Abstract
OBJECTIVES
Bacterial infections are the major cause of acute exacerbation of COPD (AE-COPD). The relationship between lung functions and respiratory failure (arterial blood gas parameters) with the etiology of AE-COPD has not been clearly understood. We conducted this study to determine the bacterial profile in AE-COPD and to identify the associated risk factors and drug sensitivity pattern.
MATERIAL AND METHODS
Seventy-two patients hospitalized for AE-COPD were prospectively evaluated. Quantitative sputum culture, blood gas analysis, and drug sensitivity testing were performed at the time of admission, and pulmonary function testing was performed 6 weeks after discharge as per standard guidelines.
RESULTS
Bacterial pathogens were isolated in 34 (47.22%) cases. Pathogens isolated were Pseudomonas aeruginosa (38.23%), Klebsiella pneumoniae (29.41%), Staphylococcus aureus (23.53%), Streptococcus pneumoniae (5.88%), and Acinetobacter spp. (2.94%). Isolation of bacterial pathogen was observed in patients with advancing age (p=0.02), frequent exacerbations (p<0.001), systemic steroid use (p=0.005), and deranged lung function (p=0.02). Binary logistic regression analysis revealed that higher partial pressure of carbon dioxide (PaCO2) was independently associated with isolation of K. pneumoniae (p=0.025) and P. aeruginosa (p=0.001). Additional independent factors that favor isolation of K. pneumoniae were age >55 years (p=0.017) and systemic steroid use (p=0.017). Antibiotic sensitivity testing showed that ciprofloxacin and piperacillin/tazobactum were effective in 27/34 (79.41%) of isolates followed by gentamycin in 26/34 (76%).
CONCLUSION
Hypercapnic respiratory failure is an independent risk factor for isolation of K. pneumoniae and P. aeruginosa in addition to advanced age and systemic steroid use. These findings may be an important adjunct in deciding the initial antibiotic therapy.
Keywords: Chronic obstructive pulmonary disease, acute exacerbation, bacteria, respiratory failure, lung function
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is a chronic inflammatory condition of the airways, which is associated with significant morbidity and mortality. Almost 90% deaths occur in low to middle income countries. According to World Health Organization (WHO), COPD will be the third-leading cause of death worldwide by 2030 [1]. The overall prevalence of COPD in India is 3.49% in adults with age >35 years [2].
Chronic Obstructive Pulmonary Disease is characterized by gradually progressive impairment in lung function, which is interrupted with exacerbations of varying severity. Exacerbation can be defined as “a sustained worsening of the patient’s condition, from the stable state and beyond normal day–to-day variations that is acute in onset and necessitates a change in regular medication in a patient with underlying COPD” [3]. Exacerbations are associated with significant morbidity, mortality, decreased quality of life, and increased health care resource utilization [4,5].
Acute exacerbations of COPD (AE-COPD) are caused by several factors, including respiratory tract infections, air pollution, change in temperature, allergen exposure, and interruption of medications [6]. Respiratory tract infections are the most common cause of AE-COPD implicated in 50%–80% cases [7,8]. The bacteria most frequently involved in infectious exacerbation of COPD are Streptococcus pneumoniae, Nontypable Haemophilus influenza, and Moraxella catarrhalis [8–11]. However, majority of the data are derived from outpatients and different methodologies have been used to obtain the respiratory secretions for bacterial culture across studies (sputum, protracted specimen brush). In addition, there is geographical variation in bacteriological profile in AE-COPD. Some studies have correlated lung function impairment and bacterial pathogen responsible for the exacerbation [12,13]. Enterobacteriaceae and Pseudomonas are the predominantly isolated pathogens in those with significant impairment of pulmonary function (forced expiratory volume in 1 second [FEV1] <50% of predicted]. Further, hospitalized patients usually have more severely compromised lung function, and the spectrum of the causative organisms could be different.
One of the major hurdle in defining the role of bacterial pathogen as causative agent of AE-COPD is the colonization of the airways by bacteria even in absence of an exacerbation. Despite the ongoing debate regarding this issue, there is ample evidence that implicates bacterial infections in AE-COPD indicated by high bacterial load [8].
In this context, it would be helpful to determine patient characteristics that are associated with specific bacterial infection to guide empirical antibiotic therapy. Thus, we aimed to prospectively investigate the etiological agent (bacteria) implicated in AE-COPD of hospitalized patients, their relation to lung function, factors associated with isolation of particular pathogen and antibiotic sensitivity pattern.
MATERIAL AND METHODS
Study Participants
All hospitalized patients diagnosed with AE-COPD admitted to Pulmonary Medicine department of a tertiary care hospital in western India from January 2012 to December 2012, were prospectively evaluated. COPD was diagnosed according to the Global Initiative for Obstructive Lung Disease (GOLD) guidelines. AE-COPD was assumed when a patient presented with at least two of the three following symptoms, proposed by Anthonisen et al. [14]: (a) worsening dyspnea, (b) increased sputum volume, and (c) increased sputum purulence. Written informed consent was obtained from all the study participants. This study was approved by the institutional ethics committee.
The need for hospitalization was assessed according to the patient’s clinical condition or the presence of other complicating factors, such as advanced age and lack of social support. Patients were excluded for the study if (1) they had an outpatient status; (2) received antibiotic within last 48 hours of hospital admission; (3) infiltrates were seen on chest radiograph; (4) other known chronic respiratory disorder; (5) active malignancy; (6) immunosuppression; and (7) long-term steroid use (>5 mg prednisolone or equivalent per day for more than 3 months). Patients were included only once in study even if they hospitalized frequently during study period.
