Abstract
Background
Polycystic ovary syndrome (PCOS) is the commonest endocrine disorder at a reproductive age. It is associated with a high risk of metabolic syndrome (MS) and cardiovascular diseases (CVD).
Objective
To measure the prevalence of MS in women with PCOS and to assess the global cardiovascular risk (CVR) among them.
Methods
This cross-sectional study was conducted at King Khalid Hospital, Tabuk, Saudi Arabia during the period from February through December 2014. A total of 404 infertile women were randomly selected, and checked for diagnosing PCOS, MS and estimated CVD probability. Data were analyzed by IBM-SPSS version 22, using independent-samples t-test, Chi-square, and conditional logistic regression. A p-value of <0.05 was considered significant.
Results
MS was diagnosed in 58% and 32% of women with and without PCOS respectively (p<0.00). Results showed a statistically significant association between the two syndromes. Patients with the two syndromes showed high averages of clinical and biochemical values (p<0.00), high rate of predicted CVR, a high percentage of clustering of MS factors, and that weight-waist circumference - HDL are predictive for the occurrence of MS.
Conclusion
PCOS is associated with the risk of development of MS, and CVD. Screening for early detection of PCOS and MS and the application of cohort studies are recommended to better explore the role of PCOS in the development of CVD and to assess the significance of interventions.
Keywords: Polycystic ovary syndrome, Metabolic syndrome, Cardiovascular disease
1. Introduction
Polycystic ovary syndrome (PCOS) affects 1 in 15 women worldwide. It is a heterogeneous clinical condition, and is the commonest endocrine disorder at reproductive age, varying from 6–10%, according to diagnosing criteria (1–3). In Saudi Arabia, the prevalence of PCOS is still unknown, but it is believed that it might be similar to that of other reports (4). Along with classic chronic anovulation and hyperandrogenism, PCOS also leads to a higher risk of cardiovascular problems associated with insulin resistance such as type 2 diabetes mellitus, systemic arterial hypertension, glucose intolerance, obesity, dyslipidemia, and metabolic syndrome (MS) (5, 6). Metabolic syndrome is another cluster of endocrine disturbances, including insulin resistance, dyslipidemia, obesity, and hypertension. Individuals with MS often have the phenomena of clustering of these major cardiovascular risks (CVR) factors, which dramatically increases the risk for development of cardiovascular disease (CVD) (7). In Saudi Arabia, the prevalence of MS in women with PCOS was estimated to be 52% (8). Clinical trials and epidemiologic studies support the necessity to detect PCOS in women to determine their risk of cardiometabolic disorders in order to prevent and treat its serious consequences (9). Many studies have underlined the connection between PCOS and the metabolic/CVR profile of such female patients, but results of these studies are controversial regarding whether the development of MS in these patients was dependent or independent on abdominal obesity (10–12). Stepto et al. indicate that the adverse impact of obesity was more in PCOS. Overweight women had similar insulin resistance to slim women with PCOS, so women with PCOS are effectively metabolically equal to obese, non-PCOS women (12). Investigating the clinical and biomedical profile in Saudi Arabian women with PCOS will allow for better understanding of changes in their metabolic parameters and will help to determine the most important risk factors that would predict for MS and CVR in these patients. Previous studies have indicated that individuals with MS encompass a broad range of CVD risk levels (13). Assessment of the global risk of CVD among women with MS associated PCOS who showed different clustering of MS criteria will better characterize the diversity in their CVD risk and will help to most appropriately target the interventions for prevention of CVD. Little information is available, to our knowledge, on the degree at which CVR factors in patients with MS associated PCOS meet the recommended levels on the basis of evidence-based recommendations. Biochemical, morphological and functional markers of early CVD in PCOS are well-established to identify cardiovascular morbidity (14). Such information may be of use to clinicians in deciding how they should approach risk assessment in women with MS associated PCOS, as well as how aggressively to treat it. Thus, we conducted this survey to determine the prevalence of MS among patients with PCOS, investigating the metabolic profile and CVR in women with PCOS and assessing the degree at which CVR factors in patients with MS associated PCOS meet the recommended levels.
