Abstract
Mucosal immunity to re-infection with a highly virulent virus requires the accumulation and persistence of memory CD8 T cells at the site of primary infection. These cells may derive from memory precursor effector cells (MPECs), which are distinct from short-lived effector cells (SLECs) that provide acute protection but are often destined to die. Using respiratory virus infection, we identify HVEM (Herpes Virus Entry Mediator, TNFRSF14), a member of the TNF receptor superfamily, provides key signals for MPEC persistence. HVEM-deficient CD8 T cells expanded normally but were skewed away from MPECs with resultant poor development of circulating and lung-resident memory cells. HVEM was selectively expressed on MPECs whereas MPECs deficient in HVEM failed to survive in adoptive transfer recipients. As a consequence, HVEM-deficient recipients failed to afford protection against respiratory re-infection with influenza virus. HVEM therefore represents a critical signal for MPECs and development of protective mucosal CD8 T cell memory.
Introduction
During viral infection, CD8 T cells can form alternate effector cell populations with varied differentiation states, ranging from terminal effectors to precursors of central memory cells (1). These differentiation states play an important role in immediate elimination of the pathogen as well as in providing protection against subsequent re-infection (2). The terminally differentiated cells, also known as short lived effector cells (SLECs) are proficient killers but undergo rapid death during or following viral clearance. Inflammatory cytokines such as IL-2, IL-12 and type I IFN can drive generation of SLECs by regulating the expression of key transcription factors such as T-bet and Blimp1(1, 3, 4). In contrast, less differentiated memory precursors effector cells (MPECs) are regulated by a distinct set of transcription factors including Eomes (5), Bcl6 (6), Foxo1 (7, 8), Tcf-1 (9), and Bcl11b (10). These cells have increased capacity to survive long-term and go on to form the bulk of the memory pool. Moreover, SLECs and MPECs can occupy different anatomical niches within the lymphoid and peripheral tissues, which might further impact their survival and homeostasis and their contribution to protection (11–13).
The overall signal strength a CD8 T cell receives, from antigen, co-stimulation and inflammatory cytokines, is thought to influence SLEC and MPEC differentiation, and memory generation (1). Although several co-stimulatory molecules and cytokines have been described to favor the development of SLECs, little is known about specific molecules that might more directly control MPECs. Co-stimulatory molecules belonging to the tumor necrosis factor receptor (TNFR) superfamily are widely known to influence different aspects of T cell biology including regulating proliferation, survival, and functional activity but whether they are major factors in determining the balance between MPECs and SLECs is not understood well (14, 15). The Herpes Virus Entry Mediator (HVEM, CD270, TNFRSF14) is one such costimulatory molecule belonging to the TNFR superfamily that was initially discovered as the cellular entry receptor for Herpes simplex virus 1 (HSV-1) (16). Multiple cellular ligands have been discovered for HVEM, including LIGHT, BTLA, CD160 and LTα3, all of which have the potential to provide a pro-inflammatory or a survival signal by ligating HVEM on T cells (17). Complicating the biology of HVEM, it can also participate in bidirectional signaling with BTLA and CD160 inducing a number of possible activities from these molecules that can be either inhibitory or stimulatory depending on the cell type that expresses them (18). However, the general concept that has emerged over the past few years is that T cell expressed HVEM can be essential to the development of some CD8 T cell responses (19, 20) but its role in T cell fate decisions is unknown.
Here, we determined the role of HVEM expressed specifically by CD8 T cells in the context of respiratory poxvirus and influenza infection. We found that HVEM-deficient CD8 T cells expanded normally but failed to generate memory cells in the lungs. The lack of HVEM skewed the effector cell balance towards a more terminal differentiation state with a reduction in the proportion of MPECs. In line with this, we found that expression of HVEM was confined to MPECs at the peak of the effector response and HVEM-deficient MPECs were impaired in the ability to survive over time. Similar to CD8 T cells lacking HVEM, WT CD8 T cells failed to accumulate in LIGHT-deficient host but not BTLA-deficient host upon virus infection. Our study thus reveals an important role for HVEM-LIGHT signaling in the longevity of the mucosal and lymphoid memory precursor pool that is essential for optimal generation ofCD8 T cell memory to respiratory virus.
Materials and Methods
Mice
Eight to twelve week old female C57BL/6 (CD45.2) and B6.SJL-Ptprca Pepcb/BoyJ (CD45.1/Ly5.1) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). HVEM-, LIGHT-, BTLA- and RAG-knockout mice and wild type OT-I transgenic mice were bred and maintained at the University of Florida animal facility. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Florida, Gainesville (OLAW Assurance # A3377-01).
Viruses and infections
Vaccinia virus Western Reserve strain (VacV-WR) and recombinant virus expressing full-length ovalbumin protein (rVacV-WR-OVA) were grown in HeLa cells and subsequently tittered on VeroE6 cells as described previously (21). Recombinant influenza virus expressing SIINFEKL peptide (PR8-OT-I) was a generated as previously described (22). Mice were infected with 2 × 104 PFU of VacV-WR-OVA via the intra-nasal route in a volume of 10µl. For challenge experiments, PR8-SIINFEKL was administered at a normally lethal dose of 2 × 107 PFU in 40µl volume via the intra-nasal route.
CD8 T Cell Adoptive Transfer
For adoptive transfer experiments, 5 × 104 naive WT or HVEM−/− OT-I CD8 T cells were purified from spleens with MACS technology (Miltenyi Biotec), FACS sorted and transferred into WT C57BL/6 mice via the intra-venous route as described previously (23, 24). One day later, mice were infected with rVacV-WR-OVA as above.
Flow cytometry
Preparation of cells, extracellular/intracellular staining, data acquisition, and data analysis were performed as described previously (10, 25, 26).
