Abstract
OBJECTIVES
Effects of air pollution parameters of sulfur dioxide (SO2) and particulate matter (PM10) values on the respiratory system were investigated.
MATERIAL AND METHODS
Data of SO2 and PM10 were obtained daily for air pollution and classified into two groups: Group I (2006–2007), coal burning years and Group II (2008–2009), natural gas+ coal burning. Groups I and II were divided into two subgroups according to the months of combustion as combustible (November–April) and noncombustible (May–October). The number of patients with asthma and chronic obstructive pulmonary disorder (COPD) was recorded between 2006 and 2009.
RESULTS
There was no statistically significant difference between Groups I and II for PM10 and SO2 (p>0.05). Within the years, the values of SO2 and PM10 were statistically different between the groups defined by month (p<0.01). The number of patients in the combustible and noncombustible subgroups were found to be different for every 4 years, and the numbers of patients with COPD or asthma were not changed through the years. There was a strong correlation between PM10 and COPD (r=0.59, p<0.01) and a weak correlation between PM10 and asthma (r=0.25, p>0.05). A correlation was found between SO2 and COPD (p<0.01) but not between SO2 and asthma (p>0.05). The number of visits for COPD and asthma was statistically different between combustible and noncombustible subgroups (X2:58.61, p=0.000; X2:34.55, p=0.000, respectively). The r2 values for SO2 and PM10 for COPD patients were 17% and 24%, respectively, in contrast to 8% and 5%, respectivley for asthma patients.
CONCLUSION
Air pollution is known to increase respiratory disease occurrences. With decrease in the usage of solid fuel, air pollution could be reduced and may be effective in preventing respiratory diseases.
Keywords: Air pollution, respiratory system disease, PM10, SO2, asthma, COPD
INTRODUCTION
Inhalation of toxic particles and gases increases epithelial permeability, which is one of natural defense mechanisms of the lungs; decreases mucociliary activity; and depresses macrophage functions. These substances render toxic effects in healthy or unhealthy individuals and can be a component of molecular events that commonly develop [1]. In vitro experimental studies conducted on humans and animals showed that the damages caused increased inflammatory cellular activation (e.g., neutrophils, T lymphocytes, macrophages, and mast cells), increased production of inflammatory cell proteins (cytokines and chemokines), increased oxidative stress with free radical formation [2] (superoxide, hydrogen peroxide, and hydroxyl radicals), and decreased antioxidant enzyme levels (glutathione transferase and superoxide dismutase) [3].
Several studies have shown that particulate matter (PM) in the air affect short- and long-term health. In many studies, there is evidence of effects of PM10 and PM2.5 (PM diameter of 10 or 2.5 μm) on asthma and chronic obstructive pulmonary disease (COPD) and on the increased rate of hospitalization [4]. Studies have shown that particle pollution in the air has negative effects on many parameters, particularly on respiratory function tests [5], patient’s symptoms, and the rate of hospitalization [6–8].
Chronic obstructive pulmonary disease presents with progressive inflammation of the airways, pulmonary veins, and pulmonary parenchyma [9,10] and irreversibly causes airflow restriction [11]. Among all pulmonary diseases, COPD is believed to be strongly associated with exposure to polluted air, in particular to PM (black smoke, total mass, PM10 or PM2.5 μm in diameter) [12]. Epidemiological data suggest that increased level of PM pollution elevates the number of admissions to the emergency unit due to previously existing COPD or the rate of hospitalization [13,14]. According to the estimates of World Health Organization (WHO), the number of deaths due to exposure to smoke from solid fuels is approximately 1.6 million per year. Of these 693000 are associated with COPD and 910000 with acute lower respiratory tract infections [15].
Sulfur dioxide (SO2) pollution is caused by combustion of fossil fuels including sulfur and by pollutants resulting from heating and released from smokestacks. In contrast, PM pollution is mostly caused by industrial regions and partially by fossil fuels used for heating [16]. Exposure to SO2 was found to be associated with increased prevalence of respiratory symptoms, such as wheezing and shortness of breath; total and respiratory mortality [17]; increased risk of asthma [18]; and exacerbation of a previously occurred respiratory disease [19], increased prevalence of respiratory symptoms, such as wheezing and shortness of breath [20].
The aim of the study was firstly to determine the relationship between the use of solid fuels, which causes increased particles and harmful gases in air, and respiratory tract diseases and secondly to determine the effect of PM10 and SO2 in air pollution on the exacerbation of asthma and COPD, which are among the respiratory tract diseases.