2. Material and Methods
This cross-sectional study was conducted in the infertility clinic of King Khalid Hospital from February 2014 through December 2014. Infertile women aged 15–45 years who agreed to participate in our study after obtaining an oral consent were recruited. Four hundred and four infertile women were randomly selected assuming that the prevalence of metabolic syndrome is 50% probability. The sample size was calculated using the formula n=z2pq/d2 where z=95% confidence (1.96), p=prevalence of metabolic syndrome in KSA, q=100-prevalence, and d=tolerated error. Women previously diagnosed with hypertension or diabetes mellitus, women under treatment with Metformin (a drug that could alter the metabolic parameters in women with PCOS) or under any hormonal treatment were excluded. Participating women were investigated for clinically diagnosed PCOS using Rotterdam consensus (RC) (15) as a first step. Menstrual history, clinical examination, transvaginal ultrasound examination, and biochemical blood analysis were performed (blood samples were taken after 12-hour overnight fast), and women with/without PCOS were further investigated for MS using the International Diabetes Federation criteria (16). The ten-year probability of developing hard coronary heart disease outcomes (myocardial infarction and coronary death) were estimated on the basis of the Framingham risk score (FRS) (17) for all participants. Blood pressure was measured using a mercury sphygmomanometer after a 10-minute rest period in a supine position; two separate measurements were performed with a 5-minute interval. Blood samples were taken after a 12-hour overnight fast. Plasma glucose, total serum cholesterol (TC), HDL, and serum TG were determined by spectrophotometry according to standard colorimetric methods (18). Low-density lipoprotein cholesterol (LDL) value was calculated with the Friedewald equation (19). On the basis of the Framingham risk algorithm, and classified according to the American Heart Association (AHA)/National Heart, Lung, and Blood Institute (NHLBI) scientific statement on MS (20), patients were diagnosed to have low (<6%), moderate (6 to <10%), moderately high (10–20%), or high (>20%) 10-year probability for coronary heart diseases. Cardiovascular risks were stratified according to different combinations of MS criteria. Subjects with measurements that are not at the recommended levels for HDL, TG, systolic/diastolic blood pressure, fasting plasma glucose, and LDL were identified, and mean distance to recommend level was calculated. The distance from goal was determined as the difference between the actual level and recommended goal. For people with diabetes, goals for blood pressure are <130/80 mmHg, and for nondiabetics, <140/90 mmHg. The LDL cholesterol goal for those with a low risk is <4.12mmol/l, for those with a moderate to moderately high risk (6–20%) is <3.37mmol/l, and for those with a high risk (>20%, diabetes, or CVD) is <2.59 mmol/l. The goal for FG is <5.5 mmol/l, for HDL cholesterol is 1–1.5 mmol/l, and for TG is <1.7mmol/l, on the basis of revised AHA/NHLBI MS recommendations (21). The IBM© SPSS© Statistics version 22 (IBM© Corp., Armonk, NY, USA) was used for data analysis. Means ±SD, frequencies and percentages were used in the descriptive analysis. The independent-samples t-test and Chi-square were used to test for the statistical significance of differences between quantitative and qualitative data respectively. Backward Logistic regression analysis was performed to determine the factors predicting for the occurrence of MS in the studied population.
