Abstract
Mucor is a ubiquitous fungus that belongs to the family of Zygomycetes, though a noninvasive saprophyte in the normal host, it can cause life threatening infections in immunocompromised patients, including angioinvasive pulmonary mucormycosis; a disease notorious for its high mortality. This article tracks the ever-changing management of pulmonary mucormycosis over the last 130 years, and how this affected mortality.
Keywords: Pulmonary mucormycosis, posaconazole, fungal infection
INTRODUCTION
Pulmonary mucormycosis is a rare but serious fungal infection that usually occurs in the presence of a defective immune system. Pulmonary mucormycosis was first described in 1876 by Furbringer [1]. Since then only a few hundred cases have been reported.
In a classic review in 1955, Baker [2] thoroughly described all mucormycosis cases previously reported; it included six cases in the old German literature and 10 cases in the American literature. He considered mucormycosis to be a new disease in the USA and attributed its increasing incidence to the amplified use of antibiotics, cortisone, and Adrenocorticotropic hormone ACTH. The respiratory tract was recognized to be the portal of entry for mucorales, and these fungi can easily invade arteries, veins, and lymphatics and produce thrombosis and infarction. In the first half of the twentieth century, no antifungal therapy was available except for potassium iodide. In 1971, Baker [3] further expanded his review to include 49 cases of mucormycosis (including 39 pulmonary cases), which were reported in the literature till that time.
The review by Tedder et al. [4] in 1994 included 30 patients who were treated at their institution and 225 cases reported in the literature; some of their patients had disseminated mucormycosis. Of the 92 patients diagnosed antemortem, 61% underwent medical treatment with antifungal agents, 21% were surgically treated, and 18% underwent combined medical and surgical therapy.
Francis et al.[5] described 87 cases of pulmonary mucormycosis reported in the literature from 1970 to 2000 after introducing flexible bronchoscopy. In his review, 55 patients underwent antifungal therapy, in which most received amphotericin B and only seven received an Azole. The overall survival rate was 44% and was higher in patients who underwent combined medical and surgical therapy.
In this study, we describe a patient with diabetic ketosis who presented subacutely with semi-invasive pulmonary mucormycosis infection and review 22 other cases of pulmonary mucormycosis treated with posaconazole reported in the literature since 2001 [6–22].
CASE PRESENTATION
A 21-year-old male patient was referred to our hospital for elective bronchoscopy because of 4-month history of recurrent respiratory symptoms and persistent pulmonary infiltrates that failed to improve even after different antibiotic courses. His symptoms included purulent yellowish sputum on coughing, occasional streaks of blood, feverish sensation, non-specific chest pain, anorexia, and weight loss of 18 kg in 4 months.
Socially, he was a nonsmoker, worked at a gas station, and slept in a poorly ventilated work place.
Past medical history was significant for poorly controlled diabetes mellitus (DM) type 1 of 6-year duration (recurrent DKA and ketosis; HA1c, 11%).
On examination, vital signs were normal; patient was well looking, had decreased vesicular breathing sounds over both lung bases, and normal cardiovascular and abdominal examination. Laboratory test results revealed diabetic ketosis. CXR showed bilateral lower lobe infiltrates (Figure 1). He was admitted to ICU, and IV insulin and fluid infusions were initiated.
Figure 1.
