Abstract
Recording neural activity in awake freely moving mice is a powerful and flexible technique for dissecting the neural circuit mechanisms underlying pathological behavior. This unit describes protocols for designing a drive and recording single neurons and local field potentials during anxiety-related paradigms. We also include protocols for integrating pharmacologic and optogenetic means for circuit manipulations, which when combined with electrophysiological recordings demonstrate input-specific and cell specific contributions to circuit wide activity. We discuss the planning, execution and troubleshooting of physiology experiments during anxiety-like behavior.
Keywords: in vivo electrophysiology, anxiety, local field potentials, implant construction, multi-site recordings, implant design
Introduction
Neural activity fluctuates as the internal state of an individual changes, affecting thoughts, feelings and behaviors. Behavioral animal models of psychiatric anxiety disorders provide experimentally defined windows for studying cognitive processing (Bowers and Ressler, 2015; Gunaydin and Kreitzer, 2016; Nestler and Hyman, 2010). Using such windows to record dynamic neural changes during well-characterized behavior can shed light on how brain activity governs anxiety and depression.
Extracellular electrodes implanted in a specific region record neural activity in several ways: action potentials from individual neurons (“single cells” or “units”), action potentials that cannot be attributed to individual neurons (“multiunit activity” or MUA), and local field potentials (LFP) from the fluctuating currents due to synchronous synaptic activity of many neurons. Such extracellularly recorded signals collected from awake, freely moving mice correspond to intracellular action potentials (Henze et. al., 2000; Harris et. al., 2000), and thus neural activity during behavior. Regardless of the type of signal, from single unit to LFP, each can dynamically shift with anxiety (Likhtik et. al., 2014; Harris and Gordon, 2015; Karalis et. al., 2016). Simultaneous multi-site recordings of LFPs and single units can provide a better understanding of cross-regional communication during anxiety. Using chronically implanted lightweight microdrives, researchers can record single units, MUA and LFPs from different regions during tasks that model specific aspects of anxiety disorders, such as avoidance behavior, fear generalization, and impaired extinction. Moreover, an explosion in genetic engineering has led to the development of tools that significantly improve the temporal and anatomical precision for testing if an area is a necessary and sufficient component of a properly functioning circuit during behavior. Thus, in addition to pharmacological inactivation of an area, optogenetic and pharmacogenetic approaches now provide the means for temporally precise cell-type specific, molecular-subtype specific, and projection-specific control within a circuit (Kale et al., 2015; Farrell and Roth, 2013). Combining in vivo physiology with tight temporal control of firing in defined cell populations brings a powerful approach to dissecting circuit function in anxiety (Johansen et al., 2012; Allsop et al., 2014).
This unit starts with a basic protocol for implanting neural recording drives in mice to record LFP activity during anxiety tasks (Basic Protocol 1). We then discuss methods for constructing and implanting moveable drives for recording single cell activity in freely moving mice (Basic Protocol 2, Support Protocol 1). We outline the means for combining optogenetic and pharmacological tools with physiological recording to manipulate neural circuits during behavior (Support Protocol 2). Next, we discuss how to perform electrolytic lesions to identify recording sites at the end of experiments (Support Protocol 3). Finally, we describe methods for troubleshooting electrical noise, as well as considerations for planning experiments, and the types of data one can expect to collect for analysis.
Basic protocol 1
Assembling and implanting a headstage for in-vivo physiology during behavioral tasks testing anxiety
Recording neural activity during behavior involves implanting electrodes into brain regions of interest. The electrodes are connected via an electronic interface board (EIB) to a headstage, which does some initial processing of the data, such as digitizing the analog recording via a microchip. A flexible tether connects the headstage to a neural signal acquisition board, which further processes the data (e.g. adds time stamps to each data sample) and stores it on a computer hard disk. Recording of the animal’s neural activity is synchronized with video recordings of the animal’s behavior. See figure 1 for a detailed overview of the animal behavior and physiology setup and table 1 for a list of commonly used recording equipment sources. Basic Protocol 1 starts with instructions for recording LFPs from multiple sites. We then describe how to combine single unit recordings with LFPs (Basic Protocol 2), and the procedures to 3D print the parts for a moveable drive (Support Protocol 1), to add an optic fiber or cannula to the physiology recordings for circuit manipulation (Support Protocol 2), and to perform electrolytic lesions at the end of experiments for marking electrode location (Support Protocol 3).