3. Results
Of the total number of 404 infertile women, 76 were diagnosed to have PCOS (18.8%). It was found that 12 women in the PCOS group and 44 in the NO PCOS group had diabetes (15.8%, 13.4% respectively). From the total number of 404 infertile women, 148 were diagnosed to have MS (36.6%). Results revealed that 44 from the total number of 76 PCOS women were diagnosed to have MS (57.9%), From the total number of 328 NO PCOS women, 104 were diagnosed to have MS (31.7%), and this difference was found to be statistically significant (p<0.00) (Table 1). In the youngest age category, and in the PCOS group, MS was present in only 25% of the women in this age. In the middle age group, women who have MS are two times more than those who do not. In the older age category, the women who have MS are four times more than those who do not. There was no statistically significant association between the presence of PCOS and the development of MS (p>0.05) in the youngest age category. In the oldest age categories, there was a statistically significant association between PCOS and the development of MS (p<0.05) (Table 1). In all, 68% of patients who have PCOS were obese compared to 56.8 in NO PCOS patients, and this difference was found to be not statistically significant (p>0.05). In the BMI categories below 30, there was no statistically significant association between the presence of PCOS and the development of MS (p>0.05). In the BMI category ≥ 30, 85% of PCOS women have MS compared to 47% in the NO PCOS group and this difference was found to be statistically significant (Table 2).
Table 1.
Distribution of MS among different age groups for women with and without PCOS.
| Age (years) | MS | PCOS (n=76); n (%) | NO PCOS (n=328); n (%) | Total (n=404); n (%) | p-value |
|---|---|---|---|---|---|
| 15–24 | Have MS | 4 (5.9) | 20 (29.4) | 24 (35.3) | 0.075ns |
| Don’t have MS | 16 (23.5) | 28 (41.2) | 44 (64.7) | ||
| 25–34 | Have MS | 24 (10.9) | 44 (20) | 68 (30.9) | 0.000* |
| Don’t have MS | 12 (5.5) | 140 (63.6) | 152 (69.1) | ||
| 35–45 | Have MS | 16 (13.8) | 40 (34.5) | 56 (48.3) | 0.002* |
| Don’t have MS | 4 (3.4) | 56 (48.3) | 60 (51.7) |
, no statistical significant dependence relation between PCOS groups and MS groups at the 0.05 level.
Statistical significant dependence relationship between PCOS groups and MS groups at the 0.01 level.
Table 2.
Distribution of MS among studied women with different categories of BMI who have and do not have PCOS.
| BMI (kg/m2) | MS | PCOS (n=76); n (%) | NO PCOS (n=328); n (%) | Total (n=404); n (%) | p-value |
|---|---|---|---|---|---|
| <18.5 | Have MS | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | a |
| Don’t have MS | 0.0 (0.0) | 8 (100) | 8 (100) | ||
| 18.5–24.9 | Have MS | 0.0 (0.0) | 4 (5.6) | 4 (6.6%) | 0.263ns |
| Don’t have MS | 20 (27.8) | 48 (66.7) | 68 (94.4) | ||
| 25–29.9 | Have MS | 0.0 (0.0) | 8 (10.5) | 8 (10.5) | 0.635ns |
| Don’t have MS | 4 (5.3) | 64 (84.2) | 68 (89.5) | ||
| ≥30 | Have MS | 44 (17.7) | 92 (37.1) | 136 (54.8) | 0.000* |
| Don’t have MS | 8 (3.2) | 104 (41.9) | 112 (45.2) |
No statistics are computed because MS is constant.
, no statistical significant dependence relation between PCOS groups and MS groups at the 0.05 level.
Statistical significant dependence relation between PCOS groups and MS groups at the 0.01 level.