VACV titer assay
Following adoptive T cell transfer and rVacV-WR-OVA infection, tissues from individual mice were homogenized and sonicated for 1 minute with a pause every 10 seconds using ultrasonic cleaner (1210 Branson). Serial dilutions were made and viral titers were determined by plaque assay on confluent VeroE6 cells.
CFSE labeling of transgenic CD8 T cells
Splenocytes were obtained from CD8+ OT-I TCR-transgenic mice and purified using positive selection with MACS beads (Miltenyi Biotec). Enriched cells were FACS sorted and labeled with CFSE (Molecular Probes) by incubating 107 purified cells per ml with 5 µM CFSE for 10 min at 37°C. Cells were then washed three times in HBSS containing 2.5% FCS and adoptively transferred (5 × 105 cells/mice) into RAG deficient host.
Gene array
Total lung RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA was subsequently treated with DNase I (Qiagen) and further purified using an RNeasy Mini Kit (Qiagen). A total of 1 mg high-quality total RNA (RNA integrity number .7) was then reverse transcribed using the First Strand Synthesis Kit (Qiagen) and subsequently loaded onto mouse apoptotic RT2 profiler array according to the manufacturer’s instructions (Qiagen). Qiagen’s online Web analysis tool was used to produce comparative heat maps, and fold change was calculated by determining the ratio of mRNA levels to control values using the D threshold cycle (Ct) method (22DDCt). All data were normalized to an average of five housekeeping genes, Gusb, Hprt, Hsp90ab1, Gapdh, and Actb. PCR conditions used: hold for 10 min at 95°C, followed by 45 cycles of 15 s at 95°C and 60 s at 60°C.
Statistical analysis
Tests were performed using Prism 5.0 software (GraphPad, San Diego, CA). Statistics were done using two-tailed, unpaired Student’s t test with 95% confidence intervals unless otherwise indicated. Two-way ANOVA was used to determine differences in weight loss profiles and the Mantel-Cox test was utilized for survival analysis. Unless otherwise indicated, data represent the mean ± SEM; p < 0.05 considered statistically significant.
Results
T cell expressed HVEM is essential for development of memory with respiratory virus infection
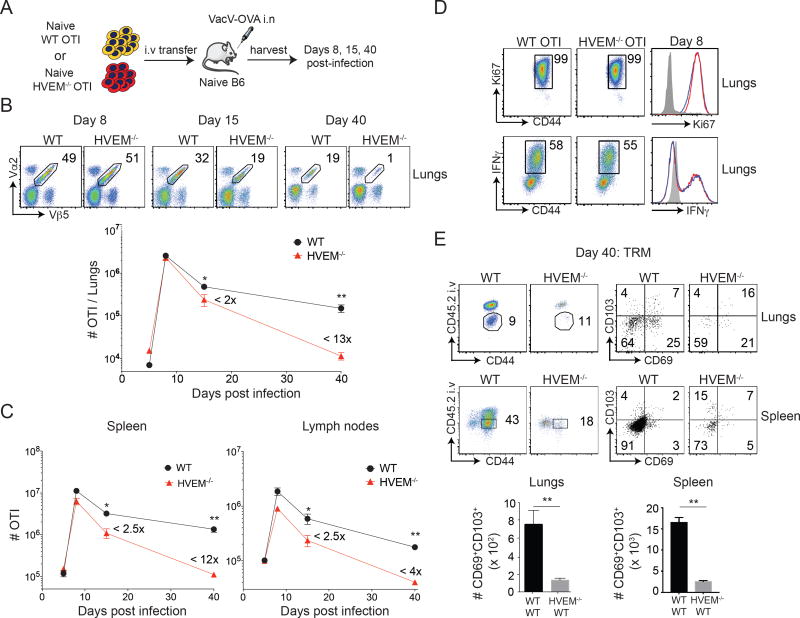
We previously showed that HVEM was important for the development of CD8 T cell memory to intraperitoneal infection with vaccinia virus Western Reserve strain (VacV-WR)(19). To further understand the role of HVEM, we used a model of respiratory infection that involves the differentiation of SLECs and MPECs, where MPECs are essential for long-term protection against virus re-infection in the lungs (13). To efficiently track CD8 T cells, and allow specific deletion of HVEM on these cells, we used a system with adoptive transfer of OT-I CD8 T cells responding to recombinant VacV-WR expressing the full-length ovalbumin protein (rVACV-WR-OVA) given intranasally. Initially, WT and HVEM−/− OT-I cells were adoptively transferred into naïve B6 mice (Fig. 1A), and expansion and persistence of these cells was analyzed in lungs (Fig. 1B), spleen (Fig. 1C), and lymph nodes (Fig. 1C) over time. Interestingly, the frequency and number of HVEM−/− OT-I cells in the lungs was similar to WT OT-I cells at day 8 post-infection (Fig. 1B), and in line with HVEM having no role in the primary effector response, expression of Ki67 and IFN-γ was equivalent in HVEM-deficient cells (Fig. 1D). However, there was ~50% reduction in the frequency and numbers of HVEM−/− OT-I cells at day 15 post-infection accompanied by a further reduction in these cells by day 40 when compared to their WT counterparts (Fig. 1B) resulting in an approximately 13-fold reduction in the accumulation of memory cells. Similar to the lungs, there was a strong defect in the accumulation of HVEM−/− OT-I memory cells both in the spleen and the lymph nodes over this time period (Fig. 1C).
Figure 1. HVEM on CD8 T cells is required for memory generation after respiratory vaccinia virus infection.
