Abstract
Secondary spontaneous pneumothorax almost always develops secondary to an underlying lung disease. A pneumothorax secondary to a malignancy is very rare, and is observed most frequently in soft tissue sarcomas. Pazopanib, a tyrosine kinase inhibitor, is used in metastatic soft tissue sarcomas treatment. The rate of pneumothorax that is caused by pazopanib is about 14% in the literature. The patient being presented in this article underwent surgery for soft tissue sarcoma, postoperatively received pazopanib (Votrient® 400 mg, oral, Glaxo Group Ltd, Brentford, UK) treatment due to widespread bilateral lung metastases, and developed synchronous spontaneous pneumothorax.
Keywords: Bilateral, pazopanib, pneumothorax, spontaneous, video-assisted thoracoscopic surgery
INTRODUCTION
Pneumothorax is defined as the presence of air in the pleural cavity, which occurs for various reasons, and lung collapse developing secondary to that. Its etiology frequently includes trauma and lung diseases. Spontaneous pneumothorax develops without any trauma or iatrogenic reason, and its incidence is reported as 7.4–28/100,000 per year for men and 1.2-6/100,000 per year for women. Pneumothorax is classified as primary spontaneous pneumothorax (PSP) and secondary spontaneous pneumothorax (SSP). PSP develops in the absence of trauma or any pathology in the structure of the lung, and SSP occurs as a result of pulmonary diseases, particularly chronic obstructive pulmonary disease (COPD) or tuberculosis [1,2]. Pneumothorax secondary to malignancy generally develops in association with soft tissue sarcoma, germ cell tumor, and primary lung metastases, and its incidence ranges between 0.03% and 0.05% [3].
Pazopanib (Votrient® 400 mg, oral, Glaxo Group Ltd, Brentford, UK), a tyrosine kinase inhibitor, is used for urothelial tumors, renal cell carcinoma, pancreatic neuroendocrine tumor, and metastasis of cervical cancer, particularly for soft tissue sarcomas. However, it is reported that pazopanib-induced pneumothorax develops only in cases of soft tissue sarcoma [4]. In this article, we aimed to present a patient who underwent mass excision due to undifferentiated pleomorphic sarcoma in the chest wall and developed bilateral pneumothorax secondary to diffuse lung metastases during postoperative pazopanib therapy.
CASE PRESENTATION
A 49-year-old male patient, who had noticed a swelling in the anterior thoracic wall 3 months before, was admitted to our outpatient clinic because of pain that started in this region. No related features were found in his medical history and family history. He had a history of smoking 25 packs/year. His laboratory findings were as follows: hemoglobin: 9.9 g/dL; hematocrit: 31.8%; leukocytes: 9240/uL; platelets: 279000/uL C-reactive protein: 44.2 mg/L; and sedimentation: 60 mm/hour.
The magnetic resonance imaging of the thorax revealed a 9x9x6.5 cm malignant-appearing soft tissue mass that was localized in the pectoral muscle in the left thoracic wall, extending to the axilla and infraclavicular region and invading adjacent muscle tissues, but not neighboring ribs. In positron emission tomography-computed tomography, pathological FDG involvement (SUV max: 15.47) was observed in this region. No significant hypermetabolic focus was detected in other parts of the body.
The mass in the thoracic wall was totally excised under general anesthesia, and the defect involving the skin, subcutaneous, and muscle tissues in the chest wall was primarily closed. The histopathological evaluation of the excised mass was reported as high-grade undifferentiated pleomorphic sarcoma. The patient was administered postoperative radiotherapy and chemotherapy, and local recurrence and multiple lung metastases were detected at the surgical site in the postoperative 6th month. The patient was begun to be given chemotherapy (ifosfamide+doxorubisin+mesna), but the treatment was continued with pazopanib (Votrient® 400 mg, oral, Glaxo Group Ltd, Brentford, UK) because his disease progressed. On the 13th day of pazopanib therapy, bilateral pneumothorax was observed in the direct chest radiography of the patient, who was developing shortness of breath and chest pain (Figure 1). The thoracic computed tomography (CT) demonstrated bilateral pneumothorax, multiple metastatic lesions in both lungs, and cavitary lesion probably secondary to tumor necrosis related to the pleura in the right upper lobe (Figure 2b). In the thoracic computed tomography (CT) that was taken before pazopanib therapy, multiple metastatic lesions were viewed in both lungs (Figure 2a).
Figure 1.
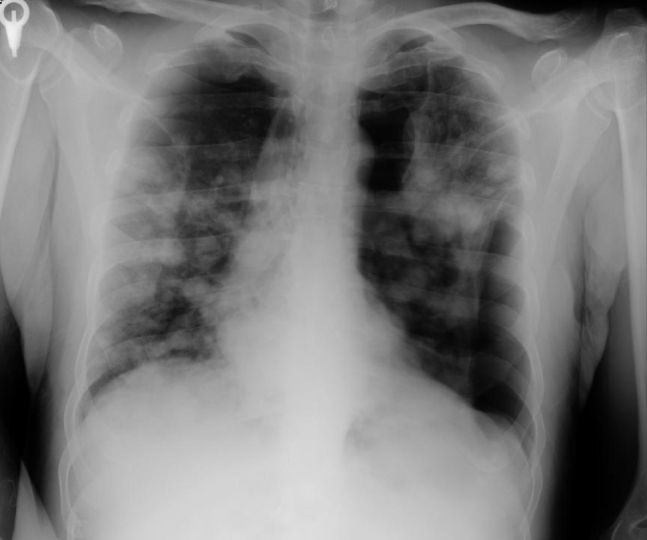
Bilateral pneumothorax and diffuse metastatic lesions in both lungs are viewed in direct chest radiography
Figure 2. a, b.