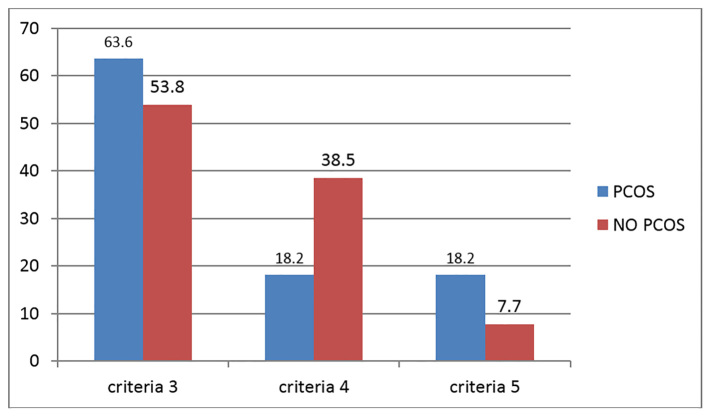
All clinical and biochemical factors in patients with MS showed statistically significant higher mean values (HDL showed significantly lower mean value) in the PCOS group compared with the NO PCOS group, except for systolic BP (Table 3). All risk factors showed a statistically significant higher mean distance to recommended levels in patients with PCOS compared to the NO PCOS group except for blood pressure and FBG (Table 4). Examined cardio-metabolic risk factors in the NO PCOS group model of backward logistic regression analysis did not show any predictive factor. In the PCOS group model, the predictive variables for the development of MS were: increased weight, large waist circumference, low HDL (Tables 5, 6). Results also revealed that 10% from a total number of NO PCOS, and 23% from a total number of PCOS patients who have MS showed a moderately high risk of developing CVD. All PCOS without MS and all NO PCOS patients showed a low risk of developing CVD. Furthermore, 63% of PCOS and 53.8% of NO PCOS women with MS have clustering of 3 metabolic risk factors. More women in PCOS with MS group have clustering of 5 criteria of MS compared with the NO PCOS with MS group (18.2% and 7.7% respectively), and the difference was found to be statistically significant (p<0.05) (Figure I).
Table 3.
Clinical and biochemical factors associated with the presence of MS in women with and without PCOS.
| Clinical & biochemical risk factors | Women with MS (n=148) | p-value | |
|---|---|---|---|
| In PCOS (n=44); Mean (±SD) | In NO PCOS (n=104); Mean (±SD) | ||
| Weight/kg | 91.6 (13.1) | 83.8 (9.6) | 0.000** |
| Waist circumference | 102.2 (12.8) | 96.5 (9.6) | 0.003** |
| BMI (kg/m2) | 37.5 (5.8) | 35.0 (4.2) | 0.004** |
| HDL (mmol) | 1.0 (0.2) | 1.1 (0.1) | 0.000** |
| LDL (mmol) | 3.7 (1.2) | 2.9 (0.8) | 0.000** |
| TC (mmol) | 5.5 (1.2) | 4.7 (0.8) | 0.000** |
| TG (mmol) | 1.8 (0.3) | 1.4 (0.6) | 0.000** |
| FBS (mmol) | 6.7 (2.7) | 5.9 (1.2) | 0.039* |
| Systolic BP | 131.6 (11.3) | 134.7 (16.5) | 0.270ns |
| Diastolic BP | 82.3 (10.1) | 78.5 (7.6) | 0.024* |
No statistically significant difference between PCOS groups at the 0.05 level,
Statistical significant difference between PCOS groups at the 0.05 level.
Statistical significant difference between PCOS groups at the 0.01 level.
Table 4.
Mean distance to recommended levels of cardio-metabolic risk factors among women with MS.
| Clinical & biochemical risk factors | Women with MS (n=148) | p-value | |
|---|---|---|---|
| In PCOS (n=44); Mean (±SD) | In NO PCOS (n=104); Mean (±SD) | ||
| Waist circumference | 22.18 (3.77) | 16.46 (2.58) | 0.000** |
| HDL (mmol) | 0.65 (0.15) | 0.43 (0.12) | 0.000** |
| LDL (mmol) | 1.40 (0.19) | 0.70 (0.1) | 0.000** |
| TC (mmol) | 1.10 (0.14) | 0.74 (0.22) | 0.000** |
| TG (mmol) | 0.56 (0.11) | 0.45 (0.05) | 0.000* |
| FBS (mmol) | 1.45 (0.32) | 1.5 (0.22) | 0.762ns |
| Systolic BP (mmhg) | 13.42 (2.53) | 9.27 (2.58) | 0.000* |
| Diastolic BP (mmhg) | 7.62 (1.07) | 7.45 (1.67) | 0.534ns |
No statistically significant difference between PCOS groups at the 0.05 level.
Statistical significant difference between PCOS groups at the 0.01level.
Table 5.