(A) Equal numbers (5 × 104) of WT and HVEM−/− naïve (CD44lo) OT-I (Vα2+Vβ5+) transgenic CD8 T cells were adoptively transferred into BL/6 mice and infected with rVacV-WR-OVA (2 × 104 PFU i.n) the following day. (B) Lungs, (C) spleen and lymph nodes were harvested at days 6, 8, 15 and 40 post-infection and stained for CD8, CD44, Vα2, and Vβ5 and frequencies of OT-I CD8 T cells determined. (D) OT-I cells from lungs of recipient mice at day 8 were stained for Ki67 intranuclearly and restimulated in vitro with OVA and stained for IFN-γ. (E) At day 40 post-infection, recipient mice were injected with anti-CD45.2 antibody intravenously, three minutes before euthanizing. Representative plots of CD45.2 and CD44 were pre-gated on CD8+OT-I (Vα2+Vβ5+) cells. The gated CD45.2 negative cells were analyzed for the expression of CD103 and CD69 using flow cytometry and total numbers of cells calculated in lungs and spleen. *, P<0.05; **, p<0.01 and results are the mean ± SEM (n = 3 mice/group). Similar results were obtained in four independent experiments.
In order to determine whether HVEM affects the tissue resident memory CD8 T cell subset, we directly injected recipients of WT and HVEM−/− OT-I cells with fluorophore conjugated CD45.2 antibody (i.v) prior to sacrifice to distinguish between circulating (CD45.2 positive fraction; vascular) and resident memory (CD45.2 negative fraction; parenchyma/airway) cells. Although the ratio of tissue resident memory cells to circulating cells was normal, there was a dramatic reduction in the total number of CD69+CD103+ tissue resident memory cells with the HVEM deficiency, seen both in the lungs and the spleen (Fig. 1E). Overall, these data suggest that HVEM signaling is dispensable for optimal CD8 effector T cell expansion to respiratory infection but necessary for the generation of high numbers of circulating and lung tissue-resident memory CD8 T cells.
HVEM is preferentially expressed by MPECs and controls the balance between SLECs and MPECs
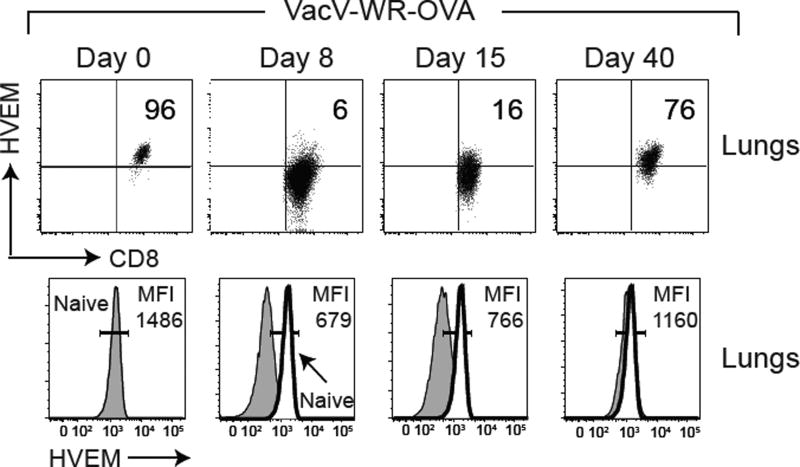
To more fully understand how HVEM might control memory development, we examined its expression on WT CD8 T cells as they transitioned from naïve to effector to memory cells. Naïve (CD44lo) CD8 T cells constitutively expressed high cell surface levels of HVEM, whereas at the peak of the effector response (day 8 post-infection), most of the CD8 T cells down-regulated HVEM (Fig. 2). However, notably, HVEM was detectable on a small percentage (5–15%) of cells at the effector time point as well as during the contraction phase of the response (between days 8 – 15). In contrast, when memory was fully established (day 40), the majority of surviving antigen-reactive cells were HVEM positive (Fig. 2). A similar expression pattern of HVEM was also observed on splenic OT-I CD8 T cells (data not shown). Therefore, unlike other TNF family receptors whose expression is maintained or increased during transition from naïve to effector cell, HVEM is dynamically downregulated, except on a minor subpopulation of cells.
Figure 2. Expression of HVEM on antigen specific CD8 T cells during the course of infection.
WT OT-I T cells from lungs of adoptive recipients infected with rVacV-WR-OVA as in Fig. 1A were stained over time for HVEM expression after gating on CD8, Vα2, Vβ5, and CD44hi. Gates determined based on expression in naïve (CD44lo) cells in the same host. Similar results were obtained in two independent experiments.
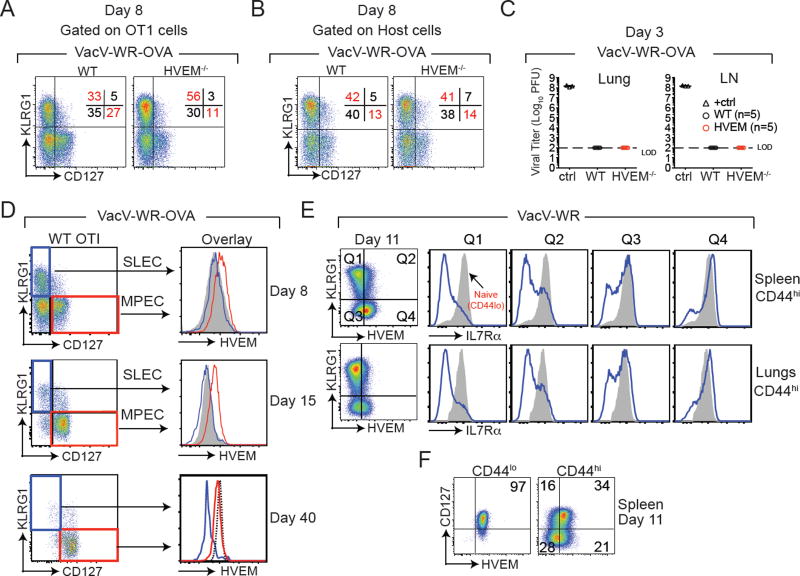
To determine if the loss of HVEM-deficient cells over time might be related to a defect in that minor population that maintained HVEM, and if this reflected the memory precursor population, we assessed the proportion of SLECs vs. MPECs in the lungs during virus infection. SLECs are characterized as KLRG1+CD127−, whereas MPECs are KLRG1−CD127+. The remaining two subsets called Early Effector Cells (EECs/ KLRG1−CD127−) and Double Positive Effector Cells (DPECs/ KLRG1+CD127+) are thought to be effector cells with intermediate states that are undergoing transition (27). At day 8 post-infection compared to WT controls, HVEM−/− OT-I cells gave rise to a significantly higher proportion of SLECs (KLRG1+CD127−) with a simultaneous reduction (~60 – 70%) in the formation of MPECs (KLRG1−CD127+) (Fig. 3A and Supplemental Fig. 1). In striking contrast, no gross changes were observed in the frequencies of host derived VacV-reactive SLEC and MPEC subsets in HVEM−/− OT-I recipient mice (Fig. 3B). Notably, recipient mice harboring WT and HVEM−/−OT-I donor cells cleared rVACV-WR-OVA equally well from the lung tissue and lung draining lymph nodes as early as day 3 post infection (Fig. 3C) with no effect on body weight (not shown). Together, these results show that the higher SLEC numbers in HVEM−/− OT-I recipients was not due to an altered inflammatory environment caused by HVEM deficiency on donor OT-I cells and/or higher viral loads. To show that the altered SLEC versus MPEC ratio was not a biased read-out of the OT-I transgenic system, we infected WT and HVEM−/− mice with VacV-WR. Similarly, we found that the immunodominant VacV-reactive B8R-specific CD8 T cell population was also skewed towards SLEC with concomitantly impaired MPECs (Supplemental Fig. 2).