MATERIAL AND METHODS
This was a retrospective observational study, and the values of SO2 and PM10 were obtained from Isparta Provincial Directorate of Environment in order to determine daily air pollution between 2006 and 2009. Data were recorded as daily measurements in the measurement station at Isparta and were evaluated considering daily SO2 and PM10 values for all years.
The protocols for the research project and survey have been approved by a suitably constituted ethics committee of our institution and conform to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000).
Of the patients who were admitted with a complaint of a respiratory tract disease to the emergency unit of the Suleyman Demirel University hospital between 2006 and 2009, data of adults (aged >15 years) with asthma and COPD attack were assessed retrospectively. The diagnosis and staging process were performed in accordance with the guidelines of Global Initiative for Asthma (GINA) for asthma patients and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for COPD patients [21,22]. The attacks were initially assessed by emergency medicine and chest diseases research assistants and on-call chest diseases professors in the emergency medical unit. The coexistence of clinical and physiological findings, such as speech disorder, agitation, confusion, cyanosis, respiratory rate >30/minute, pulse rate >120/minute, involvement of accessory respiratory muscles, oxygen saturation below 91%–92%, and CO2 retention was accepted as asthma and COPD attack [23,24]. Patients admitted to hospital were classified in accordance with the International Classification of Diseases, Revision 10 (ICD 10-CM, code J44-KOAH and J45-ASTHMA). Inclusion criteria were patients with asthma and COPD and admitted to the emergency unit for exacerbation; however, those admitted to the emergency unit due to other upper respiratory tract diseases and with coexistent cardiac diseases were excluded from the study.
Study groups were designed as follows:
Group I: According to heating state, November–April was accepted as one period and May–October as the other period. The periods were further classified based on the months in which solid fuels was used (November–April) and not used (May–October), and monthly data were subsequently evaluated between 2006 and 2007.
Group II: In addition to solid fuel usage, the periods were classified based on the months in which fuel was used (November–April) and not used (May–October) between 2008 and 2009 when natural gas usage was initiated, and monthly data were evaluated.
According to data obtained from Torosgaz at the time of the study, there were a total of 94000 houses across Isparta, and 7000 active subscribers began the use natural gas. The measurement station was downtown and there were 550 houses, 23 official institutions, and a central heating system. In the region where the measurement was performed, the total number of houses was 4000, and 1477 houses used natural gas, with the fuel usage rate of 36.9%, whereas this rate was 7.4% across the province. According to these results, the rate of natural gas use is defined as partial transition to natural gas and the use of solid fuel continues in the same region.
The mean SO2 and PM10 values for each month in the determined groups and asthma and COPD patients admitted with a complaint of respiratory tract diseases to the emergency unit of Suleyman Demirel University hospital in the related months were included in the study. Of the admissions, air pollution data of the city center where the study was performed were evaluated. In case of an attack, necessary treatment could be received in the emergency unit of any hospital. Therefore, the patients included in the study were those who were admitted in the region where air pollution was evaluated.
Statistical Analysis
The Kolmogorov-Smirnov test was used to determine whether data displayed normal distribution. The nonparametric Kruskal-Wallis test was employed to compare the months in which solid fuel was and was not used and to assess the differences. The numbers of patients were compared using ANOVA according to the months in which solid fuel was used or not used by considering even the year. Since variances were not homogeneous, patient numbers were compared using the Kruskal-Wallis test according to the months in which solid fuel was used and not used. Depending on the absence of nonhomogeneous variance, patient numbers were compared using the F-test variance analysis as years and months in which solid fuel was and was not used. Because the variance range was extremely wide, variations were found to be homogeneous as a result of homogeneous square root transformation in the F test, and the data were made reliable. In statistical evaluations, the value of p<0.05 was accepted to be significant.
RESULTS
Table 1 presents the numbers of asthma and COPD patients from those admitted due to respiratory tract diseases to the emergency unit in 2006–2007 (Group I) and 2008–2009 (Group II) and the evaluation of PM10 and SO2 values according to the months in which solid fuel was used and not used. PM10 and SO2 levels showed a statistically significant difference in the months (p<0.001). According to these findings, the levels of PM10 and SO2 significantly increased in the months in which solid fuel was used. Considering the months in which solid fuel was used and not used in a year, PM10 and SO2 levels were found to differ significantly (p<0.001), and there was a decrease in the months in which solid fuel was not used. Similarly, there was a statistically significant decrease in the frequency of asthma (p<0.001) and COPD (p<0.001) occurrence in the months in which solid fuel was not used (Table 1).
Table 1.
