Abstract
Background
The timing of intravenous antibiotic administration and lactate measurement is associated with survival of septic shock patients. Septic shock patients were admitted to the medical intensive care unit (MICU) from 2 major sources: hospital ward and emergency department (ED). This study aimed to compare the timing of antibiotic administration and lactate measurement between hospital wards and the ED.
Patients and methods
Medical data were collected from adult patients admitted to the MICU with septic shock from January 2015 to December 2016. “Time Zero” was defined as the time of diagnosis of sepsis. The associations between the times and risk-adjusted 28-day mortality were assessed.
Results
In total, 150 septic shock patients were admitted to the MICU. The median time interval (hour [h] interquartile range [IQR]) from time zero to antibiotic administration was higher in patients from the hospital wards compared to those from the ED (4.84 [3.5–8.11] vs 2.04 [1.37–3.54], P<0.01), but the lactate level measurement time interval (h [IQR]) from time zero was not different between the hospital wards and the ED (1.6 [0.2–2.7] vs 1.6 [0.9–3.0], P=0.85). In multivariate analysis, higher risk-adjusted 28-day mortality was associated with antibiotic monotherapy (odds ratio [OR]: 19.3, 95% confidence interval [CI]: 2.4–153.1, P<0.01) and admission during the weekends (OR: 24.4, 95% CI: 2.9–199.8, P<0.01).
Conclusion
Antibiotic administration in septic shock patients from the hospital wards took longer, and there was also less appropriate antibiotic prescriptions seen in this group compared with those admitted from the ED. However, neither the timing of antibiotic administration nor lactate measurement was associated with mortality.
Keywords: septic shock, antibiotic administration, lactate, ward, emergency department
Introduction
Sepsis and septic shock are crucial health problems worldwide1,2 and are the leading causes of death in many intensive care units. Since sepsis and septic shock have poor outcomes with high mortality rates, the 4 versions of the Surviving Sepsis Campaign (SSC) aim to decrease the mortality rates.3 A worldwide observational study revealed that the mortality rate decreased 0.7% per site for every 3 months of participation in the SSC.4 Thus, compliance to the bundles of the SSC is vital.
According to the SSC 2012,5 management of sepsis was divided into 2 bundles: 3-h bundle and 6-h bundle. In the 3-h SSC bundle, the administration of intravenous antibiotics should be initiated within the first hour of recognition of sepsis. A study by Kumar et al6 revealed that every 1 h of delayed antibiotic administration will reduce the survival by 7.6%. Multiple observational studies confirmed that delays in antibiotic administration were associated with mortality.7–10 In addition to antibiotic administration, lactate should be measured at the time of diagnosis of sepsis. Lactate level is used for sepsis recognition and as a guide for resuscitation. Unfortunately, these 2 components of the bundles are often omitted due to many factors that include managing the protocol and limited resources. In addition, the IMPreSS study, conducted in 2015, showed that compliance in the measurement of lactate in Asia was only 48.3%.11
The majority of septic patients admitted to the medical intensive care unit (MICU) are from 2 sources: hospital wards and the emergency department (ED).12 Since the settings of these sources are different, the timing of the compliance of appropriate antibiotic administration and lactate measurement is different. One study revealed different times of antibiotic administration between hospital wards and the ED but lacked data on the timing intervals; also, no timing of lactate measurement was reported.13
The objectives of this study were to determine the times of each step in antibiotic administration and the time of lactate measurement and to compare the results between the hospital wards and the ED in a tertiary hospital in southern Thailand.
Patients and methods
Study design and setting
This study was a single-center retrospective study conducted in septic shock patients who were transferred to the MICU from 2 sources: a hospital ward and the ED at Songklanagarind Hospital. This hospital is an 816-bed university-affiliated hospital that has 40 adult wards and a 10-bed MICU. Patient data collected from the ICU Sepsis database electronic medical records tracked the hospital number codes from January 2015 to December 2016. At the time of the study, sepsis management in our hospital followed the SSC 2012 bundles. The study protocol was approved by The Research Ethics Committee of Faculty of Medicine, Prince of Songkla University (REC 60-041-14-1). Patient consent to review their medical records was not required by the research ethics committee as the data collected from electronic medical records were anonymous, confidential, and not linked to the individuals.