Information about demographic characteristics, body mass index (BMI), dyspnea measured by the modified Medical Research Council (mMRC) was recorded. Data on smoking history in terms of pack years, number of acute exacerbations of COPD within the last year requiring hospitalization, use of systemic or inhaled corticosteroids and long-term oxygen therapy prior to admission were also collected. Arterial blood gases sample was obtained by puncture of the radial artery while patient was breathing on room air.
Bacteriological Data
At the time of hospital admission, spontaneously expectorated sputum samples were collected into a sterile container before institution of antibiotics. Gram’s stain of the sputum samples were performed and only the samples consisting <10 epithelial cells and >25 leukocytes per low power field were processed for culture [15]. The sputum sample was homogenized with sputolysin and culture inoculum was prepared using 1 μL standard loop onto blood agar, chocolate agar, and MacConkey agar which were incubated in 5% CO2 at 35°C for 18–24 hours. The culture plates were incubated further for additional 24 hours if no growth was observed after overnight incubation.
Bacterial agents were classified as potentially pathogenic microorganisms (PPMs) or non-PPMs. PPMs were only considered as significant if they achieved >106/colony forming units (CFU), except in case of Streptococcus pneumoniae for which 105/CFU was deemed sufficient [16]. A PPM had to grow in significant concentration to be considered as a causative agent of an exacerbation. The sensitivity pattern of the PPMs were carried out by minimum inhibitory concentration method and classified as sensitive, intermediate, or resistant according to Clinical Laboratory Standard Institute criteria [17].
Lung Function
Spirometry was performed at follow-up after 6 weeks of discharge from hospital as per the GOLD guidelines [18]. Post bronchodilator reversibility of FEV1 was assessed by repeating spirometry 20 min after inhalation of salbutamol (400 μg) given through a volume spacer. Based on the GOLD staging of severity, lung functions were categorized into four stages: stage 1, FEV1 ≥80% predicted; stage 2, FEV1 50%–80% predicted; stage 3, FEV1 30%–50% predicted; and stage 4: FEV1 <30% predicted.
Patients were treated with a standard management protocol consisting of systemic corticosteroids, nebulized short-acting bronchodilators (salbutamol, ipratropium bromide), and O2 was titrated according to continuous monitoring of peripheral oxygen saturation. Antibiotics were prescribed after the availability of the sputum culture results unless patient’s clinical condition necessitated an early intervention. Patients were advised to attend outpatient clinic regularly for follow-up and adhere to the treatment. All the current smokers were advised to quit smoking. Patients with GOLD stage 2 or more were provided influenza and pneumococcal vaccination as per GOLD guidelines recommendations.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for Social Sciences software for windows version 21.0 (IBM Corp.; Armonk, NY, USA). The quantitative variables were expressed as mean and standard deviation and qualitative variables were expressed as counts and percentage. For independent samples, comparison between means was performed using student’s t-test. Mann–Whitney U test was used for the variables that did not meet the normal distribution criteria. The chi-square test was used to compare categorical variables. One-way Analysis of Variance (ANOVA) was used for between-group analyses.
A logistic regression model was constructed for individual PPMs isolated in sputum. The independent variables were following: age (0=<55 years; 1=>55 years), BMI (0=<18.5; 1=>18.5), smoking (0=ex and non-smoker; 1=current smoker), FEV1 (0=<50%; 1=>50%), exacerbation history in previous year (0=≤1 exacerbation; 1=≥2 exacerbation), systemic steroid use (0=no; 1=yes). Variables with p value <0.05 were selected for the model and analyzed. A p value of <0.05 was considered to be significant.
RESULTS
During the study period, 90 patients were recruited based on the inclusion and exclusion criteria. Among them, four patients died (two as inpatient and two after discharge) and three patients defaulted during follow-up. Out of remaining 83 patients, 6 patient’s sputum sample did not meet the adequacy criteria and five patients were not fulfilling the criteria for acceptability of spirometry (2 not able to perform, three had FEV1/forced vital capacity [FVC] >0.7) and hence were excluded. Thus, we included a total 72 cases of AE-COPD in the final analysis.
The demographic details and baseline characteristics of study participants are summarized in Table 1. The majority of the patients were males (91.66%) with mean ± standard deviation (SD) age of 59±9.6 years and were current smokers (43.05%). Majority of the patients had moderate to severe stage of COPD with mean post bronchodilator FEV1 of 1.23±0.48 liters and 54±18% of predicted.
Table 1.
