Abstract
Tuberculosis is highly prevalent in our country and nontuberculous mycobacteria (NTM) are frequently found in respiratory specimens recently. A 65-year-old woman was admitted with complaints of fever, cough, weight loss, and hemoptysis. On the patient’s chest radiography an upper lobe cavity in both lungs and consolidation was detected. Acid-fast bacilli 4+ were observed in smear of sputum and culture results M. intracellulare and M. tuberculosis were observed together. The patient’s treatment was arranged. Through this case, we want to emphasize that tuberculosis and nontuberculous mycobacterial disease can coexist.
Keywords: M. tuberculosis, M. intracellulare, coinfection
INTRODUCTION
Nontuberculous mycobacteria (NTM) pulmonary diseases are being increasingly detected. Cavitary NTM pulmonary disease is radiographically and clinically indistinguishable from pulmonary tuberculosis. Risk group of NTM infection include elderly persons; alcoholics; smokers with COPD.
CASE PRESENTATION
A 65-year-old woman was admitted with complaints of fever, cough, weight loss, and hemoptysis. We observed an upper lobe cavity in both lungs and consolidation on the patient’s chest radiography and chest computed tomography scans (Figures 1 and 2). Tuberculin skin test result was 19 mm and acid-fast bacilli 4+ were observed in acid-fast bacilli smear of sputum. The patient was extremely cachectic and weighed only 28 kg. Drugs were set while considering the weight, and therapy with four drugs (INH, RIF, EMB, and PRZ) was started. COPD was a comorbid factor for tuberculosis, and HIV test was negative. Informed constent form was obtained from the patient. Mycobacterium tuberculosis was obtained from sputum cultures. In the follow-up culture results, M. intracellulare and M. tuberculosis were observed together. In order to verify the patient culture results, two sputum cultures taken on separate days were sent to another center, where the same results were obtained. INH and RIF susceptibility were detected in the culture tests. After M. intracellulare was observed in the two sputum cultures, clarithromycin treatment was also added to the first drugs. The patient regularly received the medication, and no side effects were observed. The patient came to to the outpatient clinic once a month in the follow-up period. In the third month of treatment, acid-fast bacilli smear of the sputum was negative. During subsequent follow-up, she did not give sputum sample. EMB and PRZ were discontinued in the third month; the remaining drugs were continued. During the sixth month of treatment, regression of the chest X-ray cavities and consolidation was observed, and the patient’s weight increased to 35 kg (Figure 3). The patient did not come to the hospital and she died in the eight month of treatment. Through this case, we want to emphasize that tuberculosis and nontuberculous mycobacterial disease can coexist. Therefore, culture results should be carefully monitored.
Figure 1.
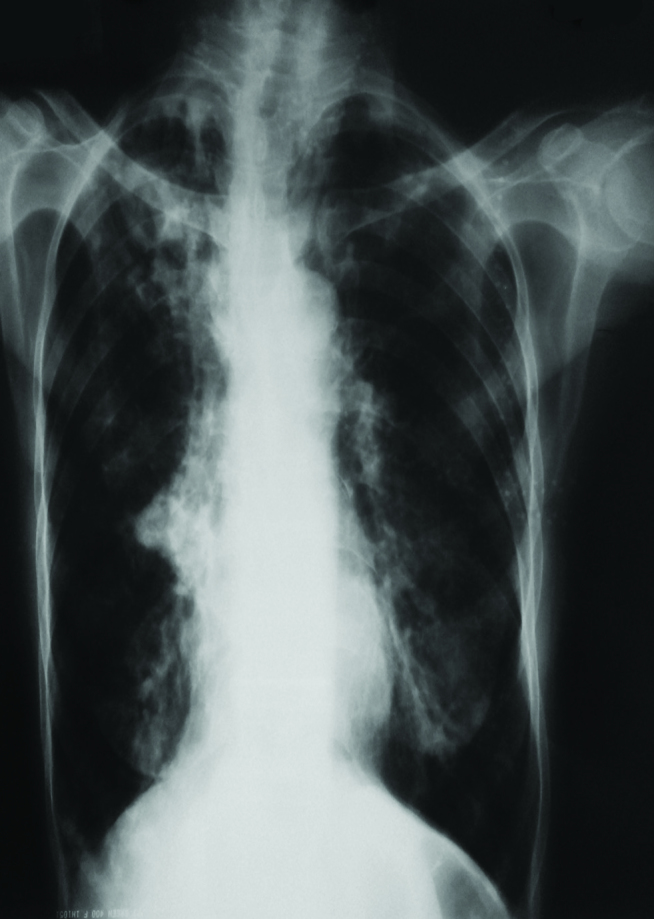
Patient’s chest X-ray before treatment
Figure 2.