Study population
Patients were included if they were 18 years old or older, diagnosed as suffering from septic shock, and transferred to the MICU. For patients transferred from the wards, we selected only patients who developed sepsis at the wards. If a patient had repeated episodes of septic shock, we chose only the first episode. Septic shock was defined as sepsis-induced hypotension that persisted despite adequate fluid resuscitation along with the presence of hypoperfusion abnormalities or organ dysfunction based on the 1992 consensus definition.14 The exclusion criteria were incomplete data, referred cases, and septic patients who did not receive antibiotics.
Data collection
The collected data included patient demographic data, Sequential Organ Failure Assessment score, source of MICU admission, presumed cause(s) of infection, date of admission, time of admission, weekend admission (admission to the MICU on Saturday or Sunday), MICU length of stay, hospital stay, and discharge type.
The time of diagnosis of sepsis was marked as “Time Zero”, defined as the systemic response to infection manifested by 2 or more of the following conditions resulting from infection: 1) temperature >38°C or <36°C; 2) heart rate >90 beats/min; 3) respiratory rate >20 breaths/min or PaCO2 <32 mmHg; and 4) white blood cell count >12,000/μL, <4,000/μL, or >10% immature (band) forms adopted from the 1992 consensus definition.14 Hemoculture time is the time the doctors ordered the hemoculture. Antibiotic prescription time was the time the doctors ordered the antibiotic(s). The time to receive antibiotics was the time patients were administered the antibiotic(s), which was recorded on the electronic medical records. In addition, hemoculture results with the sensitivity testing of the antibiotics were recorded for further analysis for the appropriateness of the type of antibiotics that were prescribed. An appropriate antibiotic was defined as susceptibility to at least one empiric antibiotic therapy by subsequent in vitro susceptibility of the pathogen.15
Outcome measures
Primary outcomes were the interval from time zero to antibiotic administration and lactate measurement. Secondary outcomes were the percentage of patients who had received appropriate antibiotic(s) and factors that affected 28-day mortality.
Statistical analysis
Categorical data were demonstrated as percentages. Continuous data are shown as mean ± standard deviation or median with minimum and maximum interquartile range (IQR) depending on the distribution of the data. The data were tested for normality using the Kolmogorov–Smirnov goodness-of-fit test. For primary outcomes, the chi-square test was performed for categorical data. Student’s t-test or Mann–Whitney U test was selected for continuous variable analysis. For secondary outcomes, selected variables with P<0.10 were introduced into a multiple logistic regression model after testing for association. Odds ratios (ORs) and their 95% confidence intervals (CIs) were used to identify the significant independent factors influencing 28-day mortality. Two-tailed values of P<0.05 were deemed statistically significant. All statistical analyses were computed with R software version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
One hundred and fifty septic shock patients admitted into our MICU were included in the study: 41.3% (62/150) from the ED and 58.7% (88/150) from the wards (Figure 1). Internal medicine wards were the predominant source with 76.1% (67/88). Patients admitted from the wards had a significantly higher number of malignancies as comorbidities and longer MICU length of stay. The most common type of infection was health care-associated infection, and the most common infection was pneumonia. There was no significant 28-day hospital mortality difference between critically ill septic patients admitted to the wards vs those admitted to the MICU from the ED (47.7% vs 35.5%, P=0.13). Details of the clinical characteristics stratified by admission sources are presented in Table 1. More than half of the patients had positive culture results, and most samples for analysis were blood. Two-thirds of organisms were Gram-negative bacteria (Table 2). The initial number of empirical antibiotics used ranged from 1 to 3, and carbapenems were frequently prescribed (Table 3).
Figure 1.
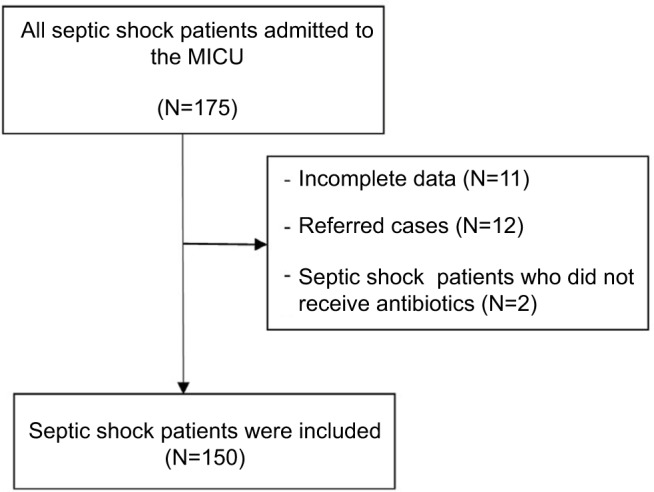
Flowchart of recruitment process.