Factors predicting the development of MS in No PCOS women as detected by backward logistic regression
| No PCOS MODEL | B | S.E. | Wald | p-value |
|---|---|---|---|---|
| Weight (kg) | −6.449 | 205.451 | 0.001 | 0.975 ns |
| HDL (mmol) | 1057.530 | 32942.823 | 0.001 | 0.974 ns |
| Constant | −734.548 | 23447.698 | 0.001 | 0.975 ns |
No statistical significant indicators of MS at the 0.05 level.
Table 6.
EXP (B) Factors predicting the development of MS in PCOS women as detected by backward logistic regression
| PCOS MODEL | B | S.E. | Wald | p-value |
|---|---|---|---|---|
| Weight (kg) | −0.033 | 0.017 | 3.871 | 0.049* |
| WC | −0.058 | 0.018 | 10.161 | 0.001** |
| HDL (mmol) | 6.713 | 0.971 | 47.833 | 0.000** |
| Constant | 11.795 | 2.768 | 18.164 | 0.000** |
statistical significant indicators of MS at the 0.05 level.
statistical significant indicators of MS at the 0.01 level.
Figure 1.
Clustering of cardio-metabolic risk factors in PCOS and NO PCOS women who have MS
4. Discussion
Polycystic ovary syndrome is the most common endocrine disorder in reproductive age women. It affects approximately 7–12% of the population worldwide (22). In our study, by using RC for diagnosing PCOS, the prevalence of PCOS in the examined infertile women was found to be 18.8%. This higher PCOS prevalence compared with the previously estimated one could be due to the higher sensitivity of RC diagnostic criteria used nowadays in the diagnosis of this disease. A community-based study in Australia conducted in 2010 using the RC, showed the prevalence of PCOS to be 18% (17.8 ± 2.8%) which is in agreement with the result of our study (2). Patients with PCOS showed high obesity prevalence compared to that in the NO PCOS group (68% and 58% respectively). This was in agreement with a previous study which stated that obesity is a prevalent characteristic of PCOS, and ranges from 12.5% to 100% (23–25). In our study, the prevalence of MS in PCOS women was found to be more than the NO PCOS group with statistical significance in difference. Previous studies showed a higher prevalence of MS in PCOS compared to the NO PCOS group, with a marked variation in MS prevalence in PCOS women of different countries and ethnic groups. Authors have explained this variation to be due to differences in diet, lifestyle and genetic factors (6). In the current study, the prevalence of MS increased with age, and this increase was marked in the PCOS group compared to the NO PCOS, with statistical significance in difference for the occurrence of MS between PCOS and NO PCOS in the categories of women with older age. This was in agreement with a previous study which revealed that the risk of MS increased with age (26). In categories of BMI, the prevalence of MS showed a marked increase in the obese category in both PCOS (85%) and NO PCOS (47%) groups. This was in agreement with a previous study which revealed that the risk of MS increased with age and in the obesity category (BMI ≥30 kg/m2) (27). Proper investigation of the role of PCOS in developing MS requires proper management of the effect of confounding factors associated with PCOS (age and BMI). Managing the role of these factors by using longitudinal study procedure, with stratification of the target sample and/or by recruiting age and BMI matched controls, will better allow for understanding the association between MS and PCOS independently on age and BMI. In the current study, all clinical and cardio-metabolic factors in patients with MS showed statistically significant higher mean values (HDL showed a significantly lower mean value) in the PCOS group compared with the NO PCOS group, except for systolic BP blood pressure. Also, these cardio-metabolic factors showed a statistically significant higher mean distance to recommended levels compared to the NO PCOS group, except for blood pressure and FBG. This was in agreement with other studies which showed that women with PCOS have higher levels of triglycerides (TG), LDL cholesterol and total cholesterol (TC), and lower HDL cholesterol levels compared with control women (28, 29). Authors of related studies explained the unusual biochemical features of women with PCOS to be due to insulin resistance, which may be associated with the pathogenesis of the disease (30). In a retrospective cohort study, authors found that relative to women without PCOS, women with PCOS had a higher BMI, but were not significantly different in total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, or fasting blood glucose measurements (5, 31). Studying the insulin resistance state of both PCOS and NO PCOS group together with studying the clinical and biochemical characteristics in prospective studies will enable us to prove