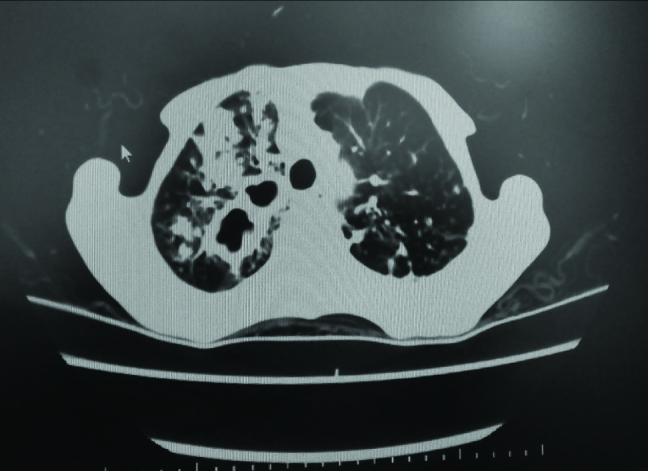
Patient’s CT scan before treatment
Figure 3.
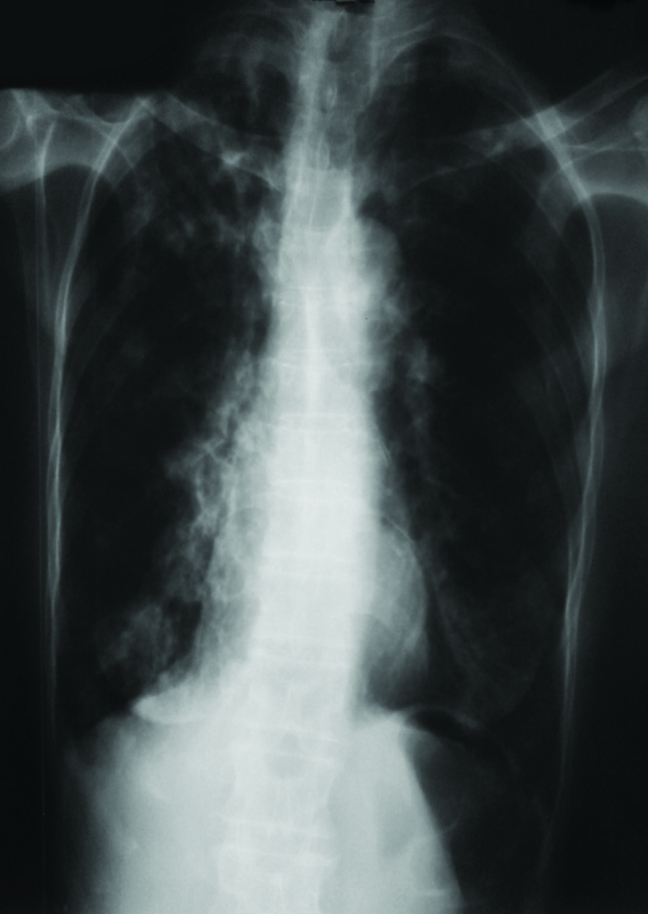
Patient’s chest X-ray after 6th month treatment
DISCUSSION
Approximately 160 known species of Nontuberculous mycobacteria (NTM) that are commonly associated with lung disease in humans [1]. Historically, significant overlap between the symptoms of NTM pulmonary disease and tuberculosis has been described, probably because clinicians were looking for a disease resembling tuberculosis [2]. Nontuberculous mycobacteria (NTM) are frequently found in respiratory specimens from patients with M. tuberculosis pulmonary disease [3,4]. NTM have been frequently isolated from water soil, dust, and plants. They are quite resistant to water disinfectants in common use, such as chlorine. Contact with contaminated environments may occasionally be responsible for infection in humans and animals, but the possibility of transmission from human to human is rare [5]. Risk group of NTM infection include elderly persons; alcoholics; smokers with COPD; and patients with chronic sinusitis, pulmonary fibrosis, gastroesophageal diseases, HIV, and a history of tuberculosis.