Numbers of patients admitted to the emergency unit due to respiratory tract diseases in 2006–2008 (Group I) and 2008–2009 (Group II) and the evaluation of PM10 and SO2 values according to the months in which solid fuel was and was not used
| PM10 (μg/m3) (mean±SD) | SO2 (μg/m3) (mean±SD) | |
|---|---|---|
| Group I | 83.49±51.66 | 55.76±56.52 |
| Months in which solid fuel was used | 121.19±49.04b | 91.26±58.32a |
| Months in which solid fuel was not used | 45.79±0.63 | 16.72±9.32 |
| Group II | 87.09±63.95 | 52.56±57.16 |
| Months in which solid fuel+natural gas was used | 123.94±54.91b | 95.38±51.70a |
| Months in which solid fuel+ natural gas was not used | 50.23±50.72 | 9.75±12.27 |
According to months in which fuel was not used;
p<0.05 for PM,
p<0.05 for SO2
For Group II, when PM10 (p<0.001) and SO2 (p<0.001) values were compared between the months in which solid fuel+natural gas were used and solid fuel+natural gas were not used, there was a statistically significant difference, and the values decreased in the months in which solid fuel+natural gas was not used.
Regardless of the classification, no significant difference was found between the groups When comparing air pollution based on the year (Table 2). When data were compared using t test, no statistically significant difference was detected (p>0.05).
Table 2.
PM10 and SO2 values according to year group. Group I: 2006–2007 and Group II: 2008–2009
| Year group | PM10 (n=24) | SO2 (n=20) |
|---|---|---|
| Group I | ||
| 2006–2007 | 83.50±51.66 | 55.77±56.53 |
| Group II | ||
| 2008–2009 | 87.09±63.95 | 52.56±57.16 |
| p value | 0.831 | 0.851 |
There was a significant difference between the months in which solid fuel was used and not used in terms of the number of COPD (p<0.001) and asthma (p<0.001) admissions. The number of patient admissions was significantly decreased in months in which solid fuel was not used.
According to single factor analysis of variance, the number of COPD patients in months in which solid fuel was used and not used differed at the same level in all years. There was a statistically significant difference between the months in which solid fuel was used and not used in terms of the number of COPD and asthma patients admitted to hospital (p<0.001 and p<0.05, respectively), and the numbers of COPD patients and asthma patients were lower in the months in which solid fuel was not used (Table 3).
Table 3.
Comparison of the numbers of patients admitted to the emergency unit in months in which fuel was used and not used according to years
| 2006–2007 | 2008–2009 | |||
|---|---|---|---|---|
|
| ||||
| COPD mean±SD | Asthma mean±SD | COPD mean±SD | Asthma mean±SD | |
| Months in which solid fuel (+natural gas) was used | 13.08±7.40a | 2.16±1.89b | 12.33±5.08a | 3.66±3.14b |
| Months in which solid fuel (+natural gas) was not used | 5.66±2.67 | 1.33±1.07 | 4.66±3.77 | 1.00±1.41 |
| p value | 0.001 | 0.001 | 0.05 | 0.05 |
One-factor variance analysis was performed. According to months in which fuel was not used;
p<0.05 for COPD,
p<0.05 for asthma
The presence of a linear relationship between PM10 and SO2 values and the numbers of patients with asthma and COPD was evaluated through correlation analysis. There was an increasing linear relationship between PM10 and SO2 values, PM10 and COPD, and SO2 and COPD. There was an increasing linear relationship between PM10 and asthma and between SO2 and asthma. However, their statistical significance level was low.
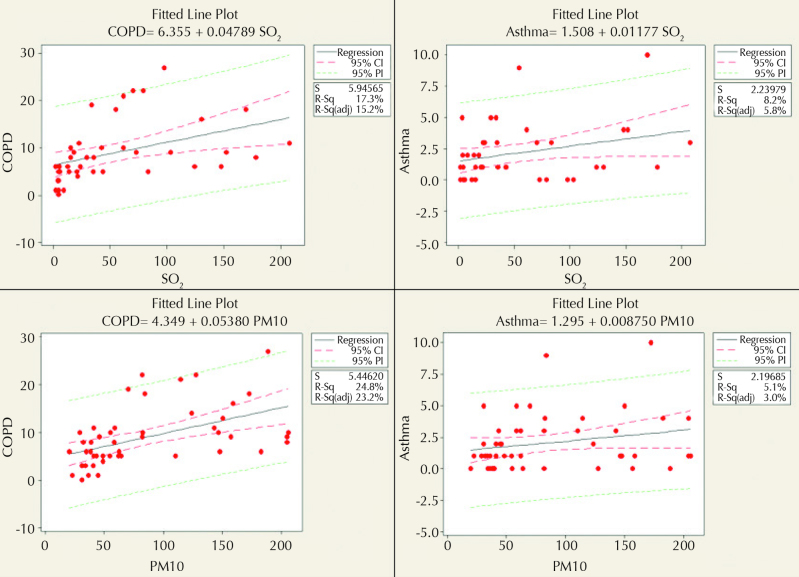
A high correlation was observed between PM10 and the number of COPD patients (r=0.59, p<0.001). There was a correlation between PM10 and SO2 (r=0.025, p<0.001). The correlation between PM10 and asthma (r=0.25, p=0.123) was found to be statistically nonsignificant. While a correlation was observed between SO2 and COPD (p<0.05), there was no significant correlation between SO2 and asthma (p>0.05). The distribution graphs for the correlation analysis between SO2 and PM10 values and asthma and COPD variables are presented in Figure 1.