Abbreviation: MICU, medical intensive care unit.
Table 1.
Characteristics of the patients
| Characteristics | All patients (N=150) | Admission source
|
P-value | |
|---|---|---|---|---|
| ED (n=62) | Wards (n=88) | |||
| Percentage of patients | 100 | 41.3 | 58.7 | – |
| Age at admission (years), median (IQR) | 64 (52–79) | 65 (60–81) | 61 (48–77) | 0.27 |
| Male | 86 (57.3) | 37 (59.7) | 49 (55.7) | 0.63 |
| Time at admission | ||||
| Night time | 45 (30) | 22 (35.5) | 23 (26.1) | 0.21 |
| Weekend | 46 (30.7) | 21 (33.9) | 25 (28.4) | 0.47 |
| Comorbidities | ||||
| Malignancy | 53 (35.3) | 11 (17.7) | 42 (47.7) | <0.01 |
| Solid | 20 (13.3) | 8 (12.9) | 12 (13.6) | 0.03 |
| Hematologic | 33 (22.0) | 3 (4.8) | 30 (34.1) | <0.01 |
| Heart disease | 35 (23.3) | 19 (30.6) | 16 (18.2) | 0.08 |
| HTN | 34 (22.7) | 21 (33.9) | 13 (14.8) | <0.01 |
| DM | 31 (20.7) | 18 (29) | 13 (14.8) | 0.03 |
| CKD | 22 (14.7) | 13 (20.9) | 9 (10.2) | 0.07 |
| Chronic lung disease | 20 (13.3) | 13 (20.9) | 7 (7.9) | 0.02 |
| Chronic liver disease | 13 (8.7) | 3 (4.8) | 10 (11.4) | 0.16 |
| Others | 36 (24) | 17 (27.4) | 19 (21.6) | 0.41 |
| Health care-associated infection | 103 (68.7) | 32 (51.6) | 71 (80.7) | <0.01 |
| Source of infection | ||||
| Pneumonia | 59 (39.3) | 28 (45.2) | 31 (35.2) | 0.22 |
| Intra-abdominal infection | 31 (20.7) | 13 (20.9) | 18 (20.4) | 0.94 |
| UTI | 19 (12.7) | 11 (17.7) | 8 (9.1) | 0.12 |
| Skin and soft tissue | 11 (7.3) | 2 (3.2) | 9 (10.2) | 0.10 |
| Catheter-related infection | 3 (2) | 1 (1.6) | 2 (2.3) | 0.78 |
| IE | 2 (1.3) | 1 (1.6) | 1 (1.1) | 0.80 |
| CNS infection | 1 (0.7) | 0 (0) | 1 (1.1) | 0.49 |
| Others | 2 (1.3) | 0 (0) | 2 (2.3) | 0.23 |
| Unknown | 22 (14.7) | 7 (11.3) | 15 (17.0) | 0.33 |
| Lactate measurement at time zero | 60 (40) | 27 (43.5) | 33 (37.5) | 0.45 |
| Lactate level (mmol/L), median (IQR) | 3.3 (1.6–6.6) | 3.4 (1.2–6.6) | 3.3 (1.9–6.7) | 0.49 |
| SOFA score, median (IQR) | 8 (6–10) | 8 (6–10) | 8 (6–10) | 0.20 |
| Outcomes | ||||
| ED LOS (h) | – | 5 (3–6) | – | – |
| MICU LOS (days), median (IQR) | 4 (1–7) | 2 (1–7) | 5 (2–7) | 0.01 |
| Ward LOS after events (days), median (IQR) | 8 (1–23) (N=110) | 5 (3–12) (N=31) | 8 (1–30) (N=79) | 0.07 |
| 28-day mortality | 64 (42.7) | 22 (35.5) | 42 (47.7) | 0.13 |
| In-hospital death | 74 (49.3) | 26 (41.9) | 48 (54.5) | 0.13 |
Note: Data are presented as n (%) unless otherwise indicated.