Baseline characteristics of the patients hospitalized with AE-COPD
| Parameters | Total (n=72) |
|---|---|
| Age, mean (SD), years | 59 (9.6) |
| Male, n (%) | 66 (91.66) |
| Smoking status, n (%) | |
| Non-smoker | 8 (11.11) |
| Ex-smoker | 33 (45.83) |
| Current smoker | 31 (43.05) |
| Pack years of smoking, mean (SD) | 33.06 (21.73) |
| BMI, mean (SD) | 19.73 (2.45) |
| Number of acute exacerbation 2 (1) in previous year, mean (SD) | |
| Home oxygen use, n (%) | 8 (11.11) |
| Inhaled corticosteroids use, n (%) | 52 (72.22) |
| Systemic steroid use, n (%) | 10 (13.89) |
| Post bronchodilator lung function, mean (SD) | |
| FEV1, L | 1.23 (0.48) |
| FEV1, % predicted | 54 (18) |
| FVC, L | 2.51 (0.56) |
| FVC, % predicted | 84 (14) |
| FEV1/FVC ratio | 48.21 (12.79) |
| Arterial blood gas analysis | |
| pH | 7.41 (0.06) |
| pO2, mmHg | 73.7 (11.9) |
| pCO2, mmHg | 40.9 (6.45) |
| Exacerbation type*, n (%) | |
| Type 1 | 28 (38.39%) |
| Type 2 | 32 (44.44%) |
| Type 3 | 12 (16.67%) |
| GOLD stage (severity), n (%) | |
| Mild | 5 (6.94%) |
| Moderate | 37 (51.39%) |
| Severe | 23 (31.94%) |
| Very severe | 7 (9.72%) |
Note: values are given either in number (%) or mean (SD) AE-COPD: acute exacerbation chronic obstructive pulmonary disease; BMI: Body Mass Index; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global initiative of Obstructive Lung Diseases; SD: standard deviation
Anthonisen’s criteria
In our study, quantitative analysis of sputum culture revealed growth of PPMs in 34 (47.22%) hospitalized patients with AE-COPD (Figure 1). Of these 34 isolates, Pseudomonas aeruginosa (n=13, 38.23%) was the most predominant organism followed by Klebsiella pneumoniae (n=10, 29.41%), Staphylococcus aureus (n=8, 23.53%), Streptococcus pneumoniae (n=2, 5.88%), and Acinetobacter spp. (n=1, 2.94%). Therefore, GNBs clearly outnumbered other organisms.
Figure 1.
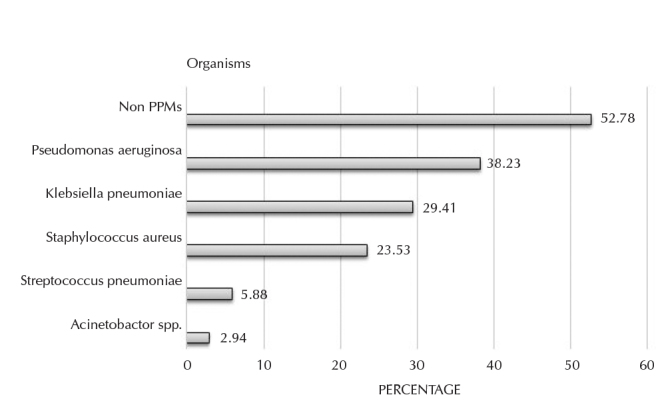
Pathogenic microorganisms isolated from sputum culture
PPMs: potentially pathogenic microorganisms
We compared the characteristics of the patients who revealed growth of PPMs versus non-PPMs (Table 2). There were statistically significant differences with regard to age (p=0.02), number of exacerbations in previous year (p<0.001), lung function (FEV1, p=0.02; FEV1/FVC, p=0.016) and systemic corticosteroids use (p=0.005). No significant difference was noted in BMI, smoking pack years, inhaled steroids use, home oxygen use, and arterial blood gas measures.
Table 2.
Comparison of characteristics of patients who revealed growth of PPMs versus non-PPMs
| Characteristics | PPMs (n=34) | Non-PPMs (n=38) | p |
|---|---|---|---|
| Age, years, mean±SD | 61.53±9.20 | 56.55±9.48 | 0.027 |
| BMI, mean±SD | 19.57±2.61 | 19.87±2.30 | 0.608 |
| Smokers, n (%) | 33 (86.84) | 31 (91.17) | 0.714 |
| Smoking pack years, mean±SD | 33.37±23.19 | 32.79±20.64 | 0.911 |
| No. of exacerbations in previous year, mean±SD | 2.26±1.23 | 1.34±0.71 | 0.001 |
| Lung function, mean±SD | |||
| FEV1 | 1.09±0.52 | 1.35±0.40 | 0.021 |
| FEV1, % predicted | 49.03±20.37 | 58.55±13.97 | 0.022 |
| FVC | 2.37±0.56 | 2.61±0.52 | 0.06 |
| FVC, % predicted | 80.35±15.76 | 86.82±12.05 | 0.053 |
| FEV1/FVC | 44.39±14.15 | 51.62±10.48 | 0.016 |
| Arterial blood gas analysis, mean±SD | |||
| pO2 | 67.81±11.71 | 71.22±6.32 | 0.124 |
| pCO2 | 43.09±7.27 | 40.64±3.75 | 0.072 |
| pH | 7.40±0.07 | 7.41±0.05 | 0.751 |
| Inhaled steroids use, n (%) | 25 (73.5) | 25 (65.8) | 0.610 |
| Systemic steroid use, n (%) | 9 (26.5) | 1 (2.6) | 0.005 |
| Home oxygen use, n (%) | 5 (14.7) | 3 (7.89) | 0.35 |
PPM: potentially pathogenic microorganisms; BMI: Body Mass Index; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; SD: standard deviation
Isolation of P. aeruginosa was significantly more common in patients with frequent exacerbations requiring hospitalization (p=0.005) and systemic steroid use (p=0.041) as compared to other pathogens (Appendix 1).
Appendix 1.