the association between PCOS and the consequent development of clinical and biochemical changes. The inability of our study to find an association between hypertension and the presence of PCOS was in agreement with results of a previous study which failed to show any association of hypertension, whether it is systolic or diastolic, during sitting or lying down between the two groups of women (31). This was in contrast to three previously conducted studies in women with and without PCOS which showed (the first was a cross sectional-study, the second was a case-control study, and the third was a review study) an elevation of blood pressure in women with PCOS (32–34), which indicates the association between hypertension and this endocrine disease. Conducting longitudinal studies in women with and without PCOS will solve this controversy by enabling us to better understand the effects of PCOS on the development of hypertension. The higher mean distance of cardio-metabolic risk factors to recommended levels in the PCOS group indicated the importance of screening using these elements for identifying women with this syndrome, for applying the proper intervention to prevent the development of MS and CVD. The higher mean difference for FBG in the NO PCOS group could be due to the greater number of diabetic cases presented in this group. In our study, weight, waist circumference, and the HDL level were found to be the predictors for the development of metabolic syndrome in the PCOS group. A study conducted for the same purpose found that BMI was one of the predictor variables for the development of MS in PCOS patients (35). Applying screening measures for women with PCOS using these simple indicators will allow for the early identification of PCOS women who have a higher risk for the development of MS and a consequently higher risk of CVD compared to those who have PCOS but with normal levels of these indicators. The risk for CVD was low as detected by the FRS, in all PCOS without MS and in all NO PCOS patients. Only 23% of patients with PCOS who have MS was shown to have a moderately high risk for the development of CVD compared to 0% in the NO PCOS with MS group. The low CVR as detected by the FRS, despite the high biochemical factors in PCOS women with MS, could be due to the relatively young age of the participated women. In the FRS model, age has the most heavily weighted variable as derived from populations that span the adult age spectrum, in younger adults (men <45 years of age and women <65 years of age), which makes modest elevations in risk factors to have little effect on 10-year risk (36, 37). Even younger adults with substantial risk factor burden (same as in our study) may still have 10-year risk estimates well below 10%, although their remaining lifetime risks may exceed 50% on the basis of these risk factors (38). The higher CVR in patients with PCOS and MS compared to their controls indicated the effects the disease imposes on the heart, and supports previous studies which stated that PCOS patients clearly present a higher risk of CVD which is linked to metabolic dysfunction due to its peculiar hormonal pattern, which is characterized by hyperandrogenism, insulin resistance, dyslipidemia, and inflammatory state (10). Follow-up studies aiming to determine the absolute risk for the development of cardiovascular events in patients with and without PCOS who have MS, could better identify the cardiovascular burden of this disease. More women in the PCOS group have clustering of 5 criteria of metabolic syndrome compared with the NO PCOS group. This supports a previous study which states that PCOS is also associated with a clustering of CVR factors (39). In fact, more rigorous cohort studies of long-term cardiovascular outcomes and clinical trials of risk factor modification are required for women with PCOS, to correctly determine the role of PCOS in the development of CVD and to assess the significance of interventions.
5. Conclusions
PCOS is associated with the risk of development of metabolic syndrome (MS) which is more prevalent in the categories of old age and high BMI. Patients with PCOS and MS have more clustering of metabolic factors and have a higher risk for the development of CVD compared to their counterparts. Screening for detecting PCOS in women, and applying rigorous cohort studies are required to properly determine the role of PCOS in the development of CVD and to assess the significance of risk factor modification interventions.
Acknowledgments
The research was self-funded by the authors, and not supported by any institute or organization. Grateful acknowledgement to Dr. Yassin Ibrahim who conducted statistical analyses of the manuscript.