Figure 3. HVEM is expressed preferentially by MPEC.
(A) WT and HVEM−/− OT-I cells from rVacV-WR-OVA infected recipient mice were stained for KLRG1 and CD127 at day 8 post-infection. (B) Endogenous CD8 T cells of the recipient mice (negative for Vα2Vβ5) were pre-gated on CD44hi cells and stained for KLRG1 and CD127 at day 8 post-infection. (C) Lungs and lymph nodes of recipient mice were harvested at day 3 post infection and plaque assay titers were performed on confluent Vero cells. Positive control represents serially diluted stock virus. Plaque assay experiment was performed once with n=5 mice/ group. (D) WT OT-I cells from recipient mice were harvested from the spleen at day 8, 15 and 40 post-infection with VacV-WR-OVA and HVEM expression analyzed on gated SLECS (blue line, KLRG1+, CD127−) and MPECs (red line, KLRG1−, CD127+). Gray shaded histogram HVEM−/− OT-I cells. Dotted line naïve (CD44lo) cells. (E & F) Naïve WT mice were infected with VacV-WR. At day 11, spleen and lung CD8+CD44hi cells were stained for KLRG1, HVEM, and CD127 (IL7Rα). Gray histograms, naïve (CD44lo) cells. Similar results were obtained from two independent experiments (n = 3 mice/group).
We then assessed HVEM expression on SLECs and MPECs in mice infected with rVacV-WR-OVA. At the peak of the effector cell response, HVEM was selectively expressed on MPECs and not present on SLECs (Fig. 3D). This was similarly apparent at day 15 post-infection (Fig. 3D). Not surprisingly based on our analysis of WT cells, by day 40 post-infection, all KLRG1−CD127+ memory cells expressed high levels of HVEM similar to naïve cells (Fig. 3D). To further investigate this possible selective expression of HVEM in the polyclonal CD8 T cell response, we analyzed WT mice infected with VacV-WR. As shown in Figure 3E, KLRG1+ cells that were HVEM− (Quadrant 1: Q1) were also negative for CD127, corresponding to SLECs whereas KLRG1− cells that were HVEM+(Q4) were mostly CD127 positive corresponding to MPECs (Fig. 3E). Further analysis revealed that HVEM was coordinately expressed with CD127 on naive and activated CD8 T cells (Fig. 3F).
To further investigate the idea that HVEM controls the proportion of MPECs vs SLECs that are either generated or maintained over time, we assessed the down-regulation of CXCR3 on KLRG1hi and KLRG1lo CD8 T cells that we have recently demonstrated to distinguish memory subsets that localize in particular niches in the lungs in response to respiratory VacV infection (13). Interestingly, whereas the proportion of KLRG1loCXCR3hi MPECs generated in the lungs when HVEM could not be expressed on CD8 T cells was reduced by approximately 2-fold (Supplementary Fig. 3A; upper left quadrant), HVEM−/− OT-I cells generated greater proportion of KLRG1hiCXCR3lo SLECs (Supplementary Fig. 3A; lower right quadrant), seen both at day 8 and day 15 post-infection.
Collectively, these data show that HVEM is downregulated strongly on the short-lived effector cell population during the peak of the primary response but is retained on the memory precursor effector cell population. They also suggest the expression on memory precursors is functionally relevant for this population of cells to persist and become memory cells.
HVEM expression on MPECs is necessary for their survival
To further substantiate these conclusions, we asked if HVEM was necessary for initial generation of MPECs by assessing expression of the transcription factors T-bet and Eomes that are known to be involved in the development of SLECs and MPECs respectively (4, 5). Most WT OT-I cells responding to virus (CD44hi) were T-bet positive and a proportion of these cells upregulated Eomes (Supplemental Fig. 3B). A similar percentage of cells were also Eomes positive when HVEM could not be expressed and analysis of MFI further showed that both WT and HVEM-deficient OT-I cells expressed similar levels of the two transcription factors (Supplemental Fig. 3B). This observation suggests that HVEM is unlikely to control the initial decision to become an MPEC, in line with the data that showed that some of these cells could still be found at day 8 post-infection (see Fig. 3B).