Comparison of characteristics of Pseudomonas with the remaining of PPMs
| Characteristics | Pseudomonas (n=13) | Non-Pseudomonas (n=21) | p |
|---|---|---|---|
| Age, years, mean±SD | 59.23±7.46 | 62.95±10.05 | 0.258 |
| BMI, mean±SD | 19.35±2.82 | 19.71±2.54 | 0.700 |
| Current smokers, n (%) | 7 (53.85) | 8 (38.10) | 0.369 |
| Smoking pack years, mean±SD | 38.23±26.55 | 32.33±24.24 | 0.511 |
| No. of exacerbations in previous year, mean±SD | 3.0±1.08 | 1.81±1.12 | 0.005 |
| Lung function, mean±SD | |||
| FEV1 | 0.93±0.48 | 1.20±0.54 | 0.154 |
| FEV1, % pred. | 41.23±18.97 | 53.86±20.11 | 0.079 |
| FVC | 2.25±0.51 | 2.46±0.59 | 0.298 |
| FVC, % pred. | 76.77±17.45 | 82.57±14.61 | 0.304 |
| FEV1/FVC | 39.86±14.39 | 47.21±13.59 | 0.144 |
| Arterial blood gas analysis, mean±SD | |||
| pO2 | 63.46±8.43 | 70.51±12.80 | 0.088 |
| pCO2 | 44.27±7.97 | 42.37±6.91 | 0.468 |
| pH | 7.40±0.08 | 7.41±0.07 | 0.842 |
| Inhaled steroids use, n (%) | 12 (92.3) | 13 (61.9) | 0.056 |
| Systemic steroid use, n (%) | 6 (46.15) | 3 (14.29) | 0.041 |
| Home oxygen use, n (%) | 3 (23.08) | 2 (9.52) | 0.278 |
FEV1: forced expiratory volume in one second; FVC: forced vital capacity: BMI: Body Mass Index; SD: standard deviation; PPM: potentially pathogenic microorganisms
Isolation of the individual pathogen was assessed according to deterioration in lung function (Table 3, Figure 2). There was severe impairment in lung functions (FEV1) with isolation of P. aeruginosa and K. pneumoniae compared to non-PPMs that was statistically significant (p=0.030 and p=0.038, respectively).
Table 3.
Distribution of PPMs compared to non-PPMs according to lung function
| Lung function | S. aureus (n=8) | S. pneumoniae (n=2) | K. pneumoniae (n=10) | P. aeruginosa (n=13) | Non-PPMs (n=38) | p |
|---|---|---|---|---|---|---|
| FEV1, L | 1.4 (0.88–1.93) | 1.6 (−0.57–3.75) | 0.9 (0.65–1.21) | 0.93 (0.64–1.22) | 1.4 (1.22–1.49) | 0.038, *0.030, † |
| FEV1, % | 57.6 (40.14–75.11) | 72 (−16.95–160.94) | 43.7 (33.34–54.06) | 41.2 (29.76–52.69) | 58.6 (53.96–63.14) | 0.010, † |
| FVC, L | 2.6 (2.13–3.14) | 2.5 (−3.70–8.75) | 2.3 (1.84–2.71) | 2.3 (1.94–2.56) | 2.6(2.45–2.80) | NS |
| FVC, % | 83.6 (71.65–95.60) | 87.5 (68.44–106.56) | 78.8 (67.48–90.12) | 76.8 (66.22–87.13) | 86.8 (82.86–90.78) | NS |
| FEV1/FVC | 51.5 (40.07–62.87) | 64.7 (−8.72–137.14) | 38.9 (32.69–45.03) | 39.9 (31.15–48.55) | 51.6 (48.18–55.07) | 0.020,* 0.017,† |
PPM: potentially pathogenic microorganisms; BMI: Body Mass Index; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; NS: not significant; SD: standard deviation
Values are given in mean (95% confidence interval). Inter-group comparison was conducted using one-way ANOVA
Non-PPM vs K. pneumoniae,
‡Non-PPM vs P. aeruginosa
Figure 2.
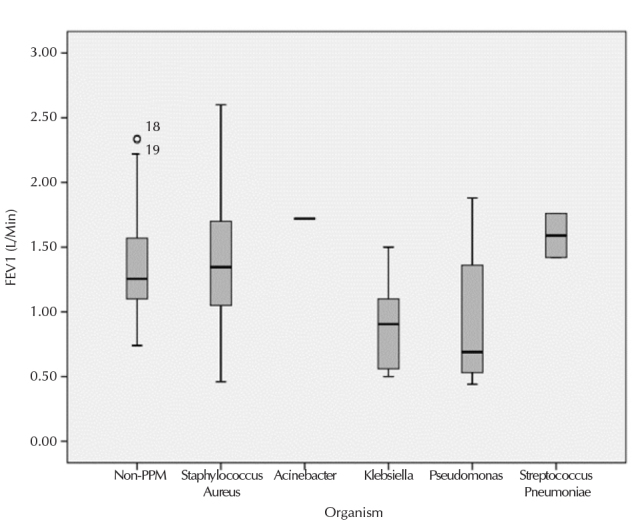
FEV1 represented as mean value, SD and range for PPMs separately and non-PPMs as whole group. Horizontal bars represent mean values, boxes represent mean±SD, and vertical bars represent 95% CI
PPMs: potentially pathogenic microorganisms; FEV1: forced expiratory volume in 1 second; SD: standard deviation; CI: confidence interval
*Acinetobacter was recovered in one sputum sample, so represent as single bar
We stratified the patients showing growth of PPMs according to GOLD stages of COPD for estimation of between-group differences among various variables using ANOVA (Table 4). The PPMs were isolated more frequently in stage 3 of COPD as compared to stage 1 (p=0.049). Current smoking and systemic steroid use was more prevalent in stage 4 COPD as compared to stage 2 and stage 3 patients, which was statistically significant. No significant difference was observed in age, BMI, dyspnea score, current smoking, exacerbations in previous year, inhaled steroid use, and arterial blood gas values across different stages of COPD.