Figure 1.
SO2 and PM10 and asthma and COPD scatter graphs and regression models (regression analyses were performed for determining SO2 and PM10 values and the predictability of having asthma and COPD, and the results are presented in Figure 1. Coefficients of determination were ranged between 5% and 25%. Although the predictability of models is not high, they provide information that shows that they are assessable
Regression Analysis
Linear regression analyses were performed for predicting SO2 and PM10 values and the numbers of COPD and asthma patients. The r2 values were 17% for SO2 and COPD (6,355+0,04789xSO2), 8% for SO2 and asthma (1,508+0,01177xSO2), 24% for PM10 and COPD (4,349+0,005380xPM10), and 5% for PM10 and asthma (1,295+0,008750xPM10). The coefficients of determination for regression models indicated that the predictive value of the model is low. However, it partially explains the relationship between the related variables (Figure 1).
DISCUSSION
Our study revealed that an increase in air pollution and accordingly in respiratory tract diseases development occurred in association with the use of solid fuels.
The relationship between the level of daily air pollution in the center of Sivas and the diagnostic rates of COPD and bronchial asthma in patients hospitalized in Sivas Chest Diseases Hospital between October 1, 1998, and September 30, 2000, was investigated. No statistically significant relationship was found in the study between daily SO2 values and all patients hospitalized at the same period and patients diagnosed with bronchial asthma and COPD. A significant relationship was found between total daily particle values and hospitalized COPD patients residing within the borders of the municipality (r=0.5, p=0.013) [25]. A study evaluating the use of coal banned in 1990, 1995, and 1998 and the patient admission rates before and after the ban in Ireland revealed that the number of admissions due to respiratory tract diseases continuously decreased after the ban, and a decrease was observed in the numbers of pneumonia, COPD, and asthma patients [26]. Similarly, a study investigating SO2- and PM-induced air pollution and the admissions of asthma and COPD patients to the emergency unit showed that high SO2 values were found to be associated with admission to the emergency unit [27]. A study by Rumana et al. [28] examined the relationship between the levels of PM2.5, PM10, nitrogen oxides (NOx), SO2, ammonia (NH3), and ozone (O3), urbanization, and air pollution and emergent respiratory and cardiac diseases and revealed that respiratory infections (25%) and the prevalence of asthma/COPD (4%) were associated with increased air pollution. In literature, studies have demonstrated a linear relationship between air pollution and respiratory tract diseases. Similar findings were obtained in our study. However, harmful effects on respiratory health will be reduced with widespread use of natural gas and elimination of other pollutants contributing to air pollution.
The current degree of air pollution was evaluated in the province of Diyarbakir. It was observed that the annual SO2 and PM concentrations were approximately 110 μg/m3 in 2000–2001, which increased in 2002 and declined in 2003. According 2004 data, the values of SO2 and PM increased to 134 and 137 μg/m3, respectively, in January; the values were 115 and 120 μg/m3, respectively, in December. These values were above the targeted limit determined by the Turkish Air Quality Protection Regulation and WHO. Similar to our study, it is observed that the factors causing air pollution are the use of solid fuels, exhaust gas, and factory emissions [29]. In the example of the province of Van, air pollution parameters (SO2 and PM10) before the use of natural gas and after transition to natural gas were examined. Coal, fuel oil, and diesel fuel were used for heating in the province. Natural gas use was initiated as of March 2008. With the use of natural gas, the use of other fuels decreased. Thus, the study thus revealed that while the SO2 value of 250 μg/m3 stated in the Air Quality Evaluation and Management Regulation (AQEMR) was exceeded in some months before transition to the use of natural gas, the value did not exceed the limit after initiation of natural gas usage. It was observed that the PM10 value exceeded the 200 μg/m3 value stated in the regulation in winter and reached 267 μg/m3. With transition to natural gas usage in March 2008, a decrease was observed in the PM10 value again [30]. The data obtained from this study, which are consistent with those in literature, show that solid fuel-induced air pollution significantly decreased with partial transition to natural gas.