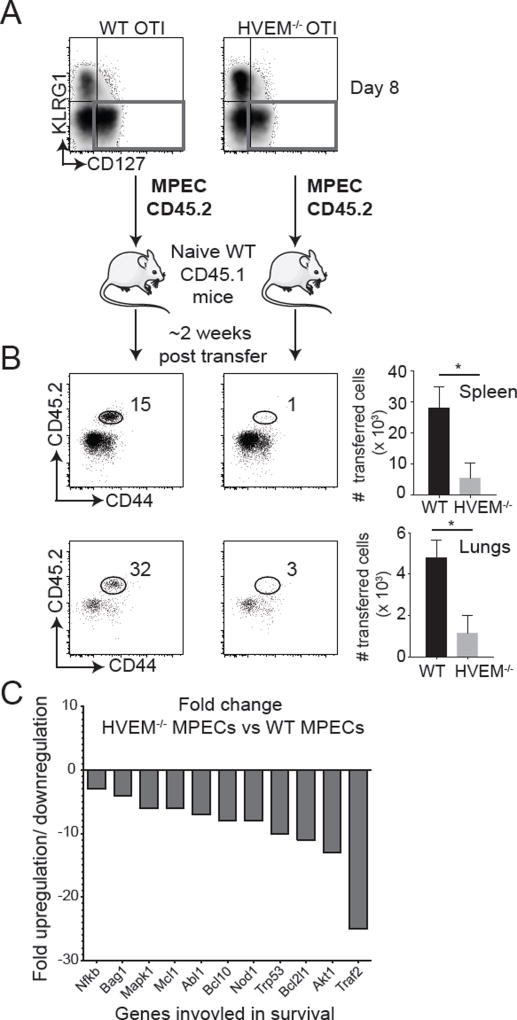
Next, we sorted WT and HVEM−/−OT-IMPECs from the spleens of recipient mice at day 8 post-infection and adoptively transferred equal numbers into separate naïve CD45.1+ congenic mice (Fig. 4A). Two-weeks post-transfer, we found dramatically reduced frequencies of HVEM−/− MPECs compared to their WT counterparts in the spleen and the lungs of these secondary recipients (Fig. 4B). To investigate whether the reduction in HVEM−/− MPECs was related to their impaired survival, a mouse apoptotic gene array was performed using RNA isolated from WT vs. HVEM−/− MPECs. This analysis showed that HVEM−/− MPECs exhibited reduced expression of a number of key T cell survival genes including Akt1, Bcl2l1, Nod1, Bcl1, Mcl1 (Fig. 4C). Moreover, there was a ~25-fold reduction in TRAF2 in MPECs lacking HVEM, which has been implicated as a direct downstream signaling target of HVEM (28). Overall, these data suggest that HVEM provides an essential survival signal to MPECs to enable their transition into the long-lived memory pool.
Figure 4. HVEM expression on MPECs is necessary for their survival.
(A) CD45.2+ MPECs (KLRG1−CD127+) from VacV-WR-OVA infected WT OT-I and HVEM−/− OT-I recipient mice were sorted at day 8 and equal numbers (2 × 106) adoptively transferred into naïve CD45.1+ congenic mice. (B) Two-weeks post-transfer, spleens and lungs were stained for CD8, Vα2, Vβ5, CD44 and CD45.2 and analyzed by flow cytometry to determine frequencies of persisting cells. *, P<0.05. (C) Total mRNA was isolated from sorted MPECs at day 8 of primary infection and transcript levels of apoptotic genes were measured using affymetrix mouse apoptotic gene array. Bars represent fold change in transcript levels between HVEM−/− MPECs compared to WT MPECs. Similar results were obtained in at least two independent experiments with (n = 3 mice/group).
The activity of HVEM might be a direct signaling effect of the receptor, as suggested by other studies of HVEM in CD4 T cells (29). It also could be indirect controlling expression of other receptors that can impact T cell survival such as CD127 (IL7Rα) (30). In line with some activity in relation to the latter, the expression of IL7Rα was less in HVEM−/− OT-I (Fig. 5A). No change in the expression of IL15Rα and IL15Rβ was observed (Fig. 5B). IL7Rα is known to activate the downstream JAK-STAT signaling pathway by phosphorylating STAT5, and HVEM−/−OT-I cells had markedly reduced pSTAT5 expression compared to WT OT-I cells (Fig. 5C) (31).
Figure 5. HVEM regulates IL7Rα expression on MPECs.
(A) WT and HVEM−/− OT-I cells from lungs of rVacV-WR-OVA infected recipient mice were stained for CD8, Vα2, Vβ5, CD44, IL7Rα, (B) IL15Rα, IL15Rβ and pSTAT5 (intranuclearly) at day 12, 14 and 40 post-infection. The projected flow cells were pre-gated on OT-I (Vα2, Vβ5, CD44). Histograms show WT OT-I cells in blue, HVEM−/− OT-I cells in red and naïve in shaded gray. Similar results are obtained from at least two independent experiments.
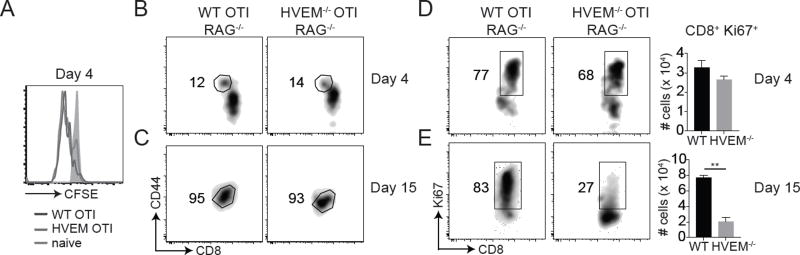
To seek direct evidence on whether intrinsic HVEM signals were required for cytokine-driven homeostatic expansion and/or survival of CD8 T cells, we sorted WT and HVEM−/− OT-I cells and adoptively transferred equal numbers into non-irradiated lymphopenic (RAG−/−) hosts (Fig. 6). By day four after transfer, the majority of donor WT OT-I CD8 T cells divided, and many underwent several rounds of division as measured by CFSE dilution and HVEM−/− OT-I cells displayed the same division profile as their WT counterparts (Fig. 6A). Few cells upregulated activation marker CD44 by day 4, however by day 15 post transfer, the majority of WT and HVEM−/− OT-I cells were CD44hi (Fig. 6B). Similar numbers of Ki67+ cells were recovered from the lung (Fig. 6C) and spleen (not shown) at day 4. Thus, direct HVEM signaling in CD8 T cells is not essential for induction of T cell division and accumulation early in response to either VacV infection or cytokine-driven proliferation. Strikingly, the overall percentage of CD8+Ki67+ cells generated from HVEM−/− donor populations was strongly reduced in the spleen (not shown) and lungs at day 15 (Fig. 6D), implying that far fewer proliferating cells survived in the absence of intrinsic HVEM signals. Again, these results mirrored the response of HVEM−/− OT-I cells to VacV infection with regards to survival of effectors and formation of memory.