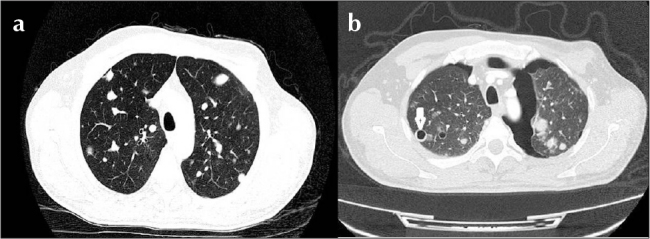
In computed tomography of the thorax, (a) multiple metastatic lesions in both lungs (before pazopanib therapy) and (b) bilateral pneumothorax, nodules consistent with diffuse metastasis in both lungs, and cavitary lesion near the pleura in the anterior segment of the right lung upper lobe (after pazopanib therapy) are viewed
The patient was applied bilateral tube thoracostomy, and pazopanib therapy was ceased. Air drainage in the left hemithorax was stopped, and chest tube was removed on the 6th day by applying chemical pleurodesis with talc. Written informed consent was obtained from the patient and video-assisted thoracoscopic surgery was performed due to prolonged air leak in the right hemithorax. It was found that metastatic nodule in the right lung anterior segment was necrosed and opened to the pleural space (Figure 3). The parenchyma was primarily repaired because of multiple metastases, and total pleurectomy was applied. The patient’s chest tube was removed on the postoperative 7th day and discharged. He was exitus in the postoperative 2nd month due to the progression of his disease.
Figure 3.
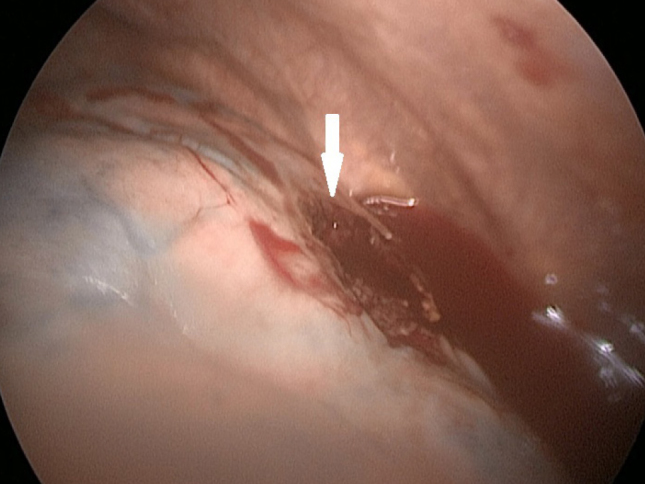
In video-assisted thoracoscopic surgery, it is viewed that cavitary lesion located in the anterior segment of the right lung upper lobe opens to the pleural space
DISCUSSION
Secondary spontaneous pneumothorax is generally seen at advanced ages, and its incidence is reported as 6.3/100.000 per year in men and 2/100.000 per year in women. The most common cause in its etiology is COPD, and metastatic lung diseases are among rare causes. Despite the fact that many tumors cause lung metastasis, the most frequent ones are malignant melanomas, genitourinary tumors, colorectal tumors, head and neck tumors, breast tumors, and soft tissue sarcomas. Sarcoma metastasis is the most common malignancy that causes SSP. Metastases occur as solitary or multiple nodules, and they are localized in the peripheral regions of the lungs because they spread hematogenically [1,2].
Pneumothorax can present as asymptomatic or with symptoms such as chest pain and shortness of breath. In rare situations, pneumothorax develops simultaneously and bilaterally, which can be life-threatening. In the literature, the rate of simultaneous bilateral spontaneous pneumothorax is specified as 1% among all types of pneumothorax. Simultaneous bilateral SSP is quite rare, and 70% of cases have an underlying disease such as malignancy, interstitial lung disease, and tuberculosis [1].
Pazopanib is an anti-angiogenic tyrosine kinase inhibitor that demonstrates its activity against vascular endothelial growth factor receptors and that is given orally for the treatment of advanced-stage or metastatic non-small cell lung cancer [4]. Nakano et al. detected pneumothorax in 3 of 32 patients given pazopanib due to soft tissue sarcomas, and they reported the incidence of pneumothorax as 1.53 per 1,000 days of pazopanib therapy [5]. Similarly, Verschoor et al. administered pazopanib for the treatment of soft tissue sarcoma metastases in their series, and they found pneumothorax in 6 of 43 cases (14%). They found the rate of pneumothorax development as high in their series compared to the rate of 3.3% in the PALETTE study [4,6]. Pneumothorax that developed in all these studies is attributed to 3 mechanisms: 1) obstruction of the proximal and distal airways by tumor nodules with the check-valve mechanism, 2) pulmonary infarct associated with tumor embolism, and 3) most important, cavitation of subpleural metastases and formation of bronchopleural fistula. Angiogenesis inhibitors such as pazopanib can lead to cavitation in metastatic lesions and also pneumothorax during treatment at high rates [4–7]. Moreover, although smoking is an important risk factor in spontaneous pneumothorax, it is reported that it is not a risk factor for patients given pazopanib [4].