Sunyer et al. [31] evaluated the admissions to the emergency unit due to COPD and daily air pollution in Barcelona, which is a Mediterranean city where motor vehicles were commonly used in 1991. They found a positive relationship between the admissions for COPD and black smoke, SO2, and carbon monoxide (CO). In other studies conducted in the USA and Canada, the significant relationship between admission or hospitalization in the emergency unit due to respiratory diseases and asthma and particles and O3 was emphasized [32–37]. These results indicate a relationship between the levels of PM and SO2 and the admissions for asthma and COPD, similar to our study.
Stieb et al. [38] evaluated 400000 emergency service visits in 14 hospitals in seven different cities between 1990 and 2000 and examined the levels of CO, NO2, SO2, PM10, and PM2.5. The levels of PM10 and PM2.5 were found to be associated with asthma attacks. In the study conducted by Canova et al. [39] regarding the effect of PM10 on hospitalization rate and its relationship with asthma and COPD, it was found that the high level of PM10 was related to hospital admission, and short-term exposure to PM10 decreased antioxidants in the blood samples of patients and increased exacerbations.
In İzmir, the relationship between asthma cases and the levels of SO2 and PM10 was investigated in six districts between 2007 and 2010. A significant correlation was noted between air pollution in the province and the number of asthma cases [40]. In an analysis conducted on adults and children in London, it was shown that PM10 and SO2 had strong effects on asthma and other lower respiratory tract diseases [41]. In our study, the findings revealed an increasing linear relationship between the levels of PM10 and SO2 and the number of patient admissions for COPD. “A study in Tokyo examining the acute effect of air pollution on pulmonary functions and airway inflammation in healthy volunteers showed that the mean 4-day PM10 concentrations increased, and PM10 was significantly associated with forced expiratory volume in 1 second (FEV1) values. In relationship with the history of asthma, the level of exhaled nitric oxide (FeNO) was found to be increased. While high level of PM10 was associated with decreased FEV1, it was emphasized that the patients with rhinitis and asthma are more susceptible to air pollution [42]. In a study investigating the effect of PM2.5 on asthma-related mortality and morbidity, experimental asthma was induced with ovalbumin in rats in two cities of the USA, and the rats were exposed to air pollution for 16 hours. Subsequently, PM2.5 analyses were performed (mass, size, fraction, and main component analyses, and trace element content), the lung lobe was removed through bronchoalveolar lavage (BAL), and airway inflammation and mucus response were evaluated. The concentration of PM, which was similar in two cities, did not cause any effect in nonasthmatic rats. On the contrary, 200% airway mucus, 250% neutrophil, and 90% eosinophil increases in BAL and 300% total protein increase were noted in the asthmatic rats. It was concluded that increased PM caused exacerbation in asthma patients sensitive to it, and exposure to PM should be considered for the protection of public health [43]. In the Italian part of the EpiAir Project, the effect of air pollution on hospital admissions was investigated in nine cities between 2001 and 2005. The relationship between PM10 and gases (NO2 and O3) and respiratory tract diseases was examined, and three pollutants were found to be associated with hospitalization for different levels of asthma, COPD, and respiratory tract infections. A high relationship was detected between NO2 and asthma, particularly in children [44]. In our study, there was an increasing linear relationship between admissions due to asthma attack and the levels of PM10 and SO2; however, the significance value was low, which could be because of increasing linear relationship can be explained with that the number of asthma patients was same in all months and pollens increased attacks as well as air pollution.
In fact, while this pollution is more associated with secondary transformation and long-range transport in hot periods, particle pollution in Isparta is strongly influenced by local traffic and factories. Moreover, high levels of PM and SO2 even in the months in which solid fuel is not used can be associated with air pollution produced by large factories downtown and near city centers as well as exhaust gases. In this case, in addition to traffic and industrial sectors, the numbers of asthma and COPD attacks increased in parallel with increased air pollution in the months in which solid fuel was used. In this study, the particles measured during the study period in Isparta were found to be a risk factor, particularly for COPD. This is associated with air pollution caused by the use of solid fuel especially in winters, exhaust gases, and smokestacks of factories. The results show that exposure to oxidants (particles) leads to exacerbation of inflammatory response symptoms that develop against infections and an increase in the number of hospitalizations due to infection [45,46].