Table 4.
Characteristics of the patients according to severity of COPD (GOLD stage)
| Parameters | GOLD stages of COPD | p | |||
|---|---|---|---|---|---|
|
| |||||
| Stage 1, n=2 | Stage 2, n=14 | Stage 3, n=11 | Stage 4, n=7 | ||
| Organisms, n (%) 0.069* | |||||
| S. aureus | 1 (50) | 4 (28.6) | 2 (18.2) | 1 (14.3) | 0.049† |
| S. pneumoniae | 0 | 2 (14.36) | 0 | 0 | 0.107‡ |
| Acinetobacter spp. | 1 (50) | 0 | 0 | 0 | 0.484|| |
| P. aeruginosa | 0 | 4 (28.6) | 5 (45.5) | 4 (57.12) | 0.494¶ |
| K. pneumoniae | 0 | 4 (28.6) | 4 (36.4) | 2 (28.6) | 0.890§ |
| Age, mean±SD | 60±12.73 | 61±9.96 | 65.3±8.78 | 57.1±6.82 | NS |
| BMI, mean±SD | 18.7±0.16 | 19.9±3.02 | 20±2.24 | 18.5±2.72 | NS |
| mMRC dyspnea score, mean±SD | 2.5±0.71 | 3.3±0.61 | 3.5±0.52 | 2.6±0.53 | NS |
| Exacerbations-per year, mean±SD | 2±0.00 | 1.9±1.44 | 2.5±1.21 | 2.6±0.98 | NS |
| Current smokers, n (%) | 2 (100) | 5 (35.7) | 2 (18.2) | 6 (85.7) | 0.021† 0.031¶ 0.005§ |
| PaO2, mmHg, mean±SD | 79.8±14.71 | 71.9±11.01 | 65.1±6.45 | 63.5±14.89 | NS |
| PaCO2, mmHg, mean±SD | 41.8±5.02 | 40.1±6.17 | 45.4±7.86 | 45.8±7.91 | NS |
| pH, mean ± SD | 7.4±0.01 | 7.4±0.04 | 7.4±0.1 | 7.4±0.07 | NS |
| Systemic steroid use, n (%) | 0 | 2 (14.3) | 2 (18.2) | 5 (71.7) | 0.009¶ 0.024§ |
| Inhaled steroid use, n (%) | 1 (50) | 8 (57.1) | 10 (90.9) | 6 (85.7) | NS |
BMI: Body Mass Index; mMRC: modified medical research council; NS: not significant; COPD: chronic obstructive pulmonary disease; GOLD: Global initiative for Lung Disease; SD: standard deviation
Data are given either in mean±SD or number (%)
GOLD stage 1 vs stage 2
GOLD stage 1 vs stage 3
GOLD stage 1 vs stage 4
GOLD stage 2 vs stage 3
GOLD stage 2 vs stage 4
GOLD stage 3 vs stage 4
Binary logistic regression analysis was used to determine factors independently associated with the isolation of PPMs (Table 5). We found that the independent predictor for isolation of K. pneumoniae and P. aeruginosa was high partial pressure carbon dioxide (PaCO2; odds ratio [OR]: 33.5; 95% confidence interval [CI]: 1.54–728.72; p=0.025) and (OR: 43.2; 95% CI: 4.48–416.12; p=0.001), respectively. Additional independent factors associated with isolation of K. pneumoniae were age >55 years (p=0.017) and systemic steroid use (p=0.011). Isolation of the S. aureus was negatively associated with rising PaCO2 (OR: 0.045).
Table 5.
Factors independently associated with isolation of PPMs: Binary logistic regression analysis
| PPMs | Independent variables | OR | 95% CI | p |
|---|---|---|---|---|
| S. aureus | PaCO2> 45 mmHg vs <45 mmHg | 0.045 | 0.004–0.521 | 0.013 |
| P. aeruginosa | PaCO2> 45 mmHg vs <45 mmHg | 43.2 | 4.48–416.12 | 0.001 |
| K. pneumoniae | PaCO2> 45 mmHg vs <45 mmHg | 33.5 | 1.54–728.72 | 0.025 |
| Age > 55 years vs < 55 years | 1.2 | 1.03–1.38 | 0.017 | |
| Systemic steroid yes vs no | 55.3 | 2.50–1224.38 | 0.011 |
*CI: confidence interval; OR: odds ratio; PPM: potentially pathogenic microorganisms
The analysis of the sensitivity pattern of different antibiotics to the PPMs revealed that majority of the isolates were showing resistance to aminopenicillins, macrolides, and tetracycline, the commonly used antibiotics in these patients (Table 6). Ciprofloxacin and piperacillin/tazobactum were effective in 79.41% of isolates followed by gentamycin in 76%. They appear to be most appropriate drugs for majority of the microorganisms isolated from hospitalized AE-COPD patients.
Table 6.