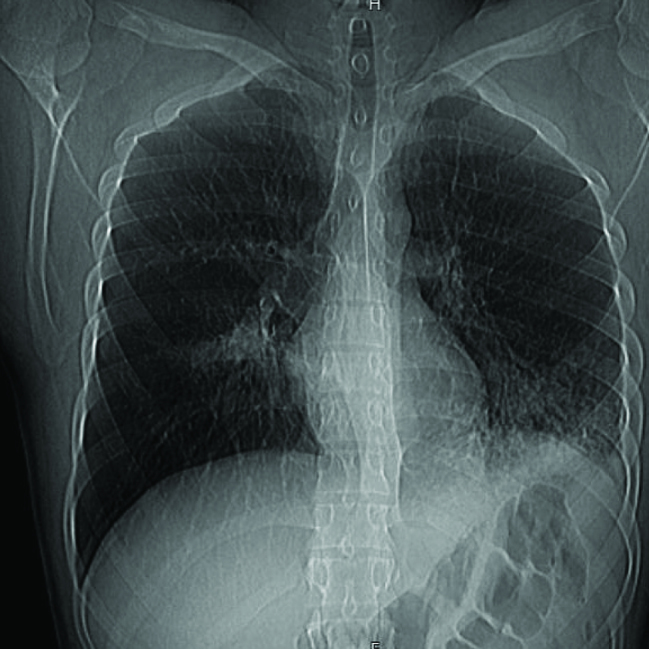
Chest xray showing bileteral lower lobe infiltrates
In light of recurrent respiratory symptoms that failed to improve, pulmonary vasculitis was suspected; chest CT was performed (Figure 2) that revealed consolidations in LLL (mainly L9) and RLL (mainly R6). Bronchoscopy showed spiral-shaped whitish gelatinous-like friable material that obstructed the orifice of the whole LLL (Figures 3 and 4). The bronchoscopist attempted to remove maximum material, restoring the patency of the left lower lobe bronchus; endobronchial and transbronchial biopsies were obtained, which revealed irregular non-septate hyphae branching at wide angles, which were typical for mucormycosis (Figure 5). The patient received dual antifungal therapy with amphotericin B and posaconazole for 2 weeks, followed by oral administration of posaconazole 400mg bid alone for another 6 months. He was followed up regularly in the out patient settings, where he continued to have residual symptoms. Repeat chest CT showed resolution of infiltrates in RLL, but significant progression of the disease in LLL. He eventually required wedge resection of L9,10 (Figure 6).
Figure 2. a, b.
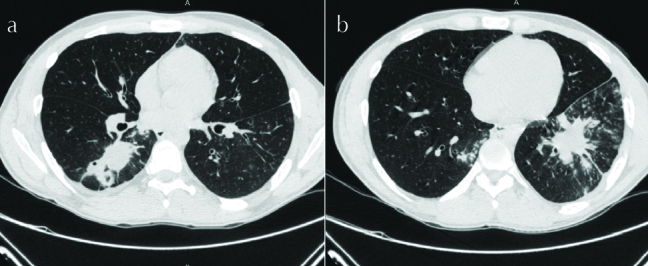
Chest CT showing consolidations in RLL and LL
Figure 3.
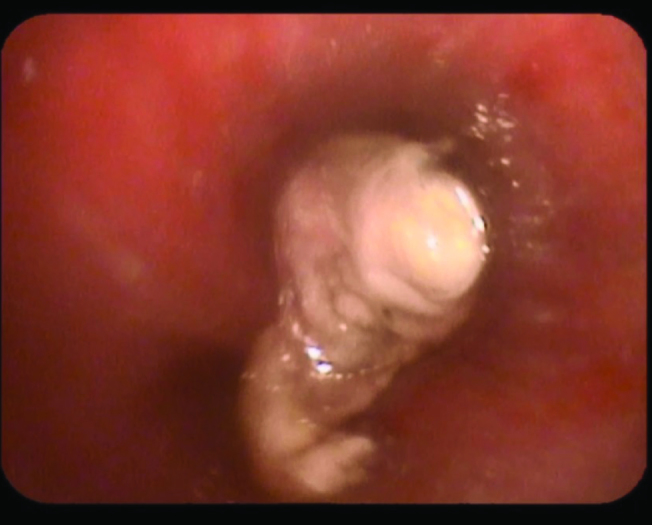
Bronchscopic view showing spiral-shaped whitish gelatinous-like friable material obstructing the orifice of LLL
Figure 4. a, b.
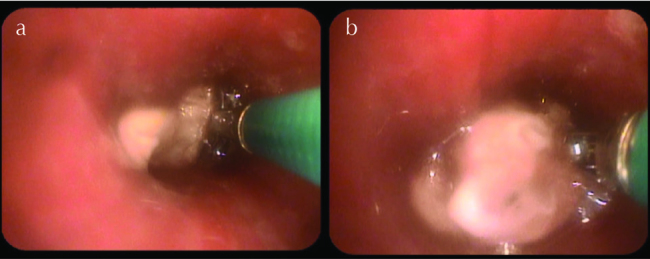
Bronchscopic view showing spiral-shaped whitish gelatinous-like friable material obstructing the orifice of LLL
Figure 5.