Figure 1.
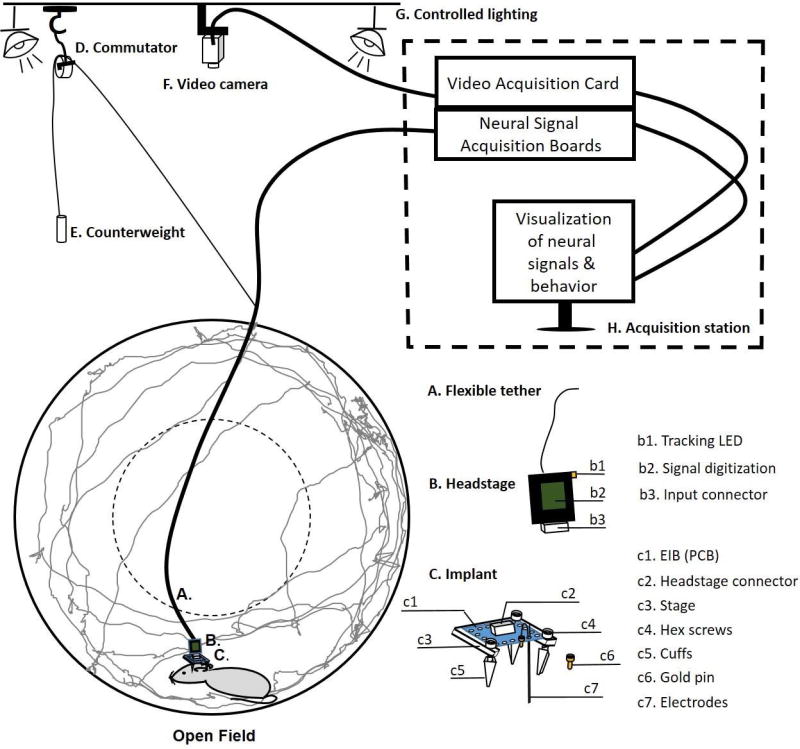
Overview of a setup for simultaneous animal behavior and physiology. A mouse is placed in an open field with an implanted set of electrodes that are connected to an electronic interface board (EIB, C) and secured to the skull with dental cement. Implant designs will vary. The shown implant has an EIB (c1) where implanted microwires (c7) and LFP wires are pinned via gold pins (c6). The EIB has a connector (Omnetics, c2) and sits on top of a custom- designed stage (Support Protocol 1, c3). The EIB attaches to the stage via three hex screws (c4) that also screw into custom-designed cuffs (c5). The screws are used to lower the headstage. The cuffs are anchored to the skull via dental cement. As the screws are rotated, the electrodes that are attached to the EIB (c1) are lowered down through the recorded area. A headstage (B) that plugs into the implant via a matching input connector (b3), and processes the recorded signals (if it is a digital headstage, it amplifies and digitizes the signal via an attached chip (b2)). The headstage (B) is connected to a Neural Signal Acquisition Board (H) via a flexible tether (A) that allows for free movement within an environment. The Acquisition Boards and associated computer are connected to a monitor that allows for setup and visualization of neural and video recordings via dedicated software. The tether is counterbalanced via a string that is attached to a commutator (D). This allows the tether to turn in all directions without tangling as the animal explores an environment. Commercial commutators will automatically turn to unwind a tether in the direction opposite to the animal’s movement. Alternatively a commutator can be made with a variety of turning pulley systems. The commutator-tether system is attached to a counterweight (E) in order to prevent the weight of the tether from pressing down on the subject. A video camera (F) and an adjustable lighting system (G) are installed above the behavioral setup to record animal behavior and to adjust lighting levels, thereby controlling anxiety (more light is anxiogenic). In the open field, cameras track animal movement via either an LED or an infrared reflective sticker attached to the headstage (b1). Tracked movement traces recorded with a ceiling-mounted video camera (F) during a typical open field exploration session are superposed on the open field (grey lines). The broken line denotes the center of the field, which is typically anxiogenic for animals, and they will spend less time exploring it than the periphery. The broken lines around the Acquisition Station (H) emphasize that while an experiment is being conducted, it’s best to separate the recording equipment and the investigator monitoring the experiment to a different room or behind a curtain.