Antibiotic sensitivity pattern of PPMs detected on sputum culture
| PPMs | Amoxicillin | Amoxycillin/clavulanate | Tetracycline | Piperacillin/tazobactum | Erythromycin | Ciprofloxacin | Gentamycin |
|---|---|---|---|---|---|---|---|
|
| |||||||
| S I R | S I R | S I R | S I R | S I R | S I R | S I R | |
| S. aureus | 4 2 2 | 6 - 2 | 5 2 1 | 8 - - | 6 1 1 | 7 - 1 | 7 - 1 |
| S. pneumoniae | 1 1 - | 2 - - | 2 - - | 2 - - | 2 - - | 2 - - | 2 - - |
| P. aeruginosa | - - 13 | 1 - 12 | - - 13 | 10 2 1 | - - 13 | 10 2 1 | 9 1 3 |
| K. pneumoniae | - 2 8 | 1 2 7 | - 1 9 | 7 2 1 | - - 10 | 8 1 1 | 8 1 1 |
| Acinetobacter | - - 1 | - - 1 | - - 1 | - 1 - | - - 1 | - - 1 | - - 1 |
| Total | 5 5 24 | 10 2 22 | 7 3 24 | 27 5 2 | 8 1 25 | 27 3 4 | 26 2 6 |
PPM: potentially pathogenic microorganisms; S: sensitive; I: intermediate; R: resistant
DISCUSSION
In the present study, bacterial pathogens (PPMs) were identified in 47% of all hospitalized patients, based on sputum culture. P. aeruginosa and K. pneumoniae were the most frequently isolated organisms. Boixeda et al. [19] reported Pseudomonas in 38.23% of cases and Eller et al. [13] reported Pseudomona sand Enterobacteriaceae spp. in 48.2% patients of their study. Thus, this is in line with the previous studies. However, we did not detect H. influenza and M. catarrhalis as pathogenic organisms in our study, although they have been reported to be a common pathogen in the literature based on the western population. This could be explained by the fact that our patients had severe exacerbation of COPD necessitating hospitalization, and we had excluded outpatients, wherein these pathogens are reported to be more prevalent. This difference could also be explained by different environment condition and inadvertent use of antibiotics in this region.
Lung function impairment has been consistently found to be a strong predictor for bacterial infection (particularly GNBs) in AE- COPD. In our study, we observed that deterioration of the lung function was significantly associated with isolation of the pathogenic organisms [FEV1% predicted (mean) 49.03 vs 58.55; p=0.022] in which P. aeruginosa and K. pneumoniae were present in 67.64% of isolates. Studies on relationship between bacterial flora and pulmonary function have consistently revealed higher prevalence of GNBs among those with significant functional impairment (FEV1, < 50% of predicted). In a retrospective study of hospitalized patients of AE-COPD, a bacterial pathogen was reported in 53% of patients [13]. Among them, Pseudomonas and other GNBs were isolated in nearly one-half of the cases. In the patients with FEV1 <50% of predicted, majority of the episodes (83.33%) were caused by these organisms. Similar observation was also made by Miravitlles et al. [12] who reported high incidence of Pseudomonas among the patients with FEV1<50% of predicted. Thus, the present study also supports the finding of previous studies that reported higher incidence of GNBs in patients with severely compromised lung function.
In the present study, another risk factor for isolation of PPMs was number of exacerbations in the previous year requiring hospitalization (p<0.001). Frequent exacerbations (>2 episodes per year) are due to the different phenotype of COPD that have susceptibility to recurrent exacerbations irrespective of severity of COPD [20]. They have been associated with severe symptoms, greater reduction in exercise capacity and a greater decline in health status as compared to patients with fewer exacerbations [20]. Recurrent exacerbations are assumed to be associated with faster decline in lung function. This decline in lung function favors colonization and/or infection with bacterial pathogen by breach in host defense, such as epithelial cell damage, mucous hypersecretion, decreased ciliary beat frequency, and inflammatory cell infiltrates which makes them susceptible for exacerbation [21]. This vicious cycle further leads to more frequent exacerbation and rapid decline in lung function.
Isolation of Pseudomonas in patients with AE-COPD has been reported to be associated with certain risk factors, including FEV1 <35%, systemic steroid use, and prior antibiotic therapy within the preceding months [22–24]. Another study stated further risk factors for Pseudomonas isolation, such as functional dependence, dyspnea score, walking distance, oral corticosteroid treatment, and the Body mass index, airflow Obstruction, Dyspnea, and Exercise (BODE) index [25]. In our study, isolation of Pseudomonas was significantly higher in patients with frequent exacerbations in the previous year requiring hospitalization and systemic steroid use. Although lung function was impaired in this patient population, the statistical significance was not observed (p=0.079).
The major risk factor independently associated with isolation of P. aeruginosa and K. pneumoniae (binary regression analysis) was hypercapnic respiratory failure (PaCO2> 45 mmHg). A similar observation was made by Soler et al. [26] that presence of GNBs and Pseudomonas must be considered in all patients presenting with acute exacerbations and respiratory failure requiring mechanical ventilation. However, they did not include arterial blood gas values in their analysis.
The higher proportion (29.41%) of isolation of K. pneumoniae in the present study also deserves special mention. Lin et al. [27] reported high prevalence of infection with K. pneumoniae (19.6%) and P. aeruginosa (16.8%) among hospitalized patients of AE-COPD. However, the isolation of Klebsiella was predominated in mild COPD. Ye et al. [28] observed that K. pneumoniae was responsible for acute exacerbation of COPD in 12.3% of patients. The present study demonstrated rather higher prevalence of isolation of K. pneumoniae (29.41% of cases). The possible reason for the frequent isolation of K. pneumoniae could be advanced underlying disease and frequent use of systemic steroids among this patient population. Multiple logistic regression analysis revealed that isolation of K. pneumoniae was independently associated with advanced age and systemic steroids use.