Figure 6. Intact early homeostatic division but defective survival of CD8 T cells in absence of HVEM.
Equal numbers (5 × 104) of CFSE labeled WT naïve (CD44lo) OT-I and HVEM−/− OT-I (Vα2+Vβ5+) cells were adoptively transferred into RAG deficient mice. (A) Day 4 post-transfer, lungs were harvested and analyzed for CFSE dilution. Histograms show WT OT-I cells in blue, HVEM−/− OT-I cells in red and naïve in shaded gray. (B – E) Cells were harvested from lungs of RAG mice and stained for CD8, Vα2, Vβ5, CD44 and Ki67 at days 4 (B & C) and 15 (D & E) post-transfer. Experiment was performed one time with results are the mean ± SEM (n = 3 mice/group) and statistical significance as p<0.05; **, p<0.01; *.
LIGHT engages with HVEM to promote MPEC survival
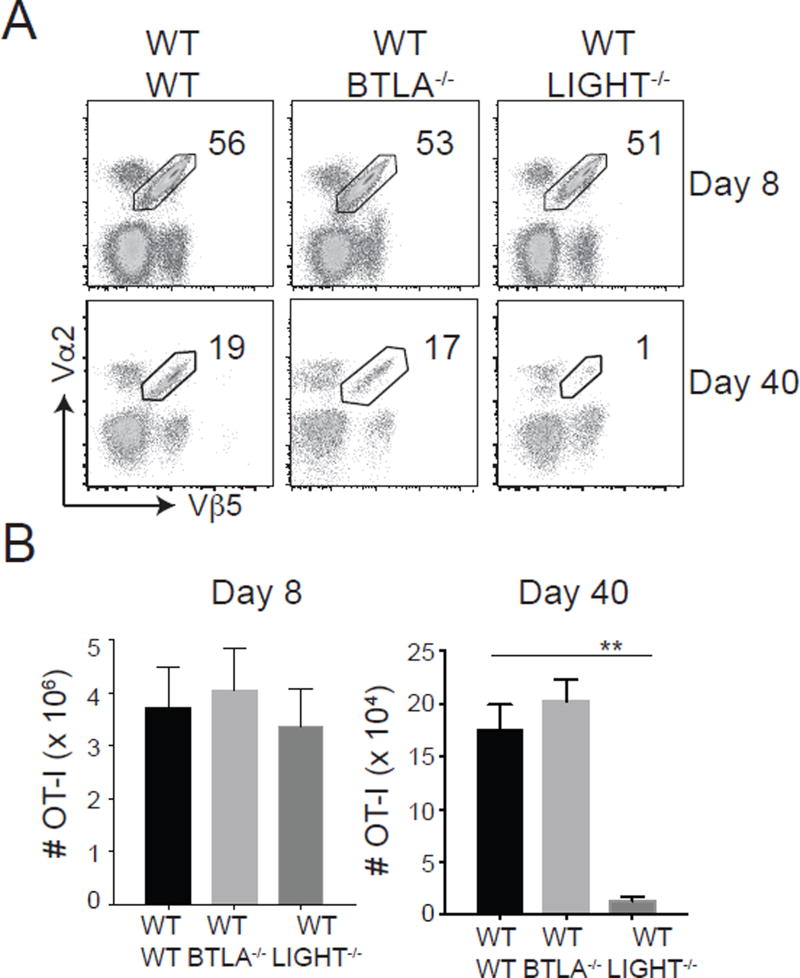
HVEM has several potential ligands that might drive its activity, namely LIGHT, BTLA, CD160 and LTα3 (32). Our prior results on HVEM controlling survival of T cells in other model systems suggested that LIGHT (29) or BTLA might be most important (19, 20, 32). To test this, we adoptively transferred WT OT-I cells into either LIGHT−/− mice or BTLA−/− mice. We observed no change in the frequencies and total numbers of effector OT-I CD8 T cell response at day 8 post rVacV-WR-OVA infection. However, we observed a dramatic defect in the accumulation of memory cells at day 40 in LIGHT−/− recipients but not in BTLA−/− recipients (Fig. 7). This result recapitulated the phenotype observed when HVEM−/− OT-I cells were transferred into WT mice, suggesting HVEM on an MPEC likely engages with LIGHT on an APC such as a dendritic cell or macrophage that can express this molecule, or alternatively HVEM engages with soluble LIGHT as it can also be cleaved from the membrane and function as a classical cytokine.
Figure 7. LIGHT controls CD8 T cell memory to respiratory virus.
Equal numbers (5 × 104) of WT naïve (CD44lo) OT-I (Vα2+Vβ5+) cells were adoptively transferred into WT, LIGHT−/− or BTLA−/− mice and infected with rVacV-WR-OVA (2 × 104 PFU; i.n) similar to Fig. 1. (A) Lungs were harvested at day 8 and 40 post-infection and stained for CD8, CD44, Vα2, and Vβ5. (B) Total OT-I CD8 T cell numbers were determined from flow data in A. *, P<0.05; **, P<0.01 and results are the mean ± SEM (n = 6 mice/group).