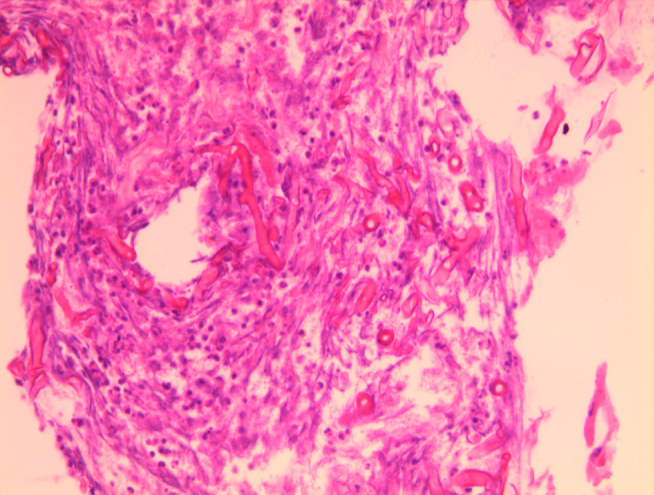
Histology showing irregular non-septate hyphae branching at wide angles typical for mucormycosis
Figure 6. a, b.
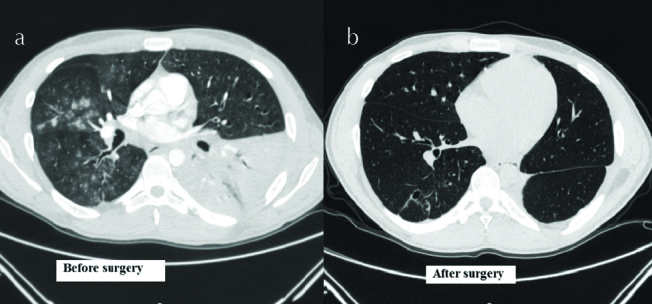
Cosolidation of LLL (Right image), Post wedge resection of L9,10 (Left image)
Review of the Literature
Methods
We searched PubMed for all pulmonary mucormycosis cases treated with posaconazole in the English literature and included only cases with pulmonary or Pulmonary or Pulmonary-plus disease. Twenty-three cases (including our patient) were identified in 18 articles published in 2000–2016.
Demographics and Underlying Conditions
Fourteen patients were males and seven were females, with a male-female ratio of 2:1; the gender was not mentioned for two cases. The mean age was 41.8 (range, 10–63) years.
Fourteen patients had hematological malignancy as their underlying condition and comprised maximum patients (60.8%), followed by six patients (26%) with organ transplantation and three (13%) with uncontrolled DM. Two patients (8.7%) had HIV, one (4.34%) had lupus nephritis, one (4.34%) had methimazole-induced neutropenia, and one (4.34%) had no known underlying condition (Note: A patient may have more than one underlying disease).
Imaging and Diagnosis
Table 1 (Radiographic manifestations of pulmonary mucormycosis and their distribution) outlines the radiographic manifestations of pulmonary mucormycosis. Most patients (26%) had infiltrates, cavity or nodules (17.39% each), consolidation (13%), and tracheitis (4.35%). In addition, in 30% of patients, radiographic manifestations were not mentioned.
Table 1.
Radiographic manifestations of pulmonary mucormycosis and their distribution
| Distribution of disease | Number of pts | % |
|---|---|---|
| RUL | 2 | 9 |
| RML | 1 | 4.25 |
| RLL | 1 | 4.25 |
| LUL | 3 | 13 |
| LLL | 1 | 4.25 |
| Bileteral | 7 | 30.5 |
| Unilateral Multilobular | 1 | 4.25 |
| Not mentioned | 7 | 30.5 |
| Total | 23 | 100 |
| Pulmonary Features | Number of pts | % |
| Inflitrate | 6 | 26.09 |
| Cavity | 4 | 17.39 |
| Consolidation | 3 | 13.04 |
| Nodules | 4 | 17.39 |
| Tracheitis | 1 | 4.35 |
| Not mentioned | 7 | 30.43 |
| Total | 23 | 100.00 |
The distribution of the disease was as follows: 30% of patients had bilateral disease, 26% had upper lobes disease (including 4% RML), 8% had lower lobes disease, 4% had unilateral multilobular, and 30% distribution was not mentioned. In addition, one patient had tracheal ulcers.