We did not perform high resolution computed tomography scan among our cases so the possibility of subtle bronchiectasis could not be ruled out, which is a common risk factor for GNBs including Klebsiella.
Another major risk factor that may contribute to higher number of GNBs in our study is systemic corticosteroid use. In our study, 10 out of 72 patients (13.89%) were on chronic steroid use, of which 90% revealed a pathogen in sputum culture. Eller et al. [13] observed that GNB isolation was more frequent among patients taking oral steroids (54.2%) as compared to those not taking them (33.3%). Long-term treatment with corticosteroids weakens adaptive immune response by down-modulating MHC class II and costimulatory molecules [29]. Systemic steroid use was also reported to be associated with poor clearance of causative microbes of acute exacerbation following antibiotic treatment [30].
Our study showed that majority of PPMs remain susceptible to ciprofloxacin and piperacillin/tazobactum. This is in line with the recent guidelines that suggest that these drugs to be considered as empirical treatment in patients with deranged lung functions [31]. The present study was not designed to evaluate whether their use can improve the prognosis in these patients. Further studies are needed to clarify the role of particular antibiotic among these cases.
There are several limitations in our study that need to be addressed. First, we have used sputum sample for culture that can be a source of contamination with nasopharyngeal organisms. To overcome this issue, we have used sputum validity criteria proposed by various authors [15,32]. Furthermore, quantitative analysis of sputum culture showed good concordance with the bronchoscopic PSB results [26]. This finding supports the use of sputum with quantitative analysis as an easy method to procure a non-invasive form of sample and was used in various studies [12,13,27].
Second, our study did not include serodiagnosis and viral culture and/or polymerase chain reaction, which could have helped in finding the etiological agents in few more patients. Some studies have shown that significant proportion of acute exacerbations in COPD may be due to atypical pathogens like Chlamydia pneumoniae, Mycoplasma pneumoniae, and respiratory viruses [26,33,34]. However, these studies have been performed either in outpatients or in mechanically ventilated patients who are not representative of hospitalized AE-COPD population included in the present study.
In conclusion, Pseudomonas and Klebsiella were the most common bacterial pathogen recovered from hospitalized AE-COPD patients. These pathogens were seen particularly in those patients with more severely compromised lung function. Hypercapnic respiratory failure is an independent risk factor for isolation of these organisms in addition to advanced age and systemic steroid use. Most isolates were resistant to the first-line antibiotics used in AE-COPD. These factors may be an important adjunct in deciding the initial antibiotic therapy. Further studies are needed for better understanding of etiology of AE-COPD and their association with respiratory failure as there is limited evidence regarding its.
Acknowledgements
The authors would like to thank Dr. Arvind Khare, Professor, Department of Microbiology, Dr S N Medical College, Jodhpur, Rajasthan for their excellent diagnostic services.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the institutional ethics committee (Dr S N Medical College, Jodhpur, Rajasthan, India).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - A.K., V.J., N.D.; Design - A.K., V.J., N.D.; Supervision - A.K., V.J., K.C.A., G.P.; Data Collection and/or Processing - A.K., V.J., S.S.; Analysis and/or Interpretation - A.K., V.J., N.D. S.S., K.C.A., G.P.; Literature Search - A.K., V.J., N.D, S.S.; Writing - A.K., V.J., N.D.; Critical Reviews - A.K., V.J., N.D. S.S., K.C.A., G.P.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.WHO. Burden of COPD [Internet] WHO; [cited 2016 Feb 7]. Available from: http://www.who.int/respiratory/copd/burden/en/ [Google Scholar]
- 2.Jindal SK, Aggarwal AN, Gupta D, et al. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH) Int J Tuberc Lung Dis. 2012;16:1270–7. doi: 10.5588/ijtld.12.0005. https://doi.org/10.5588/ijtld.12.0005. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117:398–401. doi: 10.1378/chest.117.5_suppl_2.398s. https://doi.org/10.1378/chest.117.5_suppl_2.398S. [DOI] [PubMed] [Google Scholar]
- 4.Spencer S, Calverley PM, Burge PS, et al. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23:698–702. doi: 10.1183/09031936.04.00121404. https://doi.org/10.1183/09031936.04.00121404. [DOI] [PubMed] [Google Scholar]
- 5.Kessler R, Ståhl E, Vogelmeier C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest. 2006;130:133–42. doi: 10.1378/chest.130.1.133. https://doi.org/10.1378/chest.130.1.133. [DOI] [PubMed] [Google Scholar]
- 6.White AJ, Gompertz S, Stockley RA. Chronic obstructive pulmonary disease. 6: The aetiology of exacerbations of chronic obstructive pulmonary disease. Thorax. 2003;58:73–80. doi: 10.1136/thorax.58.1.73. https://doi.org/10.1136/thorax.58.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy TF, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–83. doi: 10.1164/ajrccm/146.4.1067. https://doi.org/10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 8.Monsó E, Ruiz J, Rosell A, et al. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med. 1995;152:1316–20. doi: 10.1164/ajrccm.152.4.7551388. https://doi.org/10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–52. doi: 10.1136/thorax.57.10.847. https://doi.org/10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy TF, Brauer AL, Grant BJ, et al. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med. 2005;172:195–9. doi: 10.1164/rccm.200412-1747OC. https://doi.org/10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S, Evans N, Grant BJ, et al. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–71. doi: 10.1056/NEJMoa012561. https://doi.org/10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 12.Miravitlles M, Espinosa C, Fernández-Laso E, et al. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest. 1999;116:40–6. doi: 10.1378/chest.116.1.40. https://doi.org/10.1378/chest.116.1.40. [DOI] [PubMed] [Google Scholar]
- 13.Eller J, Ede A, Schaberg T, et al. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest. 1998;113:1542–8. doi: 10.1378/chest.113.6.1542. https://doi.org/10.1378/chest.113.6.1542. [DOI] [PubMed] [Google Scholar]
- 14.Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. https://doi.org/10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 15.Murray PR, Washington JA. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc. 1975;50:339–44. [PubMed] [Google Scholar]
- 16.Cabello H, Torres A, Celis R, et al. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J. 1997;10:1137–44. doi: 10.1183/09031936.97.10051137. https://doi.org/10.1183/09031936.97.10051137. [DOI] [PubMed] [Google Scholar]
- 17.Wayne P National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. 2004 [Google Scholar]
- 18.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of chronic pulmonary disease, 2011. [Internet] Available from: http://www.goldcopd.org/Guidelines/guidelines-resources.html.