HVEM is necessary for protection against lethal challenge with respiratory virus
Lastly, to show that HVEM activity on MPECs was essential to generating a CD8 T cell response that would be protective to future encounters with virus, recipients of WT and HVEM−/− OT-I cells that were initially infected with rVacV-WR-OVA were re-challenged via the lungs on day 40 post-infection with a normally lethal dose of recombinant influenza virus PR8 strain expressing the OVA epitope SIINFEKL (Fig. 5A). As expected, the majority of WT OT-I recipient mice were able to survive the lethal challenge, and recovered from the initial weight loss caused by the virus. However, HVEM−/− OT-I recipients were dramatically impaired in protection and were not able to recover from weight loss with 100% of mice succumbing by 6 days post-infection, similar to naïve mice that did not receive OT-I T cells (Fig. 5B & 5C). In line with this, far fewer CD8 T cells reactive with OVA were detected by day 6 post-reinfection when HVEM was absent (Fig. 5D). To understand if this was due completely to the significantly reduced precursor frequency before re-challenge, we assessed the fold expansion between the groups from day 40 to day 6 post re-challenge. This showed that HVEM−/− OT-I cells expanded only 13-fold compared to 20-fold in WT OT-I cells (Fig. 8E), suggesting that HVEM may also have been active in the secondary response of these cells to the virus as well as in the primary MPEC response. Further substantiating the latter conclusion, the re-expanded secondary memory HVEM−/− OT-I cells consisted of a higher proportion of secondary SLECs (KLRG1+CD127−) and again a lower proportion of secondary MPECs (KLRG1−CD127+) compared to WT OT-I cells (Fig. 8F). Thus defective immunity to secondary virus challenge is likely due to both fewer numbers of memory cells in the lungs before challenge as well as weaker secondary proliferation.
Figure 8. HVEM is necessary for protection against lethal challenge with influenza virus.
(A) WT and HVEM−/− OT-I recipients infected initially with rVacV-WR-OVA as in Fig. 1 were challenged at day 40 with a normally lethal dose of PR8-SIINFEKL (i.n). (B & C) Mice were monitored for weight loss and survival over 15 days (n = 10 mice/group). (D–F) Lungs were harvested before re-challenge at day 40, and after challenge at day 6 and stained with CD8, CD44, Vα2, Vβ5, KLRG1 and IL-7Rα. (D) Representative flow plots of Vα2 and Vβ5 in gated CD8+CD44hi cells. As a control for the endogenous OVA-specific response, naïve mice were infected with PR8-SIINFEKL (no transfer). (E) Frequency of OT-I cells in the lungs and fold change in OT-I cell numbers from day 40 (memory) to day 6 (re-infection) quantified from flow data. (F) KLRG1 and IL-7Rα expression in gated OT-I cells. Results are the mean ± SEM (n = 4 mice/group). Similar results were obtained in at least two independent experiments.
Discussion
This study provides the first evidence that HVEM intrinsically shapes the balance between effector and memory T cell generation following an acute respiratory virus infection. HVEM did not play a role in expansion and accumulation of effector CD8 T cells in the lungs but contributed greatly to promoting memory precursor development and survival. The critical role of HVEM on virus-reactive CD8 T cells is demonstrated by the loss of protection against a lethal respiratory challenge. Thus, such a cell-surface signaling molecule that is involved in developing protectiveCD8 T cell memory could be an attractive adjuvant target in vaccination strategies against mucosal pathogens.
Although a number of studies have established that certain members of the TNF/TNFR family such as CD27-CD70, OX40/OX40L and 4-1BB/4-1BBL can influence the magnitude of CD8 T cell effector and memory responses to respiratory viruses (14, 33), none have so far defined a specific alteration in the ratio of SLEC/MPEC subsets in their absence. Our studies break new ground in this regard with the demonstration that, following infection with an attenuated-replicating VacV-WR strain (rVacV-WR-OVA) or a highly virulent wild-type VacV-WR strain, expression of HVEM is confined to MPECs at the peak of the effector response and lack of HVEM dramatically altered the SLEC/MPEC ratio, causing effector cells to skew more towards the SLEC phenotype and away from the MPECs. HVEM thus seems to play a unique role in not only being a positive regulator of MPEC development but also in maintaining brakes on excessive terminal differentiation. This outcome is seen with both the endogenous polyclonal VacV-reactive CD8 T cells as well as with monoclonal transgenic HVEM-deficient CD8 T cells (OT-I). Additionally, we observe the same outcome in the context of a respiratory infection with influenza virus. Thus, with different responding T cells and virus strains with variable virulence, the absence of HVEM signals to CD8 T cells prevents their ability to form large pool of circulating and tissue resident memory cells that are necessary for optimal protection against reinfection.
Previous studies have suggested that HVEM expression on T cells enables their optimal primary responses to both viral and bacterial antigens (19, 20), but none has so far related this pathway to altered SLEC/MPEC ratio and the longevity of MPECs overtime. Our results demonstrate for the first time that, the absence of HVEM alone on MPECs prevents them from surviving in adoptive transfer recipients. Gene expression profiling revealed intrinsic differences in the expression of key anti-apoptotic genes such as Akt1, Bcl2l1, Bcl10 and Mcl1 suggesting HVEM deficiency likely causes an imbalance between pro and anti-apoptotic genes. It is notable that MPECs lacking HVEM express substantially less TRAF2 mRNA than their WT counterparts, and we have previously shown that TRAF2 is a major downstream adaptor molecule linking membrane engagement of HVEM with downstream signal transduction and gene-regulation events that control the survival of CD4 T cells responding to non-replicating protein antigens (29, 32). This further suggests that HVEM likely controls the survival of MPECs by providing a direct downstream signal.