Table 1.
Widely used electrophysiology recording equipment suppliers.
|
Materials
Mouse brain atlas (Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates)
Tungsten wires for LFP recordings (e.g. Straightened tungsten LFP wire; California Fine Wire, #100211)
Electrical interface board (the connector on the board has to be compatible with the connector on the headstage, example shown is Neuralynx, EIB-16)
Gold pins (Neuralynx, #S00038)
Pinner (constructed by filing down 2 mm off the bottom tip of a Halsey Needle Holder, Fine Science Tools, #12001-13)
Fine forceps (Fine Science Tools, #11294-00)
Student’s forceps (Fine Science Tools, #91110-10)
Wire for constructing ground and reference (e.g., Wire, Hook-Up; 30 AWG; 0.006 in.;0.22 in, Electronic Supply, #70134954)
Wire cutter/stripper (e.g. Ideal Industries #45-417)
Screws for anchoring to the skull (e.g., Stainless Steel Machine Screw, Plain Finish, Pan Head, Slotted Drive, Meets ASME B18.6.3, Right Hand Threads, Inch, Amazon, AMS120/1P-25)
Soldering iron (e.g. Weller, WES51)
Solder (MG Chemicals Sn99, 99.3% Tin and 0.7% Copper, No Clean Lead Free Solder, 0.032″ Diameter)
Flux (Harris SCLF4 Stay Clean Soldering Flux)
Small animal hair clippers (e.g., Braintree Scientific, CLP-9931 B)
Stereotax with stereotactic arms (e.g. David Kopf Instruments, 902)
Anesthesia induction chamber and ventilation system (isoflurane is advisable, Parkland Scientific, V3000PK)
Heating pad for maintaining temperature post-surgery (MaxHeat, SoftHeat Plus Heating Pad Moist/Dry, Full Size, 12-Inch by 15-Inch)
Temperature Controller (CWE, TC-1000, #08-13000)
Bone marker (Viscot Mini Surgical Fine Tip Markers)
Drill for making holes in the skull (Foredom Drill, #1070 High Speed Rotary Micromotor Kit, 2.35mm (3/32″) or 1/8″ Collet)
Drill burrs (0.5–0.9 mm; Fine Science Tools, 0.5mm, #19007-05; 0.7mm, #19007-07, 0.9mm, #19007-09)
Jeweler’s screwdriver (Tekton, #2985)
Bead sterilizer (Braintree Scientific, Germinator 500, #GER 5287)
Sharp scissors for making skin incision (Fine Science Tools, #14060-09)
Iris forceps for skin incision (Fine Science Tools, #11066-07)
Dumont AA Forceps (Fine Science Tools, #11210-10)
Dura pick (Fine Science Tools, #10065-15)
Betadine (Amazon)
Alcohol, 100% (Fisher Scientific, # 04-355-224)
Sterile Saline (Fisher Scientific, #NC9054335)
Dexamethasone (Henry Schein Vet, #002458)
Syringes, 1 ml (Fisher Scientific, #14-823-30)
Needles, 26 gauge (Fisher Scientific, #14-826-10)
Triple antibiotic ointment (Water Jet 2120 Bacitracin Zinc Triple Antibiotic Ointment)
Topical anesthetic (bupivicane, Pfizer Injectables, #0409-1163-01)
Analgesic (carpofen, Henry Schein Vet, #058739)
Cotton tip applicators (Fischer Scientific, #23-400-101)
Dental cement (Metabond, C&B Metabond #S380, Teets Cold Curing, AM Systems, #25000/526000)
Wooden applicators (Fisher Scientific, #23-400-104)
Cyanoacrylate (Loctite Liquid Professional, #1365882)
Kim wipes (VWR, #21905-026)
Isoflurane (Henry Schein Vet, #029405)
Preparatory steps
Prepare ground and reference screws by deinsulating 2mm (using the strippers, #9) from both sides of a 15 mm length of the silver wire (#8). Pre-tin both sides of the wire and solder one end to the machine screw (#10). Prepare two of these wires: one for the Reference screw, and one for the Ground screw. Be sure to apply flux and solder to only one-half of the head of the screw and to avoid getting solder in the slot (Supplementary Figure 1), as it’s important to fully access the slot with a screw driver (#22) when implanting.