Footnotes
iThenticate screening: October 02, 2017, English editing: October 12, 2017, Quality control: October 15, 2017
This article has been reviewed / commented by Three experts
Conflict of Interest:
There is no conflict of interest to be declared.
Authors’ contributions:
All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 2.March WA, Moore VM, Wilson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 3.Marcondes JAM, Barcellos CRG, Rocha MP. Dificuldades e armadilhas no diagnostico da syndrome dos ovlriospolicysticos. Arq Bras Endocrinol Metab. 2011;55(1):6–15. doi: 10.1590/S0004-27302011000100002. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ruhaily AD, Malabu UH, Sulimani RA. Hirsutism in Saudi females of reproductive age: a hospital-based study. Ann Saudi Med. 2008;28(1):28–32. doi: 10.4103/0256-4947.51762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iftikhar S, Collazo-Clavell ML, Roger VL, St Sauver J, Brown RD, Jr, Cha S, et al. Risk of cardiovascular events in patients with polycystic ovary syndrome. Neth J Med. 2012;70(2):74–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose intolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–63. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 7.Malik S, Wong ND. Metabolic syndrome, cardiovascular risk and screening for subclinical atherosclerosis. Expert Rev Cardiovasc Ther. 2009;7(3):273–80. doi: 10.1586/14779072.7.3.273. [DOI] [PubMed] [Google Scholar]
- 8.Rouzi A, Ardawi M. Prevalence of the metabolic syndrome in Saudi women ad its components with polycystic ovary syndrome. 8th European Congress on Menopause (EMAS) Maturitas. 2009;63(Supplement 1):S1–136. [Google Scholar]
- 9.Cobin RH. Cardiovascular and metabolic risks associated with PCOS. Intern Emerg Med. 2013;8( Suppl 1):S61–4. doi: 10.1007/s11739-013-0924-z. [DOI] [PubMed] [Google Scholar]
- 10.Scicchitano P, Dentamaro I, Carbonara R, Bulzis G, Dachille A, Caputo P, et al. Cardiovascular Risk in Women with PCOS. Int J EndocrinolMetab. 2012;10(4):611–8. doi: 10.5812/ijem.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.do Oliveira RS, Redorat RG, Ziehe GH, Mansur VA, Conceição FL. Arterial hypertension and metabolic profile in patients with polycystic ovary syndrome. Rev Bras Ginecol Obstet. 2013;35(1):21–6. doi: 10.1590/S0100-72032013000100005. [DOI] [PubMed] [Google Scholar]
- 12.Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28:777–84. doi: 10.1093/humrep/des463. [DOI] [PubMed] [Google Scholar]
- 13.Wong ND, Pio JR, Franklin SS, L’Italien GJ, Kamath TV, Williams GR. Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am J Cardiol. 2003;91:1421–26. doi: 10.1016/S0002-9149(03)00392-8. [DOI] [PubMed] [Google Scholar]
- 14.Cussons AJ, Stuckey BGA, Watts GF. Cardiovascular disease in the polycystic ovary syndrome. new insights and perspectives. Atherosclerosis. 2006;185:227–39. doi: 10.1016/j.atherosclerosis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition: a consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 17.Risk Scoring Systems. 2013. Available from: http://www.framinghamheartstudy.org/
- 18.Onaka L. Clinical Chemistry Concepts and Applications. London: W.B. Saunders; 1993. Lipids. [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 22.Panidis D, Farmakiotis D, Rousso D, Katsikis I, Kourtis A, Diamanti-Kandarakis E. Serum luteinizinghormone levels are markedly increased and significantly correlated with D4-androstenedione levels in lean women with polycystic ovary syndrome. Fertil Steril. 2005;84:538–40. doi: 10.1016/j.fertnstert.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Spritzer PM. Polycystic ovary syndrome: reviewing diagnosis and management of metabolic disturbances. Arq Bras Endocrinol Metabol. 2014;58(2):182–7. doi: 10.1590/0004-2730000003051. [DOI] [PubMed] [Google Scholar]
