Abstract
Objective
The aim of this study was to compare subepithelial angiogenesis developing within the perimatrix of the cholesteatoma between pediatric and adult patients.
Methods
Sixty-one patients who underwent mastoidectomy for the first intent because of chronic otitis media with cholesteatoma between 1993 and 2013 and from whom appropriate tissue specimens were taken were included in the study. The patients were classified in the pediatric patient group if they were under the age of 18 years and the adult patient group if they were 18 years and older. Immunohistochemical staining for CD-31 was performed on new sections taken during surgery and sections prepared from archived tissues in paraffin blocks. Results were compared between the groups.
Results
A total of 61 patients, of whom 25 were pediatric and 36 were adult patients, were included in the study. The mean CD-31 immunopositive microvessel rates were 8.8 (3–15) and 6.61 (2–14) for the pediatric and adult patient groups, respectively. The difference between the groups was statistically significant (p=0.037). Correlation analysis showed a statistically significant negative correlation between the CD-31 immunopositive microvessel rates and age (p=0.036).
Conclusion
Subepithelial angiogenesis developing within the perimatrix of the cholesteatoma of the pediatric patients was more expressed than that of the adult patients.
Keywords: Angiogenesis, CD-31, cholesteatoma, pediatric
Introduction
Cholesteatoma develops from the squamous cell epithelium in the air spaces of the temporal bone and it includes keratin debris in its center. It is a disease that displays histopathologically benign but clinically local destructive clinical course. In its pathogenesis, increased cell proliferation in the epithelial tissue and keratin production are responsible. It is a immunohistochemical marker in CD-31 microvascular vein endothelium and it is defined in tumor angiogenesis (4, 5). Hypoxic period developing in cholesteatoma, tissue damage, and increased inflammatory cells stimulate angiogenesis through vascular and endothelial growth factors. Angiogenesis increasing in the subepithelial tissue, namely in perimatrix, can be considered as one of the biological markers of cholesteatoma activity (5, 6).
In this retrospective study in which it was aimed to compare pediatric and adult patients with regard to subepithelial angiogenesis in cholesteatoma perimatrix, the density of microvessels stained with CD-31 was detected. Moreover, the relationship between the age of patients and the number of microvessels was investigated through correlation analysis.
Methods
Before beginning the study, the ethical approval was received from the Non-Invasive Research Ethics Committee with the decision number of 2013/17–15.
A total of 223 patients who had been implemented mastoidectomy for the first time due to chronic otitis media in our clinic between 1993 and 2013 were determined retrospectively. Their tissue samples taken during surgery were examined. The inclusion criteria were defined as having been taken adequate and appropriate tissue sample including epithelial and subepithelial cells for immunohistochemical staining, giving consent for the study, and having completely reachable recordings. Tissue samples of sixty one patients meeting these criteria were included in the study. The patients younger than 18 years old were put into the pediatric patient group and the patients at the age of 18 years and older were included in the adult patient group.
New sections were prepared from the paraffin-embedded blocks of the patients, which were preserved in the archive of Pathology department. The sections were kept in 950C CC1 (cell conditioning 1) (Ventana Medical Systems, Tuscon, Arizona, USA) solution for 52 minutes and then incubated in a solution prepared in the ratio of 1/50 dilution from PECAM (MAB346P) (Innovex Biosciences, Richmond, USA) for 32 minutes. Stainings were carried out in Benchmark Ultra (Ventana Medical Systems, Tuscon, Arizona, USA) device by using Ultraview Universal DAB Detection kit (Ventana Medical Systems, Tuscon, Arizona, USA). CD-31 stained sections were viewed in Eclipse Ci (Nikon, Tokyo, Japan) microscope and digitalized with DS-Fi2 camera. Then, they were transferred to the computer. The number of microvessels immunopositively stained with CD-31 in the section was determined at ×20 magnification on the computer screen (Figure 1).
Figure 1.