- 19.Boixeda R, Rabella N, Sauca G, et al. Microbiological study of patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) and the usefulness of analytical and clinical parameters in its identification (VIRAE study) Int J Chron Obstruct Pulmon Dis. 2012;7:327–35. doi: 10.2147/COPD.S30568. https://doi.org/10.2147/COPD.S30568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. https://doi.org/10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 21.Sapey E, Stockley RA. COPD exacerbations. 2: Aetiology. Thorax. 2006;61:250–8. doi: 10.1136/thx.2005.041822. https://doi.org/10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy TF, Brauer AL, Eschberger K, et al. Pseudomonas aeruginosa in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2008;177:853–60. doi: 10.1164/rccm.200709-1413OC. https://doi.org/10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 23.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26:1138–80. doi: 10.1183/09031936.05.00055705. https://doi.org/10.1183/09031936.05.00055705. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels RA, Buist AS, Calverley PM, et al. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. https://doi.org/10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Vidal C, Almagro P, Romaní V, et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. Eur Respir J. 2009;34:1072–8. doi: 10.1183/09031936.00003309. https://doi.org/10.1183/09031936.00003309. [DOI] [PubMed] [Google Scholar]
- 26.Soler N, Torres A, Ewig S, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med. 1998;157:1498–505. doi: 10.1164/ajrccm.157.5.9711044. https://doi.org/10.1164/ajrccm.157.5.9711044. [DOI] [PubMed] [Google Scholar]
- 27.Lin SH, Kuo PH, Hsueh PR, et al. Sputum bacteriology in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease in Taiwan with an emphasis on Klebsiella pneumoniae and Pseudomonas aeruginosa. Respirology. 2007;12:81–7. doi: 10.1111/j.1440-1843.2006.00999.x. https://doi.org/10.1111/j.1440-1843.2006.00999.x. [DOI] [PubMed] [Google Scholar]
- 28.Ye F, He LX, Cai BQ, et al. Spectrum and antimicrobial resistance of common pathogenic bacteria isolated from patients with acute exacerbation of chronic obstructive pulmonary disease in mainland of China. Chin Med J (Engl) 2013;126:2207–14. [PubMed] [Google Scholar]
- 29.Van de Garde MD, Martinez FO, Melgert BN, et al. Chronic exposure to glucocorticoids shapes gene expression and modulates innate and adaptive activation pathways in macrophages with distinct changes in leukocyte attraction. J Immunol. 2014;192:1196–208. doi: 10.4049/jimmunol.1302138. https://doi.org/10.4049/jimmunol.1302138. [DOI] [PubMed] [Google Scholar]
- 30.Sethi S, Anzueto A, Miravitlles M, et al. Determinants of bacteriological outcomes in exacerbations of chronic obstructive pulmonary disease. Infection. 2016;44:65–76. doi: 10.1007/s15010-015-0833-3. https://doi.org/10.1007/s15010-015-0833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez FJ, Han MK, Flaherty K, et al. Role of infection and antimicrobial therapy in acute exacerbations of chronic obstructive pulmonary disease. Expert Rev Anti Infect Ther. 2006;4:101–24. doi: 10.1586/14787210.4.1.101. https://doi.org/10.1586/14787210.4.1.101. [DOI] [PubMed] [Google Scholar]
- 32.Heineman HS, Chawla JK, Lopton WM. Misinformation from sputum cultures without microscopic examination. J Clin Microbiol. 1997;6:518–27. doi: 10.1128/jcm.6.5.518-527.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erkan L, Uzun O, Findik S, et al. Role of bacteria in acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:463–7. doi: 10.2147/copd.s2776. https://doi.org/10.2147/COPD.S2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko FW, Ip M, Chan PK, et al. A 1-year prospective study of the infectious etiology in patients hospitalized with acute exacerbations of COPD. Chest. 2007;131:44–52. doi: 10.1378/chest.06-1355. https://doi.org/10.1378/chest.06-1355. [DOI] [PubMed] [Google Scholar]