Our study provides valuable data with regard to the relationship between air pollution and respiratory tract diseases in our country. However, it has some limitations. Data on air pollution in the study reflect the state of the center of Isparta. It cannot be certainly assumed that the patients admitted to the emergency unit due to acute exacerbation were those exposed to air pollution in the city center. However, considering that respiratory emergencies triggered by air pollution could be intervened in any medical service, patients included in the study could indeed belong to the region where the study was conducted, which is one of the restrictions of the study. The misleading factor is that the measurement of air pollution was performed at specific regions of the city, and the data obtained were adapted to the whole city. In this situation, distinguishing patients from regions with and without air pollution may be difficult. Another limitation of the study is that PM2.5 could not be evaluated instead of PM10. This was attributed to the capacity of the measurement station.
In addition to its contribution to the pathogenesis of asthma and COPD, air pollution is a risk factor for those with a history of asthma and COPD. It affects individual quality of life and has a serious economic impact. This issue should be dealt with regard to public health. To ensure a comfortable and healthy life of the members of a society, local and national authorized institutions should take necessary measures, and the society should be aware of the situation. The detection of the amount and development of this pollution (exposure to pollutants and oxidative stress) will be inestimable for the correct evaluation of the efficiency of air quality policies and for decreasing the effects of air pollution on respiratory tract diseases.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Informed Consent: The number of patients used only from the application, was not take a consent.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.S., T.G., Ö.Ö.; Design - M.S., T.G., Ö.Ö.; Supervision - S.Ç., A.K.; Resources - M.H., Z.G.H.; Materials - M.S., T.G.; Data Collection and/or Processing - M.S., T.G.; Analysis and/or Interpretation - M.S., T.G., Ö.Ö.; Literature Search - M.S., T.G., M.H.; Writing Manuscript - M.S., Ö.Ö.; Critical Review - Ö.Ö., S.Ç., A.K.; Other - M.H., Z.G.H.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Andersen ZJ, Wahlin P, Raaschou-Nielsen O, et al. Size distribution and total number concentration of ultrafine and accumulation mode particles and hospitaladmissions in children and the elderly in Copenhagen, Denmark. Occup Environ Med. 2008;65:458–66. doi: 10.1136/oem.2007.033290. https://doi.org/10.1136/oem.2007.033290. [DOI] [PubMed] [Google Scholar]
- 2.Mudway IS, Stenfors N, Duggan ST, et al. An in vitro and in vivo investigation of the effects of diesel exhaust on human airway lining fluid antioxidants. Arch Biochem Biophys. 2004;423:200–13. doi: 10.1016/j.abb.2003.12.018. https://doi.org/10.1016/j.abb.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Behndig A, Mudway IS, Brown JL, et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. European Respir J. 2006;27:359–65. doi: 10.1183/09031936.06.00136904. https://doi.org/10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–42. doi: 10.1080/10473289.2006.10464485. https://doi.org/10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 5.Penttinen P, Vallius M, Tiittanen P, et al. Source-specific fine particles in urban air and respiratory function among adult asthmatics. Inhal Toxicol. 2006;18:191–8. doi: 10.1080/08958370500434230. https://doi.org/10.1080/08958370500434230. [DOI] [PubMed] [Google Scholar]
- 6.Meng YY, Rull RP, Wilhelm M, et al. Outdoor air pollution and uncontrolled asthma in the San Joaquin Valley, California. J Epidemiol Community Health. 2010;64:142–7. doi: 10.1136/jech.2009.083576. https://doi.org/10.1136/jech.2009.083576. [DOI] [PubMed] [Google Scholar]
- 7.Halonen JI, Lanki T, Yli-Tuomi T, et al. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax. 2008;63:635–41. doi: 10.1136/thx.2007.091371. https://doi.org/10.1136/thx.2007.091371. [DOI] [PubMed] [Google Scholar]
- 8.Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy. 2011;41:1059–71. doi: 10.1111/j.1365-2222.2011.03776.x. https://doi.org/10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 9.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–21. doi: 10.1016/S0140-6736(04)16900-6. https://doi.org/10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 10.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. https://doi.org/10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 11.Pauwels R. Global initiative for chronic obstructive lung diseases (GOLD): time to act. Eur Respir J. 2001;18:901–2. https://doi.org/10.1183/09031936.01.0027401. [PubMed] [Google Scholar]