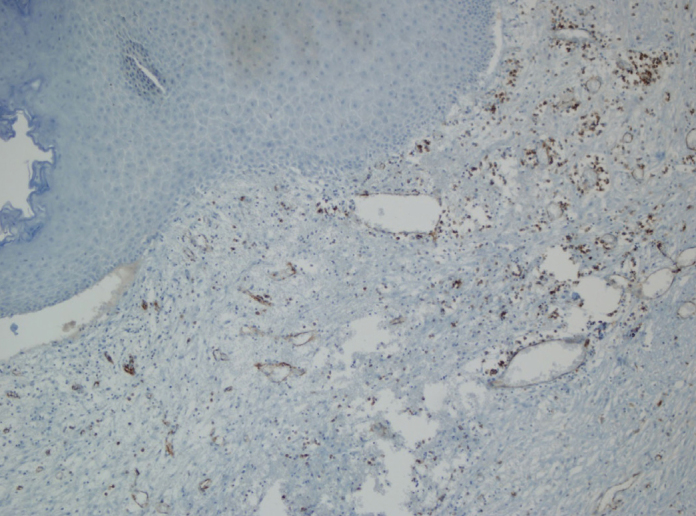
Structures of microvessels observed at ×20 magnification
While analyzing the data obtained, SPSS 15.0 statistical software (IBM, New York, USA) was used. The mean values and standard deviations were calculated. The mean values of the variables were compared using Mann Whitney U test. Kendall’s tau-b test was employed for correlation analysis. The level of significance was accepted to be p<0.05.
Results
A total of 61 patients, including 25 pediatric and 36 adult patients, were involved in the study. Of them, 29 (47,5%) were female and 32 (52,5%) were male. The mean age of patients, who ranged from 3 years to 71 years old, was found to be 27.31 years [mean 27,31 years (SD +/−18.345 years)]. The pediatric patient group included 25 (13 female and 12 male) patients and the mean age was 10,6 years [mean 10,6 years (SD +/−4.103 years)]. In the adult patient group, there were 36 (16 female and 20 male) patients and their mean age was detected to be 38,92 years [mean 38,92 years (SD +/−15.08 years)].
The mean number of microvessels was 8,8 [mean 8,8 (SS +/− 4,61)] in the pediatric patient group and 6,61 [mean 6,61 (SS:3,659)] in the adult patient group.
In comparison of the pediatric and adult groups, the number of microvessels immunopositevely stained with CD-31 was found to be significantly higher in the pediatric patient group (p:0,037, Mann-Whitney U test) (Table 1).
Table 1.
Findings obtained from pediatric and adult patient groups
| Pediatric group | Adult group | p | |
|---|---|---|---|
|
| |||
| Total | 25 (13 female and 12 male) | 36 (16 female and 20 male) | |
| Age | mean | mean | |
| 10.6 (SD +/−4.103) | 38.92 (SS +/−15.08) | ||
| median 10 (3–17) | median 36.5 (19–71) | ||
| The number of microvessels | mean | mean | |
| 8.8 (SS +/− 4.61) | 6.61 (SS +/− 3.659) | ||
| median 8 (3–15) | median 6 (2–14) | *0.037 | |
Mann-Whitney U
The correlation analysis performed using data of all patients revealed a negative and statistically significant correlation between the patient age and the number of microvessels (p: 0,036, Kendall’s tau-b) (Table 2).
Table 2.
Findings of correlation analysis
| Age | The number of microvessels | |
|---|---|---|
|
|
||
| Correlation Coefficient | −0.192 | |
| p | **0.036 |
Kendall’s tau-b
Discussion
Cholesteatoma comprises of squamous cell epithelium developing in the air spaces of the temporal bone. The epithelium constitutes cholesteatoma matrix in the periphery and includes an acellular layer associated with increased keratin production in its center (1). The perimatrix layer that is rich in inflammatory cells and has histopathological features similar to granulation tissue is observed around the matrix. Inflammation and enzymatic reactions developing in the perimatrix are held accountable for the destructive course of cholesteatoma (1). Especially in pediatric cholesteatoma that is thought to be more aggressive, the structure of the perimatrix and its histochemical characteristics have been investigated in many studies.
Quaranta et al. (7) considered the inflammation of the perimatrix responsible for the clinical features of pediatric cholesteatoma. They examined histomorphological features of the perimatrix in the cholesteatoma tissues of 30 pediatric and 30 adult patients and they found that the number of mononuclear cells in the perimatrix was higher in the pediatric group. Based on these findings, they suggested that the perimatrix was thicker and more active in pediatric cholesteatoma.
Mayot et al. (8) evaluated tissue samples of 14 pediatric patients having undergone surgery for otitis media with cholesteatoma and observed a severe inflammatory response in the junction of epidermis and middle ear mucosa. They determined CD-1 positive Langerhans cells, mast cells, and the IgA-producing cells and obtained findings consistent with delayed-type hypersensitivity. And, they suggested that this inflammation developing in the mucosa could be responsible for the pathogenesis of cholesteatoma.
