of interest was declared by the author.
Financial Disclosure: The author declared that this study has received no financial support.
References
- 1.Neskey D, Eloy JA, Casiano RR. Nasal, septal and turbinate anatomy and embryology. Otolaryngol Clin N Am. 2009;42:193–205. doi: 10.1016/j.otc.2009.01.008. https://doi.org/10.1016/j.otc.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Akoğlu E, Karazincir S, Balci A, Okuyucu S, Sumbas H, Dağli AS. Evaluation of the turbinate hypertrophy by computed tomography in patients with deviated nasal septum. Otolaryngol Head Neck Surg. 2007;136:380–4. doi: 10.1016/j.otohns.2006.09.006. https://doi.org/10.1016/j.otohns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Karatas A, Salviz M, Dikmen B, Yüce T, Acar G. The effects of different radiofrequency energy magnitudes on mucociliary clearance in cases of turbinate hypertrophy. Rhinology. 2015;53:171–5. doi: 10.4193/Rhino14.208. [DOI] [PubMed] [Google Scholar]
- 4.Gunhan K, Unlu H, Yuceturk AV, Songu M. Intranasal steroids or radiofrequency turbinoplasty in persistent allergic rhinitis: effects on quality of life and objective parameters. Eur Arch Otorhinolaryngol. 2011;268:845–50. doi: 10.1007/s00405-010-1462-1. https://doi.org/10.1007/s00405-010-1462-1. [DOI] [PubMed] [Google Scholar]
- 5.Gunel C, Basak HS. Evaluation of the effect of intranasal corticosteroid sprays on radiofrequency tissue ablation in the treatment of hypertrophied inferior turbinate. Kulak Burun Bogaz Ihtis Derg. 2011;21:10–4. [PubMed] [Google Scholar]
- 6.Mortuaire G, de Gabory L, François M, Massé G, Bloch F, Brion N, et al. Rebound congestion and rhinitis medicamentosa: nasal decongestants in clinical practice. Critical review of the literature by a medical panel. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130:137–44. doi: 10.1016/j.anorl.2012.09.005. https://doi.org/10.1016/j.anorl.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Brunworth J, Holmes J, Sindwani R. Inferior turbinate hypertrophy: Review and graduated approach to surgical management. Am J Rhinol Allergy. 2013;27:411–5. doi: 10.2500/ajra.2013.27.3912. https://doi.org/10.2500/ajra.2013.27.3912. [DOI] [PubMed] [Google Scholar]
- 8.Nease CJ, Krempl GA. Radiofrequency treatment of turbinate hypertrophy: A randomized, blinded, placebo controlled clinical trial. Otolaryngol Head Neck Surg. 2004;130:291–9. doi: 10.1016/j.otohns.2003.11.003. https://doi.org/10.1016/j.otohns.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Rhee CS, Kim DY, Won TB, Lee HJ, Park SW, Kwon TY, et al. Changes of nasal function after temperature-controlled radiofrequency tissue volume reduction for the turbinate. Laryngoscope. 2001;111:153–8. doi: 10.1097/00005537-200101000-00026. https://doi.org/10.1097/00005537-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Farmer SE, Eccles R. Chronic inferior turbinate enlargement and the implications for surgical intervention. Rhinology. 2006;44:234–8. [PubMed] [Google Scholar]
- 11.Jang TY, Kim YH. Recent updates on the systemic and local safety of intranasal steroids. Curr Drug Metab. 2016;17:992–6. doi: 10.2174/1389200218666161123123516. https://doi.org/10.2174/1389200218666161123123516. [DOI] [PubMed] [Google Scholar]
- 12.Chaaban M, Corey JP. Assessing nasal air flow: options and utility. Proc AmThorac Soc. 2011;8:70–8. doi: 10.1513/pats.201005-034RN. https://doi.org/10.1513/pats.201005-034RN. [DOI] [PubMed] [Google Scholar]
- 13.Ottaviano G, Scadding GK, Iacono V, Scarpa B, Martini A, Lund VJ. Peak nasal inspiratory flow and peak expiratory flow. Upright and sitting values in an adult population. Rhinology. 2016;54:160–3. doi: 10.4193/Rhino15.180. [DOI] [PubMed] [Google Scholar]