The lungs are easily affected by inhalation of aerosolized mycobacteria and is by far the most frequent site of human mycobacteriosis. In HIV() patients, the disease is indistinguishable from tuberculosis and is characterized by a very slow progression [6]. Cavitary NTM pulmonary disease is radiographically and clinically indistinguishable from pulmonary tuberculosis. Manifestations range from absence of symptoms to cavitary disease, and an X-ray may reveal fibrosis, upper lobe cavitation, nodular or parenchymal opacity, and pleural thickening. The most affected population is elderly patients with predisposing pulmonary conditions (such as silicosis, obstructive pulmonary disease, pneumoconiosis, previous tuberculosis, bronchiectasis, or cancer). Symptoms include cough, fever, weight loss, weakness, and respiratory insufficiency [7].
Interestingly, the increase in the proportion of pulmonary disease caused by NTM seems to be associated with a simultaneous decrease in the incidence of tuberculosis [8]. The guidelines of the American Thoracic Society provide strict criteria that are applicable in the presence of pneumopathy, for which any cause other than NTM has been excluded [9].
The most common types of NTM are M. avium complex (M. avium and M. intracellulare), M. Kansasii, and M. abscessus. Although species differentiation between M. intracellulare and M. avium in terms of clinical features and prognosis were not clearly defined. A recent large retrospective cohort study showed that patients with M. intracellulare pulmonary disease present more severe manifestations: lower body mass index, more frequent presence of respiratory symptoms and fibrocavitary disease, higher rate of smear-positive sputum, and worse prognosis, including more frequent initiation of antibiotic treatment during follow-up period and higher unfavourable treatment response than those in patients with M. avium pulmonary disease [10]. The same group also reported that patients with M. intracellulare pulmonary disease showed evidence of a more extensive disease on chest computed tomography scan than did patients with M. avium pulmonary disease [11].
Cavitary NTM pulmonary disease is radiographically and clinically indistinguishable from pulmonary tuberculosis, which may lead to misdiagnosis in low-resource tuberculosis-endemic regions [12,13]. Patients with fibrocavitary disease usually require immediate treatment because cavitary disease is associated with a higher rate of mortality due to NTM pulmonary disease [14,15].
In some Asian countries where the mainstay of tuberculosis diagnosis is the acid-fast smear, there are concerns that a number of patients diagnosed with tuberculosis, especially with putative drug-resistant tuberculosis, might actually have NTM pulmonary disease (30.7% of isolates that tested resistant to isoniazid and rifampicin and 4% of tuberculosis retreatment cases in one study from China, similar to the African data previously mentioned) [16,17]. Some studies have shown that HIV(+) patients tend to have NTM and M. tuberculosis coinfection In publications made in this regard and in our country there is no case reviewed NTM and Mycobacterium tuberculosis are in same culture, also our patient was HIV (−).
Treatment of NTM pulmonary disease is difficult due to the uncertainty regarding when treatment should be started and which regimen is most likely to achieve successful treatment [18]. Initiation of NTM treatment should be individualized considering disease types, comorbid conditions, and age. Patients with fibrocavitary disease usually require immediate treatment because the presence of cavitary disease is associated with higher mortality rate [19,20]. A study demonstrated that clarithromycin and capreomycin have high antimicrobial activities against M. intracellulare isolates; clarithromycin and amikacin resistance could be more readily and rapidly detected using molecular scanning of corresponding drug target than conventional drug susceptibility testing.
In conclusion, tuberculosis is highly prevalent in our country. Recently, NTM pulmonary diseases are being increasingly detected. As in our case, tuberculosis and nontuberculous mycobacterial disease can coexist; therefore, culture results should be carefully monitored.