In the study of Dornelles et al. (9), the thickness of the perimatrix, which is the center of inflammatory reaction in pediatric and adult patients, was measured and the results differed between 27 and 1277 micrometer. Although the mean thickness of the perimatrix was higher in adult patients, moderate but reverse correlation was found between age and perimatrix thickness in correlation analyses. In short, according to the result of Dornelles et al.’s study, as the age of patient increases, the thickness of the perimatrix decreases.
The most important feature of the perimatrix is probably its metabolic activity apart from its dimensional features. Dornelles et al. conducted a study with a population including pediatric and adult patients on epithelial thickness, perimatrix thickness, and the density of inflammatory cells in the perimatrix and they detected a strong correlation between the matrix and perimatrix thickness (10). However, they found no correlation between the density of inflammatory cells in the perimatrix and patient age or perimatrix thickness.
In another study, Dornelles et al. examined MP-2 and MP-9 levels for metalloproteinase (MP) activity and CD-31 levels for angiogenesis in the sections of pediatric and adult cholesteatoma tissues. They found the CD-31 and MP levels to be significantly higher in the pediatric group (5).
It has been focused on that hypoxic period developing in cholesteatoma increases inflammatory reactions and growth factors secreted from inflammatory cells stimulate angiogenesis. In the studies conducted on increased angiogenesis in the perimatrix, Stammberger et al. (11) examined factor-8 levels and found increased levels of factor-8 in cholesteatoma compared to normal skin. In the study of Sudhoff et al., they detected that growth factors such as β-FGF, TGF-α, TGF-β1 and VEGF, which are responsible for increased angiogenesis, were elevated in cholesteatoma tissue compared to both the skin of the external auditory canal and the middle ear mucosa (12).
In the studies performed about tumor angiogenesis, CD-31 was defined as an immunomarker in the vascular endothelium. Different from other angiogenesis immunomarkers, it is not found in lymphatic vessels (4, 5). The severity of increased inflammation in cholesteatoma, especially in the perimatrix, and the density of angiogenesis can be demonstrated through determination of the number of microvessels immunopositively stained with CD-31. Therefore, increased CD-31 immunopositivity and the number of microvessels were accepted to be biological markers of chelesteatoma activity.
Dornelles et al. compared 60 pediatric and 60 adult patients in their study and they found the density of CD-31 as 7 (4–11) in the pediatric patient group and as 4 (0–10) in the adult patient group (5). They evaluated this difference to be statistically significant. Since metalloproteinases (cytoplasmic and nuclear MP-2, MP-9) were also detected to be high in the same study, they associated CD-31 with the degree of inflammation.
In the study of Jin et al. on epidermal growth factor receptor and CD-31 levels, 32 cholesteatoma tissues were compared to 6 postauricular skin tissues (6). Epidermal growth factor receptor was found to be higher in cholesteatoma tissue, although it was statistically insignificant. CD-31 levels were detected to be significantly higher in cholesteatoma tissues than in skin tissues and they were correlated with growth factor receptor levels.
In our study, the number of microvessels stained with CD-31 immunomarker was revealed to be significantly higher in the pediatric patient group than in the adult patient group. Moreover, as a result of correlation analysis, it was found that as the age of patient increased, the number of microvessels decreased. This demonstrated that inflammatory mechanisms developing in the perimatrix increased angiogenesis and this characteristic was more remarkable in pediatric patients.
Not having taken tissue sample from each patient who had been undergone tympanpmastoidectomy previously or the absence of sufficient epithelial tissue for advanced immunohistochemical staining in the tissue samples taken caused the number of participants to be lower than expected. This is a limitation of our study. In some tissue samples, only keratin clusters were observed and their epithelial tissues could not be determined. This shows that wider biopsy specimen covering matrix and perimatrix should be taken from the neighbor tissue, not only from the content of cholesteatoma. Moreover, samples being preserved in formaldehyde and being archived in paraffin blocks make studying with some techniques (such as microRNA and gene expression), which have been begun to be used more frequently in current studies, impossible for now.
Conclusion
Angiogenesis encountered in cholesteatoma perimatrix is a result of inflammatory period and it can be accepted to be associated with the severity of inflammation. The high number of microvessels in the pediatric patient group and even the increase in the number of microvessels in parallel to decreased age show that increased inflammatory period in this age group can be one of the mechanisms responsible for the destructive course of cholesteatoma activity.