- 14.Ottaviano G, Fokkens WJ. Measurements of nasal airflow and patency: a critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy. 2016;71:162–74. doi: 10.1111/all.12778. https://doi.org/10.1111/all.12778. [DOI] [PubMed] [Google Scholar]
- 15.Midilli R, Gode S, Karci B, Orhan M, Saylam CY. The clinical value of the novel cauterization procedure for the inferior turbinate artery during turbinate surgery. Eur Arch Otorhinolaryngol. 2012;269:1629–33. doi: 10.1007/s00405-011-1869-3. https://doi.org/10.1007/s00405-011-1869-3. [DOI] [PubMed] [Google Scholar]
- 16.Kısmalı E, Göde S, Turhal G, Öztürk K, Raşit M. A new insight for evaluation of the inferior turbinate with ultrasound elastography. J Ultrasound Med. 2015;34:777–82. doi: 10.7863/ultra.34.5.777. https://doi.org/10.7863/ultra.34.5.777. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira RU, Zappelini CE, Alves FS, da Costa EA. Peak nasal inspiratory flow evaluation as an objective method of measuring nasal airflow. Braz J Otorhinolaryngol. 2011;77:473–80. doi: 10.1590/S1808-86942011000400011. https://doi.org/10.1590/S1808-86942011000400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bermüller C, Kirschek H, Rettinger G, Riechelmann H. Diagnostic accuracy of peak nasal inspiratory flow and rhinomanometry in functional rhinosurgery. Laryngoscope. 2008;118:605–10. doi: 10.1097/MLG.0b013e318161e56b. https://doi.org/10.1097/MLG.0b013e318161e56b. [DOI] [PubMed] [Google Scholar]
- 19.Berger G, Gass S, Ophir D. The histopathology of the hypertrophic inferior turbinate. Arch Otolaryngol Head Neck Surg. 2006;132:588–94. doi: 10.1001/archotol.132.6.588. https://doi.org/10.1001/archotol.132.6.588. [DOI] [PubMed] [Google Scholar]
- 20.Naclerio RM, Bacher C, Baraniuk JN. Pathophysiology of nasal congestion. Int J Gen Med. 2010;8:47–57. doi: 10.2147/ijgm.s8088. https://doi.org/10.2147/IJGM.S8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scadding GK. Optimal management of allergic rhinitis. Arch Dis Child. 2015;100:576–82. doi: 10.1136/archdischild-2014-306300. https://doi.org/10.1136/archdischild-2014-306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Sha Q, Zuo K, Jiang H, Cheng L, Shi J, et al. Nasal saline irrigation facilitates control of allergic rhinitis by topical steroid in children. ORL J Otorhinolaryngol Relat Spec. 2009;71:50–5. doi: 10.1159/000178165. https://doi.org/10.1159/000178165. [DOI] [PubMed] [Google Scholar]
- 23.Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128:1139–50. doi: 10.1016/j.jaci.2011.09.005. https://doi.org/10.1016/j.jaci.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Church MK, Maurer M, Simons FE, Bindslev-Jensen C, van Cauwenberge P, Bousquet J, et al. Risk of first-generation H(1)-antihistamines: a GA(2)LEN position paper. Allergy. 2010;65:459–66. doi: 10.1111/j.1398-9995.2009.02325.x. https://doi.org/10.1111/j.1398-9995.2009.02325.x. [DOI] [PubMed] [Google Scholar]
- 25.Portnoy JM, Van Osdol T, Williams PB. Evidence-based strategies for treating allergic rhinitis. Curr Allergy Asthma Rep. 2004;6:439–46. doi: 10.1007/s11882-004-0009-1. https://doi.org/10.1007/s11882-004-0009-1. [DOI] [PubMed] [Google Scholar]
- 26.Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317:1624–9. doi: 10.1136/bmj.317.7173.1624. https://doi.org/10.1136/bmj.317.7173.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yá-ez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002;89:479–84. doi: 10.1016/S1081-1206(10)62085-6. https://doi.org/10.1016/S1081-1206(10)62085-6. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein SF. Combination therapy in the treatment of allergic rhinitis. Allergy Asthma Proc. 2002;23:1–3. [PubMed] [Google Scholar]