The diagnosis was established using histology alone in most patients (83%), microbiology alone in 4% of patients, and both histology and microbiology in 13% of patients.
Therapy and Outcome
Of the 23 patients, 12 developed infection while undergoing prophylactic antifungal therapy (10 patients with malignancy and two with organ transplantation). Most patients (70%) underwent medical therapy alone, 30% underwent combined medical and surgical therapies, and none underwent surgical therapy alone.
All 23 patients received oral posaconazole therapy as part of their medical regimen. Posaconazole doses ranged from 600 to 800 mg/day in divided doses, and the therapy duration ranged from 2 weeks (because of patient death) up to 7 months. Oral posaconazole has poor bioavailability; however, if administered with a high-fat meal, the bioavailability increases by 400%. In one patient, administering the drug with high-fat meals and the use of 2 mg loperamide twice daily (for chemotherapy-related diarrhea) increased the posaconazole level from 262 to 708 ng/mL [20].
Other antifungals used were IV liposomal amphotericin B in 82% of patients and an echinocandin in 8.7% of patients. Table 2: (Therapy and outcomes). Liposomal amphotericin B was administered IV at a dose of 5–10 mg/kg for a duration that ranged from 1 week to 3 months. The primary reason for discontinuation was the development or fear of acute kidney injury. One patient also received amphotericin B deoxycholate by aerosol up to 5.1 g of accumulated doses, in addition to four sessions of endobronchial instillations of amphotericin B deoxycholate (20 mg in 10 mL normal saline in 5-mL aliquotes) through the working channel of the bronchoscope [8]. Surgical treatments were performed if the antifungal therapies were believed to be inadequate in controlling the disease; they mainly comprised local debridement with wedge resection or lobectomy.
Table 2.
Therapy and outcomes
| Therapy | Number of pts (%) | Survival (%) |
|---|---|---|
| Medical+ Surgical | 7 (30%) | 7 (100) |
| Medical alone | 16 (70%) | 5 (31.2) |
| Total | 23 pts | 12 (52.1) |
| Antifungals used | ||
| Amph | 19 (82.6) | 9 (47.3) |
| Posaconazole | 23 (100) | 12 (52.1) |
| Echinocandin | 2 (8.7) | 1 (50) |
The overall survival was 52.1% (12 patients survived); a large gap in survival rates was noted between medical therapy alone group (survival, 31.2%) and combined medical and surgical therapy group (survival, 100%). The distribution of survival according to the underlying disease is shown in Table 3 (Survival according to underlying disease). The lowest survival rate at 42.85% was noted among patients with malignancy.
Table 3.
Survival according to underlying disease
| Underlying Disease* | Underlying disease and survival rates | |
|---|---|---|
|
| ||
| Patient No.(%) | Patients survved No.(%) | |
| DM | 3 (13) | 3 (100) |
| Malignancy | 14 (60.8) | 6 (42.85) |
| HIV | 2 (8.7) | 1 (50) |
| Organ Transplantation | 6 (26) | 3 (50) |
| Others | 2 (8.7) | 1 (50) |
| None | 1 (4.34) | 1 (100) |
A patient may have more than one underlying condition
In conclusion, pulmonary mucormycosis is a life-threatening condition caused by molds in the order of mucorales, they usually present as an angio-invasive disease. Though widely spread in nature -especially in soil and decay matter- they rarely cause human disease, luckily because our immune system is so effective at eliminating them. However with the increasing number of patients who receive immunosuppressive medications or organ transplantation more cases of pulmonary mucormycosis are expected to be seen. In all four mentioned reviews 2–12% of patients had no known underlying disease Table 4 (Comparison between four literature reviews). To our knowledge this is the first review of the use of Posaconazole in pulmonary mucormycosis infections. The survival rate appears to be increasing with the ever expanding armamentarium of antifungal therapy, it was 10% in Baker`s review, 35% in Tedder et al. [5] 44% in Lee et al.[4] and 52.1% in our review Table 4.