Abbreviations: ED, emergency department; IQR, interquartile range; HTN, hypertension; DM, diabetes mellitus; CKD, chronic kidney disease; UTI, urinary tract infection; IE, infective endocarditis; CNS, central nervous system; SOFA, sequential organ failure assessment; LOS, length of stay; h, hour; MICU, medical intensive care unit.
Table 2.
Microbiological results in our septic shock patients
| Culture data | Number (%) of patients or organisms |
|---|---|
| Total number of patients | 150 |
| With negative culture results | 65 (43) |
| With positive culture results | 85 (57) |
| Sample type of positive culture results (n=85 patients) | |
| Blood | 40 (48) |
| Sputum | 32 (38) |
| Urine | 19 (23) |
| Wound | 5 (6) |
| Body fluid | 3 (4) |
| Total number of organisms isolateda | 104 |
| Gram-positive organisms | 23 (22) |
| MSSA | 10 (10) |
| Enterococcus faecalis | 7 (6) |
| Streptococcus pneumoniae | 3 (3) |
| MRSA | 3 (3) |
| Gram-negative organisms | 78 (75) |
| Escherichia coli | 29 (28) |
| Klebsiella pneumoniae | 19 (18) |
| Pseudomonas aeruginosa | 13 (12) |
| Acinetobacter baumannii | 12 (11) |
| Proteus mirabilis | 2 (2) |
| Others | 3 (3) |
| Fungus | 3 (3) |
Note:
Percentages of organisms were calculated using the total number of organisms isolated as the dominator.
Abbreviations: MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
Table 3.
Initial empiric antibiotic use
| Variable | Number (%) of patients or antibiotics |
|---|---|
| Number of initial antibiotics (N=150 patients) | |
| 1 | 92 (61.3) |
| 2 | 52 (34.7) |
| 3 | 6 (4) |
| Initial drug, by class (N=213 antibiotics) | |
| Aminoglycosides | 1 (0.5) |
| Carbapenems | 90 (42.3) |
| Cephalosporins | 42 (19.7) |
| Clindamycin | 3 (1.4) |
| Penicillins | 3 (1.4) |
| Piperacillin–tazobactam | 15 (7.0) |
| Fluoroquinolones | 12 (5.6) |
| Metronidazole | 7 (3.3) |
| Vancomycin | 17 (7.9) |
| Colistin | 18 (8.4) |
| Others | 5 (2.3) |
Primary outcomes
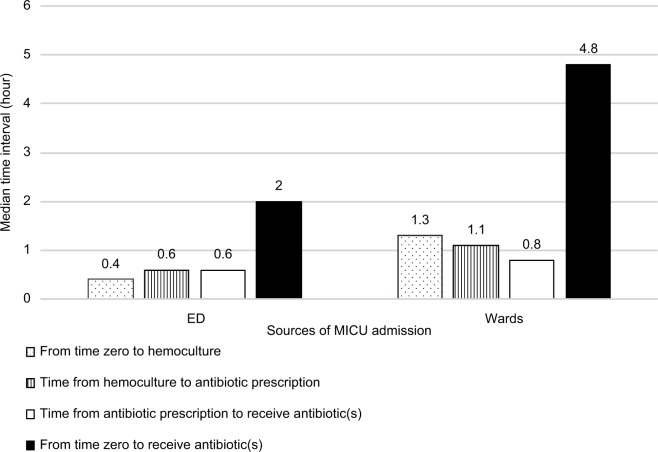
Patients admitted from wards had a significantly longer median interval (h [IQR]) from the onset of severe sepsis or septic shock or both to receive the antibiotic(s) compared with patients admitted from the ED (4.84 [3.5–8.11] vs 2.04 [1.37–3.54], P<0.01). Likewise, we found significantly longer time intervals (h [IQR]) in patients from the wards vs patients from the ED from time zero to hemoculture (1.3 [0.2–5.4] vs 0.4 [0.1–1.1], P=0.01), time from hemoculture to antibiotic prescription (1.1 [0.3–3.0] vs 0.6 [0.2–1.3], P=0.03), and time from antibiotic prescription to time patients received the antibiotics (0.8 [0.5–1.5] vs 0.6 [0.3–1.0], P=0.02) (Figure 2). Overall lactate measurement was done in only 40% of patients at time zero (Table 1). However, the lactate level measurement time intervals (h [IQR]) from time zero were not different between those admitted from the wards and those from the ED (1.6 [0.2–2.7] vs 1.6 [0.9–3.0], P=0.85).