The treatment of SSP is planned considering the overall condition of the patient, the presence of first or recurrent attack, underlying lung disease, and the state of air leakage. The treatment choices that are implemented are observation, aspiration with catheter, tube thoracostomy, pleurodesis, video-assisted thoracoscopic surgery, and thoracotomy [1]. There is scarcely any case of simultaneous bilateral spontaneous pneumothorax that develops secondary to pazopanib therapy in lung metastasis described in the literature. In the literature, cases of pneumothorax developing with the invasion of subpleural metastases of the lung to the pleural space are reported, and most of these cases are soft tissue sarcoma metastases. In pneumothorax that occurs secondary to drug, tissue necrosis develops in association with treatment in metastases of the lung parenchyma, and pneumothorax or pneumopericardium occurs due to bronchopleural or bronchopericardial fistula. When pazopanib and derivative drugs are added to the treatment, necrosis in metastatic lesions becomes more rapid, and pneumothorax develops with the spread of metastases near the visceral pleura to the pleura. In our case, simultaneous bilateral pneumothorax associated with tissue necrosis developing in bilateral lung metastasis during pazopanib therapy was detected. Surgical treatment of the right hemithorax was required. The patient was exitus in the postoperative 2nd month due to the progression of the disease and poor general health condition after surgical treatment. In this period, the patient was not given pazopanib, and only nutritional support was provided.
In conclusion, pneumothorax can develop during the treatment of metastatic diseases of the lung, and it is life-threatening when bilateral. Pneumothorax generally occurs due to tissue necrosis that develops in association with the treatment of nodules with subpleural localization. Therefore, in case of the sudden onset of chest pain and shortness of breath in patients undergoing drug therapy for lung metastasis, pneumothorax should be considered, and radiological imaging techniques should be utilized.
Footnotes
This study was presented at the 19th Annual Congress of the Turkish Thoracic Society, 6–10 April 2016, Antalya, Turkey.
Informed Consent: Written informed consent was obtained from the patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - B.Ç., V.Y., H.K.Ç. Z.P.S:; Design - B.Ç., Z.P.S., H.K.Ç; V.Y.; Supervision - B.Ç., V.Y., Z.P.S. H.K.Ç.; Resource - B.Ç., Z.P.S., H.K.Ç..; Materials - B.Ç., V.Y., Z.P.S.; Data Collection and/or Processing - B.Ç., Z.P.S., H.K.Ç; Analysis and/or Interpretation - B.Ç., V.Y., H.K.Ç..; Literature Search - B.Ç.., Z.P.S., H.K.Ç.; Writing - B.Ç., Z.P.S., H.K.Ç.; Critical Reviews - B.Ç, Z.P.S., V.Y.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Karamustafaoğlu A. Pnömotoraks ve pnömomediastinum. In: Yüksel M, Balcı AE, editors. Göğüs Cerrahisi “Kırmızı Kitap”. Nobel Tıp Kitapevleri Tic. Ltd. Şti; İstanbul: 2015. pp. 555–562. [Google Scholar]
- 2.Melton LJ, 3rd, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;29:1379–82. doi: 10.1164/arrd.1979.120.6.1379. [DOI] [PubMed] [Google Scholar]
- 3.Vencevicius V, Cicenas S. Spontaneous pneumothorax as a first sign of pulmonary carcinoma. World J Surg Oncol. 2009;7:57. doi: 10.1186/1477-7819-7-57. https://doi.org/10.1186/1477-7819-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verschorr AJ, Gelderblom H. Pneumothorax as adverse event in patients with lung metastases of soft tissue sarcoma terated with pazopanib: a single reference centre case series. Clin Sarcoma Res. 2014;4:14. doi: 10.1186/2045-3329-4-14. https://doi.org/10.1186/2045-3329-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakano K, Inagaki L, Tomomatsu J, et al. Incidence of pneumothorax in advanced and/or metastatic soft tissue sarcoma patients during pazopanib treatment. Clin Oncol. 2014;26:357. doi: 10.1016/j.clon.2014.02.010. https://doi.org/10.1016/j.clon.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 6.van der Graaf WT, Blay JY, Chawla SP, et al. EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–86. doi: 10.1016/S0140-6736(12)60651-5. https://doi.org/10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas S, Varadhachary G. Spontaneous pneumothorax in malignancy: a case report and review of the literature. Ann Oncol. 2000;11:887–9. doi: 10.1023/a:1008323632078. https://doi.org/10.1023/A:1008323632078. [DOI] [PubMed] [Google Scholar]