- 12.Sunyer J, Basagana X. Particles, and not gases, are associated with the risk of death in patients with chronic obstructive pulmonary disease. Int J Epidemiol. 2001;30:1138–40. doi: 10.1093/ije/30.5.1138. https://doi.org/10.1093/ije/30.5.1138. [DOI] [PubMed] [Google Scholar]
- 13.Pope CA, 3rd, Kanner RE. Acute effects of PM10 pollution on pulmonary function of smokers with mild to moderate chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;147:1336–40. doi: 10.1164/ajrccm/147.6_Pt_1.1336. https://doi.org/10.1164/ajrccm/147.6_Pt_1.1336. [DOI] [PubMed] [Google Scholar]
- 14.Ko FW, Tam W, Wong TW, et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax. 2007;62:780–5. doi: 10.1136/thx.2006.076166. https://doi.org/10.1136/thx.2006.076166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith K, Mehta S, Maeusezahl-Feuz M. Indoor air pollution from household use of solid fuels. In: Ezzati M, Lopez A, Rodgers A, Murray C, editors. Comparative quantifi cation of health risks Global and regional burden of disease attributable to s elected major risk factors. Geneva, Switzerland: World Health Organization; 2004. pp. 1435–93. [Google Scholar]
- 16.Bayram H. Türkiye’de Hava Kirliliği Sorunu: Nedenleri, Alınan Önlemler ve Mevcut Durum. Turk Thorac J 2017; 18. 2005;6:159–65. [Google Scholar]
- 17.Chen R, Huang W, Wong CM, et al. Shortterm exposure to sulfur dioxide and daily mortality in 17 Chinese cities: the China air pollution and health effects study (CAPES) Environ Res. 2012;118:65–71. doi: 10.1016/j.envres.2012.07.003. https://doi.org/10.1016/j.envres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Clark NA, Demers PA, Karr CJ, et al. Effect of early life exposure to air-pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–90. doi: 10.1289/ehp.0900916. https://doi.org/10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen TM, Gokhale J, Shofer S, et al. Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci. 2007;333:249–56. doi: 10.1097/MAJ.0b013e31803b900f. https://doi.org/10.1097/MAJ.0b013e31803b8e8c. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, Zhang Z, Wang Z, et al. Asthmatic symptoms among pupils in relation to winter indoor and outdoor air pollution in schools in Taiyuan, China. Environ Health Perspect. 2008;116:90–7. doi: 10.1289/ehp.10576. https://doi.org/10.1289/ehp.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulet LP, FitzGerald JM, Reddel HK. The revised 2014 GINA strategy report: opportunities for change. Curr Opin Pulm Med. 2015;21:1–7. doi: 10.1097/MCP.0000000000000125. https://doi.org/10.1097/MCP.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 22.Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186:975–81. doi: 10.1164/rccm.201207-1299OC. https://doi.org/10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 23.Watase H, Hagiwara Y, Chiba T, et al. Japanese Emergency Medicine Network Investigators. Multicentre observational study of adults with asthma exacerbations: who are the frequent users of theemergency department in Japan? BMJ Open. 2015;5:e007435. doi: 10.1136/bmjopen-2014-007435. https://doi.org/10.1136/bmjopen-2014-007435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow L, Parulekar AD, Hanania NA. Hospital management of acute exacerbations of chronic obstructive pulmonary disease. J Hosp Med. 2015;10:328–39. doi: 10.1002/jhm.2334. https://doi.org/10.1002/jhm.2334. [DOI] [PubMed] [Google Scholar]
- 25.Koç Y, Karagöz N, Seven AS. Hava kirliliğinin Sivas Göğüs Hastalıkları Hastanesine yatışlar üzerine etkisi. J Kartal TR. 2002;13:75–8. [Google Scholar]
- 26.Dockery DW, Rich DQ, Goodman PG, et al. Effect of air pollution control on mortality and hospital admissions in Ireland. Res Rep Health Eff Inst. 2013;176:109. [PubMed] [Google Scholar]
- 27.Guillén Pérez JJ, Guillén Grima F, Medrano Tortosa J, et al. Unusual attendance at Hospital Emergency Services for asthma and chronic obstructive pulmonary disease andSO2 air pollution in Cartagena (Spain) Rev Esp Salud Publica. 1995;69:305–14. [PubMed] [Google Scholar]
- 28.Rumana HS, Sharma RC, Beniwal V, et al. A retrospective approach to assess human health risks associated with growing air pollution in urbanized area ofThar Desert, western Rajasthan, India. J Environ Health Sci Eng. 2014;12:23. doi: 10.1186/2052-336X-12-23. https://doi.org/10.1186/2052-336X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayram H, Dörtbudak Z, Fişekçi FE, et al. Hava Kirliliğinin İnsan Sağlığına Etkileri, Dünyada, Ülkemizde ve Bölgemizde Hava Kirliliği Sorunu” Paneli Ardından. Tıp Dergisi. 2006;33:105–12. [Google Scholar]