Table 4.
Comparison between four literature reviews
| This Review | Lee et al. [4] | Tedder et al. [5] | Baker [3] | ||
|---|---|---|---|---|---|
| Number of patients | 23 | 87 | 255** | 39*** | |
| Mean Age | 41.8 | 44 | 41 | 44 | |
| M:F ratio | 2 to 1 | 3 to 1 | 7 to 3 | 2.4 to 1 | |
| Underlying Disease* | DM | 13% | 56% | 32% | 23% |
| Malignancy | 61% | 32% | 83.50% | 46% | |
| HIV | 8.70% | 0 | 0 | 0 | |
| Organ Transplantation | 26% | 11.40% | 8% | 0 | |
| Others | 8.75 | 24% | 23.50% | 13% | |
| None | 4% | 12.60% | 0 | 2% | |
| Reported Survival | 52.10% | 44.00% | 35.00% | 10.00% |
A patient may have more than one underlying disease
Including patients with disseminated mucormycosis
Pulmonary mucormycosis cases only
Our patient described here was mildly immune suppressed due to poorly controlled diabetes mellitus type 1, and recurrent ketosis, had been exposed to damp poorly ventilated work place. Surprisingly he was relatively clinically well despite the presence of semi-invasive pulmonary disease on lung pathology. This highlights the importance of considering mucormycosis in patients at risk even with mild immunosupression and subacute presentation [23].
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.S.Y.; Design - H.S.Y.; Supervision - A.Y.A.; Resources - H.S.Y., I.B.; Materials - H.S.Y., I.B.; Data Collection and/or Processing - H.S.Y., I.B.; Analysis and/or Interpretation - H.S.Y., A.Y.A., I.B.; Literature Search - H.S.Y.; Writing Manuscript - H.S.Y., A.Y.A.; Critical Review - H.S.Y.; Other - H.S.Y.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Furbringer P. Beobachtungen uber lungenmycose beim menschen. Arch Pathol Anat Physiol Klin Med. 1876:66330–65. [Google Scholar]
- 2.Baker RD. Mucormycosis-a new disease? JAMA. 1957;163:805–8. doi: 10.1001/jama.1957.02970450007003. [DOI] [PubMed] [Google Scholar]
- 3.Baker RD. The Pathologic Anatomy of Mycoses Human Infection With Fungi, Actinomyces, and Algae. New York Springer-Verlag; NY Inc: 1971. Mucormycosis; pp. 832–918. [Google Scholar]
- 4.Tedder M, Spratt JA, Anstadt MP, et al. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57:1044–50. doi: 10.1016/0003-4975(94)90243-7. https://doi.org/10.1016/0003-4975(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 5.Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med. 1999;159:1301–9. doi: 10.1001/archinte.159.12.1301. https://doi.org/10.1001/archinte.159.12.1301. [DOI] [PubMed] [Google Scholar]
- 6.Pacheco P, Ventura AS, Branco T, et al. Clinical Experience in Invasive Fungal Infections. Clinical Drug Investigation. 2013;33(Suppl 1):23–6. doi: 10.1007/s40261-012-0017-1. [DOI] [PubMed] [Google Scholar]
- 7.Juan YH, Saboo SS, Lin YC, et al. Reverse halo sign in pulmonary mucormyosis. QJM. 2014;107:777–8. doi: 10.1093/qjmed/hcu031. https://doi.org/10.1093/qjmed/hcu031. [DOI] [PubMed] [Google Scholar]
- 8.Alfageme I, Reina A, Gallego J, et al. Endobronchial instillations of amphotericin B: complementary treatment for pulmonary mucormycosis. J Bronchology Interv Pulmonol. 2009;16:214–5. doi: 10.1097/LBR.0b013e3181aa2583. https://doi.org/10.1097/LBR.0b013e3181aa2583. [DOI] [PubMed] [Google Scholar]
- 9.Patel A, Bishburg E, Nagarakanti S. Mucormycosis in an HIV-infected renal transplant patient: A case report and review of the literature. Am J Case Rep. 2014;15:74–8. doi: 10.12659/AJCR.890026. https://doi.org/10.12659/AJCR.890026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colón-Santos E, González-Ramos M, Bertrán-Pasarell J, et al. Disseminated nocardiosis masking an atypical zygomycosis presentation in a kidney transplant recipient. Transpl Infect Dis. 2011;13:380–4. doi: 10.1111/j.1399-3062.2011.00606.x. https://doi.org/10.1111/j.1399-3062.2011.00606.x. [DOI] [PubMed] [Google Scholar]