Footnotes
Ethics Committee Approval: Approved by Ethical Committee for Non-Invasive Human Research at Dokuz Eylul University. Approval number: 2013/17-15
Informed Consent: Written informed consent was not obtained due to the retrospective nature of this study.
Peer-review: Externally peer-reviewed.
Conflict of Interest: No conflict of interest was declared by the authors.
Author Contributions: Concept - M.A., T.K.E.; Design - M.A., T.K.E., E.Ö., S.S.; Supervision - E.A.G. A.Ö.İ., E.Ö., S.S.; Funding - M.A. T.K.E.; Materials - T.K.E., E.A.G., A.Ö.İ.; Data Collection and/or Processing - M.A., E.Ö., S.S., E.U.; Analysis and/or Interpretation - T.K.E., M.A., S.S., E.Ö. E.U.; Literature Review - M.A., T.K.E., S.S.; Writing - M.A.; Critical Review - E.A.G., A.ö.İ. E.Ö., S.S.
Financial Disclosure: This study was financially supported by a grant from Dokuz Eylül University Research Foundation.
References
- 1.Lim DJ, Saunders WE. Acquired cholesteatoma: light and electron microscopic observations. Ann Otol. 1972;81:2–12. http://dx.doi.org/10.1177/000348947208100102. [PubMed] [Google Scholar]
- 2.Louw L. Acquired cholesteatoma: summary of the cascade of molecular events. J Laryngol Otol. 2013;127:542–9. doi: 10.1017/S0022215113000601. http://dx.doi.org/10.1017/S0022215113000601. [DOI] [PubMed] [Google Scholar]
- 3.Ferlito O, Devaney KO, Rinaldo A, Milroy C, Wenig B, Iurato S. Clinicopathological consultation ear cholesteatoma versus cholesterol granuloma. Ann Otol Rhinol Laryngol. 1997;106:79–85. doi: 10.1177/000348949710600114. http://dx.doi.org/10.1177/000348949710600114. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. http://dx.doi.org/10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 5.de Dornelles CC, da Costa SS, Meurer L, Rosito LP, da Silva AR, Alves SL. Comparison of acquired cholesteatoma between pediatric and adult patients. Eur Arch Otorhinolaryngol. 2009;266:1553–61. doi: 10.1007/s00405-009-0957-0. http://dx.doi.org/10.1007/s00405-009-0957-0. [DOI] [PubMed] [Google Scholar]
- 6.Jin BJ, Min HJ, Jeong JH, Park CW, Lee SH. Expression of EGFR and microvessel density in middle ear cholesteatoma. Clin Exp Otorhinolaryngol. 2011;4:67–71. doi: 10.3342/ceo.2011.4.2.67. http://dx.doi.org/10.3342/ceo.2011.4.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quaranta A, Ressa L, Santangelo A. Otomastoid cholesteatoma in children: histopathological findings. Int J Pediatric Otorhinolaryngol. 1986;12:121–6. doi: 10.1016/s0165-5876(86)80069-6. http://dx.doi.org/10.1016/S0165-5876(86)80069-6. [DOI] [PubMed] [Google Scholar]
- 8.Mayot D, Béné MC, Faure GC, Wayoff M, Perrin C. Immunohistologic analysis of the cholesteatoma matrix in children. Int J Pediatr Otorhinolaryngol. 1991;22:115–24. doi: 10.1016/0165-5876(91)90031-6. http://dx.doi.org/10.1016/0165-5876(91)90031-6. [DOI] [PubMed] [Google Scholar]
- 9.Dornelles C, da Costa SS, Meurer L, Schweiger C. Correlation of cholesteatomas perimatrix thickness with patient’s age. Braz J Otorhinolaryngol. 2005;71:792–7. doi: 10.1016/S1808-8694(15)31250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dornelles C, Meurer L, Selaimen da Costa S, Schweiger C. Histologic description of acquired cholesteatomas: comparison between children and adults. Braz J Otorhinolaryngol. 2006;72:641–8. doi: 10.1016/S1808-8694(15)31020-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stammberger M, Bujía J, Schulz P. Correlation of vascular morphology with clinical types in cholesteatoma of the middle ear. Am J Otol. 1994;15:380–2. [PubMed] [Google Scholar]
- 12.Sudhoff H, Dazert S, Gonzales AM, Borkowski G, Park SY, Baird A, et al. Angiogenesis and angiogenic growth factors in middle ear cholesteatoma. Am J Otol. 2000;21:793–8. [PubMed] [Google Scholar]