- 24.de Vries L, Karasik A, Landau Z, Phillip M, Kiviti S, Goldberg-Stern H. Endocrine effects of valproate in adolescent girls with epilepsy. Epilepsia. 2007;48:470–7. doi: 10.1111/j.1528-1167.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- 25.Peppard HR, Marfori J, Iuorno MJ, Nestler JE. Prevalence of polycystic ovary syndrome among premenopausal women with type 2 diabetes. Diabetes Care. 2001;24:1050–2. doi: 10.2337/diacare.24.6.1050. [DOI] [PubMed] [Google Scholar]
- 26.Moini A, Javanmard F, Eslami B, Aletaha N. Prevalence of metabolic syndrome in polycystic ovarian syndrome women in a hospital of Tehran. Iran J Reprod Med. 2012;10(2):127–30. [PMC free article] [PubMed] [Google Scholar]
- 27.Paul S, Smith L. The metabolic syndrome in women: a growing problem for cardiac risk. J Cardiovasc Nurs. 2005;20(6):427–32. doi: 10.1097/00005082-200511000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95:1073–9. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Luo X, Xu L. Association of fat distribution with metabolic syndrome in patients with polycystic ovary syndrome. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1325–7. [PubMed] [Google Scholar]
- 30.Hudecova M, Jan H, Christian B, PoromaaInger S. Longterm Reproductive and Metabolic Consequences of PCOS. Curr Diabetes Rev. 2012;8:444–51. doi: 10.2174/157339912803529913. [DOI] [PubMed] [Google Scholar]
- 31.Al Mulhim AA, Abul Heija AA, Al Talib AA, Al Turki HA, Gasim TG. Hormonal, Metabolic and Clinical Profile of Saudi Women with Polycystic Ovary Syndrome. Saudi Journal of Medicine & Medical Sciences. 2013;1(1):30–34. doi: 10.4103/1658-631X.112920. [DOI] [Google Scholar]
- 32.Zachurzok Buczynska A, Szydlowski L, Gawlik A, Wilk K, Malecka-Tendera E. Blood pressure regulation and resting heart rate abnormalities in adolescent girls with polycystic ovary syndrome. Fertil Steril. 2011;96:1519–23. doi: 10.1016/j.fertnstert.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 33.Azevedo MF, Costa EC, Oliveira AI, Silva IB, Marinho JC, Rodrigues JA, et al. Elevated blood pressure in women with polycystic ovary syndrome: Prevalence and associated risk factors. Rev Bras Gynecol Obstet. 2011;33:31–6. [PubMed] [Google Scholar]
- 34.Martins WdP, Soares GM, Vieira CS, Reis RMd, Sá MFSd, Ferriani RA. Radiographic Assessment of Maxillary Sinus Lateral Wall Thickness in Edentulous Posterior Maxilla. Revista Brasileira de Ginecologia e Obstetrícia. 2009;31:111–6. [Google Scholar]
- 35.Li R, Yu G, Yang D, Li S, Lu S, Wu X, et al. Prevalence and predictors of metabolic abnormalities in Chinese women with PCOS: a cross- sectional study. BMC Endocr Disord. 2014;14:76. doi: 10.1186/1472-6823-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavanaugh-Hussey MW, Berry JD, Lloyd-Jones DM. Who exceeds ATP-III risk thresholds? Systematic examination of the effect of varying age and risk factor levels in the ATP-III risk assessment tool. Prev Med. 2008;47:619–23. doi: 10.1016/j.ypmed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham Heart Study general cardiovascular risk profile. Circulation. 2009;120:384–90. doi: 10.1161/CIRCULATIONAHA.108.835470. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–8. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 39.Di Domenico K, Wiltgen D, Nickel FJ, Magalhães JA, Moraes RS, Spritzer PM. Cardiac autonomic modulation in polycystic ovary syndrome: does the phenotype matter? Fertil Steril. 2013;99(1):286–92. doi: 10.1016/j.fertnstert.2012.08.049. [DOI] [PubMed] [Google Scholar]