Figure 2.
Intervals for stages in the process of antibiotic administration and lactate measurement by sources of MICU admission.
Abbreviations: ED, emergency department; MICU, medical intensive care unit.
Secondary outcomes
In a subgroup of 85 patients with positive culture results, the overall appropriate use of antibiotics was 66/85 (77.6%). The numbers of patients from the ED and the hospital wards who received the appropriate antibiotic(s) were 36/42 (90%) and 30/43 (69.8%), respectively (P=0.01). Patients who received the appropriate antibiotic regimen had a lower 28-day mortality compared with patients who did not (21/66 [31.8%] vs 12/19 [63.2%], P=0.03). We also analyzed factors that affected 28-day mortality adjusted for severity. Multivariate analysis showed that a higher risk-adjusted 28-day mortality was associated with antibiotic monotherapy (OR: 19.3, 95% CI: 2.4–153.1, P<0.01) and admission on a weekend (OR: 24.4, 95% CI: 2.9–199.8, P<0.01) (Table 4).
Table 4.
Results of the multivariate analysis of parameters and 28-day mortality.
| Variables | Variables | Reference | Adjusted OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Number of antibiotics | ≤1 (50th percentile) | >1 (50th percentile) | 19.3 | 2.4–153.2 | <0.01 |
| SOFA score | >8 (50th percentile) | ≤8 (50th percentile) | 13.2 | 2.1–83.8 | <0.01 |
| Admission on weekend | Yes | No | 24.4 | 2.9–199.8 | <0.01 |
Notes: Only significant variables are shown (N59). The risk factors and confounders considered were age (years), lactate level (mmol/L), time zero to antibiotic administration (h), time zero to lactate measurement (h), health care-associated infection, and appropriateness of antibiotic.
Abbreviations: OR, odds ratio; CI, confidence interval; SOFA, sequential organ failure assessment.
Discussion
To the authors’ knowledge, this is the first study to explore the time intervals of the stages in the process of antibiotic administration in Thailand. Our study found that a significantly longer time interval occurred from sepsis to antibiotic administration in septic shock patients from the wards compared with those from the ED. The time interval from sepsis to lactate measurement was insignificantly different between the wards and the ED.
The SSC recommends that patients should receive the appropriate antibiotic within 1 h. However, many worldwide surveys showed failure to accomplish this goal. However, this recommendation is not impossible to accomplish. For example, a study from the New York State Department of Health reported that the median time interval to the administration of antibiotics in the ED was 0.95 h.16 However, a study in northern California revealed that the median time interval for administration of antibiotics was 2.1 h (IQR: 1.4–3.1) in an ED.17 The median time interval from first medical contact to receive an antibiotic in another study in the United States was 4.2 h (IQR: 2.7–8.0).18
The interval of antibiotic administration depends on the level of the hospital. A study by Mok et al13 in a university-affiliated hospital in Canada showed a similar trend to our study. That study revealed that the intervals between ordering and administration differed significantly for patients in the wards (5.7 h) compared with those who had disease onset in the intensive care unit (4.0 h) and those who had disease onset in the ED (3.3 h).13 A retrospective study in a district-level hospital in South Africa reported that the median time delay in administration of antibiotic(s) was 4.2 h.19 Timing of antibiotic administration at the ED in our study was longer than previously reported at our institution (58–62 min).20 The explanation is that the definition of “Time Zero” in the previous study was the time severe sepsis or septic shock was diagnosed.