- 11.Bethge WA, Schmalzing M, Stuhler G, et al. Mucormycoses in patients with hematologic malignancies: an emerging fungal infection. Haematologica. 2005;90(Suppl):ECR22. [PubMed] [Google Scholar]
- 12.Dai Y, Walker JW, Halloush RA, Khasawneh FA. Mucormycosis in two community hospitals and the role of infectious disease consultation: a case series. Int J Gen Med. 2013;6:833–8. doi: 10.2147/IJGM.S52718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leithauser M, Kahl C, Aepinus C, et al. Invasive zygomycosis in patients with graft-versus-host disease after allogeneic stem cell transplantation. Transpl Infect Dis. 2010;12:251–7. doi: 10.1111/j.1399-3062.2009.00480.x. https://doi.org/10.1111/j.1399-3062.2009.00480.x. [DOI] [PubMed] [Google Scholar]
- 14.Capria S, De Angelis F, Gentile G, et al. Complete remission obtained with azacitidine in a patient with concomitant therapy related myeloid neoplasm and pulmonary mucormycosis. Mediterr J Hematol Infect Dis. 2013;5:e2013048. doi: 10.4084/MJHID.2013.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mousset S, Bug G, Heinz WJ, et al. Breakthrough zygomycosis on posaconazole prophylaxis after allogeneic stem cell transplantation. Transpl Infect Dis. 2010;12:261–4. doi: 10.1111/j.1399-3062.2009.00479.x. https://doi.org/10.1111/j.1399-3062.2009.00479.x. [DOI] [PubMed] [Google Scholar]
- 16.Kwan LP, Choy CB, Chan TM, et al. Successful treatment of pulmonary rhizopus infection with surgical resection and posaconazole in a renal transplant recipient. Nephrology (Carlton) 2013;18:74–5. doi: 10.1111/j.1440-1797.2012.01616.x. https://doi.org/10.1111/j.1440-1797.2012.01616.x. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez JF, Maselli DJ, Simpson T, Restrepo MI. Pulmonary mucormycosis: what is the best strategy for therapy? Respir Care. 2013;58:e60–3. doi: 10.4187/respcare.02106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lekakis LJ, Lawson A, Prante J, et al. Fatal rhizopus pneumonia in allogeneic stem cell transplant patients despite posaconazole prophylaxis: two cases and review of the literature. Biol Blood Marrow Transplant. 2009;15:991–5. doi: 10.1016/j.bbmt.2009.04.007. https://doi.org/10.1016/j.bbmt.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Brugière O, Dauriat G, Mal H, et al. Pulmonary mucormycosis (zygomycosis) in a lung transplant recipient: recovery after posaconazole therapy. Transplantation. 2005;80:1361–2. [PubMed] [Google Scholar]
- 20.Schneidawind D, Nann D, Vogel W, et al. Allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia and pulmonary mucormycosis. Transpl Infect Dis. 2012;14:E166–72. doi: 10.1111/tid.12019. [DOI] [PubMed] [Google Scholar]
- 21.Lee JS, Kim HC, Park SW, et al. A case of isolated pulmonary mucormycosis in an immunocompetent host. Tuberc Respir Dis (Seoul) 2013;74:269–73. doi: 10.4046/trd.2013.74.6.269. https://doi.org/10.4046/trd.2013.74.6.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng TF, Ho MW, Lin HC, et al. Successful treatment of disseminated mixed invasive fungal infection after hematopoietic stem cell transplantation for severe aplastic anemia. Pediatr Transplant. 2012;16:E35–8. doi: 10.1111/j.1399-3046.2010.01406.x. [DOI] [PubMed] [Google Scholar]
- 23.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–69. doi: 10.1128/CMR.18.3.556-569.2005. https://doi.org/10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