- 30.Çay Y, Yıldız A. Fosil Kaynaklı Yakıtların Neden Olduğu Hava Kirliliğinin Doğal Gaz Kullanımı İle Değişimi, Van İli Örneği. Makine Teknolojileri Elektronik Dergisi. 2011;8:45–52. [Google Scholar]
- 31.Sunyer J, Anto JM, Murillo C, et al. Effects of urban air pollution on emergency room admissions for chronic obstructive pulmonary disease. Am J Epidemiol. 1991;134:277–86. doi: 10.1093/oxfordjournals.aje.a116081. https://doi.org/10.1093/oxfordjournals.aje.a116083. [DOI] [PubMed] [Google Scholar]
- 32.Health effects of outdoor air pollution. Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. Am J Respir Crit Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.1.8542133. https://doi.org/10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz J. Air pollution and hospital admissions for the elderly in Birmingham, Alabama. Am J Epidemiol. 1994;139:589–98. doi: 10.1093/oxfordjournals.aje.a117048. https://doi.org/10.1093/oxfordjournals.aje.a117048. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz J. Air pollution end hospital admissions for the elderly in Detroit, Michigan. Am J Resp Crit Care Med. 1994;150:648–55. doi: 10.1164/ajrccm.150.3.8087333. https://doi.org/10.1164/ajrccm.150.3.8087333. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz J. PM10, ozone and hospital admissions for the elderly in Minneapolis-St Paul, Minnesota. Arch Environ Health. 1994;49:366–74. doi: 10.1080/00039896.1994.9954989. https://doi.org/10.1080/00039896.1994.9954989. [DOI] [PubMed] [Google Scholar]
- 36.Burnen RT, Dales R, Kreuski D, et al. Associations between ambient particulate sulfate and admissions to Ontario hospitals for cardiac and respiratory diseases. Am J Epidemiol. 1995;142:15–22. doi: 10.1093/oxfordjournals.aje.a117540. https://doi.org/10.1093/oxfordjournals.aje.a117540. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz J. Air pollution and hospital admissions for respiratory disease. Epidemiology. 1996;7:20–8. doi: 10.1097/00001648-199601000-00005. https://doi.org/10.1097/00001648-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Stieb DM, Szyszkowicz M, Rowe BH, et al. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-seriesanalysis. Environ Health. 2009;8:25. doi: 10.1186/1476-069X-8-25. https://doi.org/10.1186/1476-069X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canova C, Dunster C, Kelly FJ, et al. PM10-induced hospital admissions for asthma and chronic obstructive pulmonary disease: the modifying effectof individual characteristics. Epidemiology. 2012;23:607–15. doi: 10.1097/EDE.0b013e3182572563. https://doi.org/10.1097/EDE.0b013e3182572563. [DOI] [PubMed] [Google Scholar]
- 40.Ozcan NS, Cubukcu KM. Evaluation of Air Pollution Effects on Asthma Disease: The case of Izmir. Procedia - Social and Behavioral Sciences. 2015;202:448–55. https://doi.org/10.1016/j.sbspro.2015.08.201. [Google Scholar]
- 41.Hajat S, Haines A, Goubet SA, et al. Association of air pollution with daily GP consultations for asthma and other lower respiratory conditions in London. Thorax. 1999;54:597–605. doi: 10.1136/thx.54.7.597. https://doi.org/10.1136/thx.54.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoda Y, Otani N, Sakurai S, et al. Acute effects of summer air pollution on pulmonary function and airway inflammation in healthy young women. J Epidemiol. 2014;24:312–20. doi: 10.2188/jea.JE20130155. https://doi.org/10.2188/jea.JE20130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner JG, Morishita M, Keeler GJ, et al. Divergent effects of urban particulate air pollution on allergic airway responses in experimental asthma: acomparison of field exposure studies. Environ Health. 2012;11:45. doi: 10.1186/1476-069X-11-45. https://doi.org/10.1186/1476-069X-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colais P, Serinelli M, Faustini A, et al. Air pollution and urgent hospital admissions in nine Italian cities. Results of the EpiAir Project. Epidemiol Prev. 2009;33(6 Suppl 1):77–94. [PubMed] [Google Scholar]
- 45.Chauhan AJ, Krishna MT, Frew AJ, et al. Exposure to nitrogen dioxide (NO2) and respiratory disease risk. Rev Environ Health. 1998;13:73–90. [PubMed] [Google Scholar]
- 46.Becker S, Soukup JM. Exposure to urban air particulates alters the macrophage-mediated inflammatory response to respiratory viral infection. J Toxicol Environ Health. 1999;13:445–57. doi: 10.1080/009841099157539. [DOI] [PubMed] [Google Scholar]