Gram-negative bacteria were obtained predominately in our study, and the antibiotic prescriptions favored monotherapy. The leading organism was Escherichia coli, which was similar to previous reports from our hospital.20,21 However, in resource-limited settings, the expert consensus recommendation22 and the latest SSC23 suggest that in sepsis a combination of antibiotics should be used, especially in septic shock patients. The results of our study complement this recommendation as nearly 70% of septic shock patients admitted in the MICU had health care-associated infection, and empirical antibiotic monotherapy was associated with 28-day mortality. According to the studies of Kumar et al,24,25 combination antibiotic therapy improved the mortality in septic shock patients. A previous study in Thailand reported that combination antibiotic therapy in nosocomial infection reduced the chance of inappropriate antibiotics.26 However, our results did not show a significant benefit of the appropriateness of antibiotics in mortality outcome (Table 4).
In addition to the time interval of antibiotic administration, the time interval of lactate measurement is also a predictor of mortality. The lactate level encourages physicians to recognize sepsis early and initiate prompt intervention. The SSC recommends that the physician order a lactate level measurement as the initial laboratory investigation. However, the compliance in this regard was poor. According to the IMPreSS study,11 compliance in the measurement of lactate in Asia was only 48.3%, while the compliance in our study was even lower at 40%. Moreover, in a Thai shock survey of 2013,27 533 physicians stated that lactate measurement was done in only 16% of cases.
An impeding factor of the appropriate timing of antibiotic administration and lactate measurement is believed to involve the complex illness of the patient which contributes to the difficulty of identifying a patient with sepsis. A low patient-to-nurse ratio and a lack of awareness of the clinicians to recognize sepsis are also the postulated factors.
Overall, the in-hospital mortality rate of septic shock patients was 49.3%, which was similar to the result from a previous study.12 Surprisingly, in our multivariate analysis, time from the diagnosis of sepsis to antibiotic administration was not associated with 28-day mortality. However, the results confirmed outcomes of a recent meta-analysis of 11 studies that showed no mortality benefit of antibiotic administration within 3 h of the ED triage or within 1 h from septic shock recognition.28
We also found that hospital admission of septic shock patients on the weekends was related to mortality. Few studies revealed the same results.29,30 The possible explanation of the “weekend effect” may be the limited resources, including physicians and nursing staff personnel, and higher rates of nonadherence to the protocols during the weekend.
Our study has some limitations. The study is retrospective in nature and was conducted at a single center. In addition, the protocol and infrastructure were not similar to other institutes, thereby making comparison difficult. Since this study is retrospective in nature, some incomplete patient data were excluded, which led to bias and the incapability to establish direct cause and effect. We reviewed data from charts and electronic medical records. Therefore, the diagnosis of sepsis and the exact time of “Time Zero” in some patients were missed. Due to our sample size limitation, this study could not clearly determine the effects on the 28-day mortality rate in terms of the antibiotic administration and lactate measurement time intervals.
Conclusion
In conclusion, septic shock patients from the ED who were admitted to the MICU had a shorter duration of sepsis to antibiotic administration time and received more appropriate antibiotics than those from the hospital wards. The combination of antibiotic therapy and hospital admission on a weekend were associated with mortality outcome. These findings suggest that systemic intervention needs to be considered to improve the quality of care, especially in hospital wards. Future qualitative studies should be done to examine and explain the causes of delayed antibiotic administration at each stage, lactate measurement timing, and also the “weekend effect”. There are relatively few published studies concerning the specific effects of weekend admission in sepsis and septic shock.