In addition, it is plausible that HVEM could also function indirectly by regulating cytokine signals such as IL-7 and IL-15 that are known to play prominent roles in T cell survival and homeostatic proliferation (30). We show evidence that in the context of respiratory virus infection, HVEM control optimal expression of IL7Rα but not IL15Rα or IL15Rβ in maturing MPECs. Moreover, reducedSTAT5 expression level substantiates that suboptimal IL7Rα expression has functional consequences on downstream intracellular signaling events. These findings are further corroborated by studies in lymphopenic RAG deficient mice where transferred T cells are thought to be largely dependent on cytokines for their proliferation and survival (34–36). Although, the transferred naive cells lacking HVEM divided normally and underwent intact proliferation, proliferated cells with an activated memory-like phenotype (CD44hi) had profound survival defects. These observations add to the accumulating evidence suggesting that cytokines alone are insufficient and additional feedback survival signals in the form of TNF receptor ligand interactions are needed for full maintenance of T cell memory pool (37).
In summary, our findings identify HVEM as an important costimulatory molecule that enables activated MPECs to survive and transition into long-lived memory pool, and enhance our understanding of the molecular interactions through which environmental cues establish a memory state in CD8 T cells. Vaccination strategies exploiting CD8 T cell immunity against mucosal pathogens would thus require HVEM engagement to promote MPEC development and survival
Supplementary Material
Acknowledgments
This study was supported by NIH grants AI77079 and AI087734 to S.S.-A, AI67341 to MC, CA164679 and AI067890 to CFW. P.D. was supported through The American Association of Immunologists Careers in Immunology Fellowship Program.
References
- 1.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol. 2009;27:61–82. doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- 3.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 6.Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 7.Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation. Immunity. 2012;36:374–387. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MV, Ouyang W, Liao W, Zhang MQ, Li MO. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity. 2013;39:286–297. doi: 10.1016/j.immuni.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abboud G, Stanfield J, Tahiliani V, Desai P, Hutchinson TE, Lorentsen KJ, Cho JJ, Avram D, Salek-Ardakani S. Transcription Factor Bcl11b Controls Effector and Memory CD8 T cell Fate Decision and Function during Poxvirus Infection. Front Immunol. 2016;7:425. doi: 10.3389/fimmu.2016.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J Immunol. 2010;185:5315–5325. doi: 10.4049/jimmunol.1001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8(+) T cells in the memory population mediate potent protective immunity. Immunity. 2013;38:1250–1260. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abboud G, Desai P, Dastmalchi F, Stanfield J, Tahiliani V, Hutchinson TE, Salek-Ardakani S. Tissue-specific programming of memory CD8 T cell subsets impacts protection against lethal respiratory virus infection. J Exp Med. 2016;213:2897–2911. doi: 10.1084/jem.20160167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croft M. The TNF family in T cell differentiation and function--unanswered questions and future directions. Semin Immunol. 2014;26:183–190. doi: 10.1016/j.smim.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goulding J, Tahiliani V, Salek-Ardakani S. OX40:OX40L axis: emerging targets for improving poxvirus-based CD8(+) T-cell vaccines against respiratory viruses. Immunol Rev. 2011;244:149–168. doi: 10.1111/j.1600-065X.2011.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 17.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 18.Murphy KM, Nelson CA, Sedy JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6:671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 19.Flynn R, Hutchinson T, Murphy KM, Ware CF, Croft M, Salek-Ardakani S. CD8 T cell memory to a viral pathogen requires trans cosignaling between HVEM and BTLA. PloS one. 2013;8:e77991. doi: 10.1371/journal.pone.0077991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinberg MW, Huang Y, Wang-Zhu Y, Ware CF, Cheroutre H, Kronenberg M. BTLA interaction with HVEM expressed on CD8(+) T cells promotes survival and memory generation in response to a bacterial infection. PLoS One. 2013;8:e77992. doi: 10.1371/journal.pone.0077992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salek-Ardakani S, Moutaftsi M, Crotty S, Sette A, Croft M. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181:7969–7976. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins MR, Webby R, Doherty PC, Turner SJ. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J Immunol. 2006;177:2917–2925. doi: 10.4049/jimmunol.177.5.2917. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, De Trez C, Flynn R, Ware CF, Croft M, Salek-Ardakani S. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J Immunol. 2009;182:6278–6286. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salek-Ardakani S, Arens R, Flynn R, Sette A, Schoenberger SP, Croft M. Preferential use of B7.2 and not B7.1 in priming of vaccinia virus-specific CD8 T cells. J Immunol. 2009;182:2909–2918. doi: 10.4049/jimmunol.0803545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salek-Ardakani S, Flynn R, Arens R, Yagita H, Smith GL, Borst J, Schoenberger SP, Croft M. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J Clin Invest. 2011;121:296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goulding J, Abboud G, Tahiliani V, Desai P, Hutchinson TE, Salek-Ardakani S. CD8 T Cells Use IFN-gamma To Protect against the Lethal Effects of a Respiratory Poxvirus Infection. J Immunol. 2014 doi: 10.4049/jimmunol.1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutishauser RL, Kaech SM. Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol Rev. 2010;235:219–233. doi: 10.1111/j.0105-2896.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg MW, Cheung TC, Ware CF. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev. 2011;244:169–187. doi: 10.1111/j.1600-065X.2011.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soroosh P, Doherty TA, So T, Mehta AK, Khorram N, Norris PS, Scheu S, Pfeffer K, Ware C, Croft M. Herpesvirus entry mediator (TNFRSF14) regulates the persistence of T helper memory cell populations. J Exp Med. 2011;208:797–809. doi: 10.1084/jem.20101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 31.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, D'Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg M, Spear PG, Ware CF. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A. 2009;106:6244–6249. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 34.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 35.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 37.Sabbagh L, Snell LM, Watts TH. TNF family ligands define niches for T cell memory. Trends Immunol. 2007;28:333–339. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.