Footnotes
Informed Consent: Written informed consent was obtained from patient who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Y.N.; Design - Y.N., U.Y.E.; Supervision - S.L.; Resources - Y.N., U.Y.E.; Materials - Y.N.; Data Collection and/or Processing - Y.N., U.Y.E.; Analysis and/or Interpretation - Y.N., U.Y.E., S.L.; Literature Search - Y.N.; Writing Manuscript - Y.N.; Critical Review - U.Y.E., S.L.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Tortoli E. The new mycobacteria: an update. FEMS Immunol Med Microbiol. 2006;48:159–78. doi: 10.1111/j.1574-695X.2006.00123.x. https://doi.org/10.1111/j.1574-695X.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 2.Evans SA, Colville A, Evans AJ, et al. Pulmonary Mycobacterium kansasii infection: comparison of the clinical features, treatment and outcome with pulmonary tuberculosis. Thorax. 1996;51:1248–52. doi: 10.1136/thx.51.12.1248. https://doi.org/10.1136/thx.51.12.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang SM, Lim MS, Hong YJ, et al. Simultaneous detection of Mycobacterium tuberculosis complex and nontuberculous myco-bacteria in respiratory specimens. Tuberculosis (Edinb) 2013;93:642–6. doi: 10.1016/j.tube.2013.07.007. https://doi.org/10.1016/j.tube.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Damaraju D, Jamieson F, Chedore P, Marras TK. Isolation of non-tuberculous mycobacteria among patients with pulmonary tuberculosis in Ontario, Canada. Int J Tuberc Lung Dis. 2013;17:676–81. doi: 10.5588/ijtld.12.0684. https://doi.org/10.5588/ijtld.12.0684. [DOI] [PubMed] [Google Scholar]
- 5.Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126:566–81. doi: 10.1378/chest.126.2.566. https://doi.org/10.1378/chest.126.2.566. [DOI] [PubMed] [Google Scholar]
- 7.Piersimoni C, Scarparo C. Pulmonary infections associated with nontuberculous mycobacteria in immunocompetent patients. Lancet Infect Dis. 2008;8:323–34. doi: 10.1016/S1473-3099(08)70100-2. https://doi.org/10.1016/S1473-3099(08)70100-2. [DOI] [PubMed] [Google Scholar]
- 8.Brode SK, Daley CL, Marras TK. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis. 2014;18:1370–7. doi: 10.5588/ijtld.14.0120. https://doi.org/10.5588/ijtld.14.0120. [DOI] [PubMed] [Google Scholar]
- 9.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;165:367–416. doi: 10.1164/rccm.200604-571ST. https://doi.org/10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 10.Koh WJ, Jeong BH, Jeon K, et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest. 2012;142:1482–8. doi: 10.1378/chest.12-0494. https://doi.org/10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, Kim HS, Lee KS, et al. Serial CT findings of nodular bronchiectatic Mycobacterium avium complex pulmonary disease with antibiotic treatment. AJR Am J Roentgenol. 2013;201:764–72. doi: 10.2214/AJR.12.9897. https://doi.org/10.2214/AJR.12.9897. [DOI] [PubMed] [Google Scholar]
- 12.Maiga M, Siddiqui S, Diallo S, et al. Failure to recognize nontuberculous mycobacteria leads to misdiagnosis of chronic pulmonary tuberculosis. PLoS One. 2012;7:e36902. doi: 10.1371/journal.pone.0036902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YK, Hahn S, Uh Y, et al. Comparable characteristics of tuberculous and non-tuberculous mycobacterial cavitary lung diseases. Int J Tuberc Lung Dis. 2014;18:725–9. doi: 10.5588/ijtld.13.0871. https://doi.org/10.5588/ijtld.13.0871. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary Mycobacterium avium-intracellulare complex disease. Int J Tuberc Lung Dis. 2012;16:408–14. doi: 10.5588/ijtld.11.0148. https://doi.org/10.5588/ijtld.11.0148. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi M, Takayanagi N, Kanauchi T, et al. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;185:575–83. doi: 10.1164/rccm.201107-1203OC. https://doi.org/10.1164/rccm.201107-1203OC. [DOI] [PubMed] [Google Scholar]
- 16.Jing H, Wang H, Wang Y, et al. Prevalence of nontuberculous mycobacteria infection, China, 2004–2009. Emerg Infect Dis. 2012;18:527–8. doi: 10.3201/eid1803.110175. https://doi.org/10.3201/eid1803.110175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khann S, Mao ET, Rajendra YP, et al. Linkage of presumptive multidrug resistant tuberculosis (MDR-TB) patients to diagnostic and treatment services in Cambodia. PLoS One. 2013;8:e59903. doi: 10.1371/journal.pone.0059903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. https://doi.org/10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary Mycobacterium avium-intracellulare complex disease. Int J Tuberc Lung Dis. 2012;16:408–14. doi: 10.5588/ijtld.11.0148. https://doi.org/10.5588/ijtld.11.0148. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M, Takayanagi N, Kanauchi T, et al. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;185:575–83. doi: 10.1164/rccm.201107-1203OC. https://doi.org/10.1164/rccm.201107-1203OC. [DOI] [PubMed] [Google Scholar]