Acknowledgments
The authors gratefully acknowledge Dr. Polathep Vichitkunakorn from the Epidemiology Unit for the statistical analysis assistance and the International Affairs Department, Faculty of Medicine, Prince of Songkla University for the language correction services. The abstract of this paper was presented at the Sepsis 2017, Paris, France, during September 11–13, 2017 as a poster presentation with interim findings. The abstract of the poster was published in “Poster Abstracts” in Intensive Care Medicine Experimental. 2017; 5(Suppl 1):37. doi: 10.1186/s40635-017-0149-y.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vincent JL, Marshall JC, Namendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 2.SepNet Critical Care Trials Group Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. 2016;42(12):1980–1989. doi: 10.1007/s00134-016-4504-3. [DOI] [PubMed] [Google Scholar]
- 3.De Backer D, Dorman T. Surviving sepsis guidelines: a continuous move toward better care of patients with sepsis. JAMA. 2017;317(8):807–808. doi: 10.1001/jama.2017.0059. [DOI] [PubMed] [Google Scholar]
- 4.Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015;43(1):3–12. doi: 10.1097/CCM.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 7.Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 8.Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 9.Barie PS, Hydo LJ, Shou J, Larone DH, Eachempati SR. Influence of antibiotic therapy on mortality of critical surgical illness caused or complicated by infection. Surg Infect (Larchmt) 2005;6(1):41–54. doi: 10.1089/sur.2005.6.41. [DOI] [PubMed] [Google Scholar]
- 10.Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med. 2010;38(2):668–678. doi: 10.1097/CCM.0b013e3181cb0ddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes A, Phillips G, Beale R, et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study) Intensive Care Med. 2015;41(9):1620–1628. doi: 10.1007/s00134-015-3906-y. [DOI] [PubMed] [Google Scholar]
- 12.Khwannimit B, Bhurayanontachai R. The direct costs of intensive care management and risk factors for financial burden of patients with severe sepsis and septic shock. J Crit Care. 2015;30(5):929–934. doi: 10.1016/j.jcrc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Mok K, Christian MD, Nelson S, Burry L. Time to administration of antibiotics among inpatients with severe sepsis or septic shock. Can J Hosp Pharm. 2014;67(3):213–219. doi: 10.4212/cjhp.v67i3.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 15.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54(11):4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour CW, Kahn JM, Martin-Gill C, et al. Delays from first medical contact to antibiotic administration for sepsis. Crit Care Med. 2017;45(5):759–765. doi: 10.1097/CCM.0000000000002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhikoo R, Versfeld S, Basson MMV, Oosthuizen AH. A retrospective study evaluating the efficacy of identification and management of sepsis at a district-level hospital internal medicine department in the Western Cape Province, South Africa, in comparison with the guidelines stipulated in the 2012 Survivi. S Afr Med J. 2017;107(8):674–678. doi: 10.7196/SAMJ.2017.v107i8.11019. [DOI] [PubMed] [Google Scholar]
- 20.Worapratya P, Joraluck J, Wanjaroenchaisuk A, Wuthisuthimethawee P. Appropriateness of broad spectrum antibiotics for severe sepsis and septic shock in the emergency department. J Med Assoc Thai. 2016;99(5):477–483. [PubMed] [Google Scholar]
- 21.Hortiwakul T, Nagij S, Chusri S, Silpapojakul K. Nosocomial bloodstream infection in Songklanagarind Hospital: outcome and factors influencing prognosis. J Med Assoc Thai. 2012;95(2):170–174. [PubMed] [Google Scholar]
- 22.Thwaites CL, Lundeg G, Dondorp AM. Recommendations for infection management in patients with sepsis and septic shock in resource-limited settings. Intensive Care Med. 2016;42(12):2040–2042. doi: 10.1007/s00134-016-4415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Zarychanski R, Light B, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010;38(9):1773–1785. doi: 10.1097/CCM.0b013e3181eb3ccd. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Safdar N, Kethireddy S, Chateau D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med. 2010;38(8):1651–1664. doi: 10.1097/CCM.0b013e3181e96b91. [DOI] [PubMed] [Google Scholar]
- 26.Santimaleeworagun W, Wongpoowarak P, Chayakul P, Pattharachayakul S, Tansakul P, Garey KW. Clinical outcomes of patients infected with carbapenem-resistant Acinetobacter baumannii treated with single or combination antibiotic therapy. J Med Assoc Thai. 2011;94(7):863–870. [PubMed] [Google Scholar]
- 27.Chittawatanarat K, Patjanasoontorn B, Rungruanghiranya S. Thai-shock survey 2013: survey of shock management in Thailand. J Med Assoc Thai. 2014;97(Suppl 1):S108–S118. [PubMed] [Google Scholar]
- 28.Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: A systematic review and meta-analysis. Crit Care Med. 2015;43(9):1907–1915. doi: 10.1097/CCM.0000000000001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih YN, Chen YT, Shih CJ, et al. Association of weekend effect with early mortality in severe sepsis patients over time. J Infect. 2017;74(4):345–351. doi: 10.1016/j.jinf.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641–648. doi: 10.1016/j.jemermed.2013.04.042. [DOI] [PubMed] [Google Scholar]