-
Deinsulate and pre-tin two more pieces of silver wire. For an LFP-only implant, 10–15mm length of wire will suffice. If making an implant with a microwire bundle (Basic Protocol 2), the implant will sit higher up off the skull, and thus longer pieces of wire are necessary (typically around 25–30mm). Solder these two pieces into the Ground and Reference inputs on the EIB, respectively (see Figure 1c1, 3C–D). These will ultimately be soldered to the ground and reference screws from Step 1.
Note: Use rosin flux, rather than no-clean flux on the EIB as the latter can cause cross-connections. Note: If you buy EIBs commercially, make sure the Ground and Reference are not shorted anywhere on the EIB or the headstage.
-
Using a mouse brain atlas (#1), identify the stereotactic coordinates for regions of interest. Make sure that there is ample room on the skull for the implantation sites. These include any LFP wires that you plan to implant as well as the placement of the Reference and Ground skull screws that were prepared in Step 1.
Note: During surgery, the Ground and Reference wires (Supplementary Figure 1, 3C–D) that are soldered into the EIB will be soldered to wire that is attached to the skull screws above the head. Thus their length depends on the amount of space needed between the skull and the EIB. With a moveable drive, more space is needed for cuffs that lift the EIB higher off the surface of the skull.
-
Plan out the location of all the wires. There is an oval approximately 10 mm × 8 mm on an adult mouse’s head which has to accommodate the electrodes, a reference screw, a ground screw, the EIB and any additional drive materials (see Support Protocols 1–2). To allow the mouse to comfortably navigate, the implant should not protrude past the front of the mouse’s head and should not weigh more than 2.2 grams.
Note: When recording from multiple brain regions, keep in in mind that burr holes less than 0.5 mm apart are difficult to manage in the dental cementing step of the surgical implantation described below (Step #13).
Use an autoclave and/or bead sterilizer to sterilize the surgical equipment: fine forceps (#6), student’s forceps (#7), scissors (#24), iris forceps (#25), Dumont AA forceps (#26), dura pick (#27).
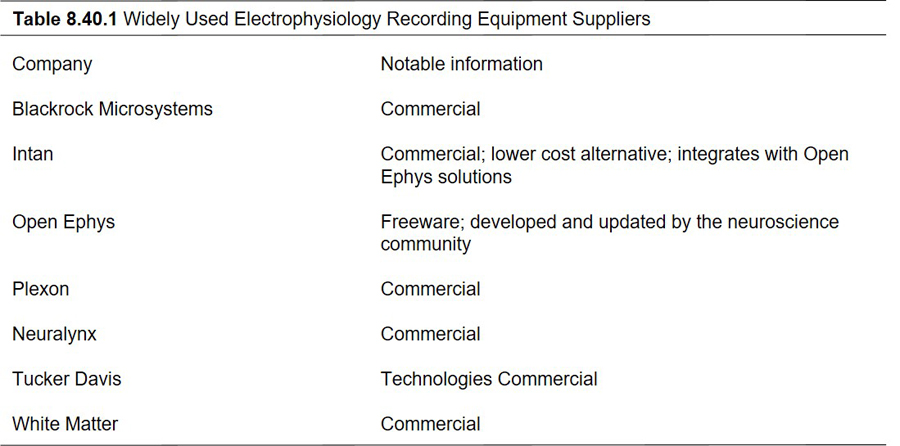
Figure 3.
Drive construction. (A1.B1). Designs (constructed in Blender) for 3D printing the stage (A1) and cuff (B1). These designs were optimized for a Neuralynx 16-channel EIB, and are available as Supplementary Material, and at likhtiklab.com/tools (A2,B2.) The 3D printed stage and cuff. Micrcoelectrodes will be carried on the open side of the stage (C.) The 16-channel EIB attached to the stage and cuffs by hex screws (side view). The reference and ground wires are soldered into the board prior to being mounted on the stage. (D.) Top view of the drive at this stage of assembly. Note that there are two hex screws that attach the board to the stage and a third screw that is providing stability. The Ground wire is in the back, and will be soldered to a screw above the cerebellum. The reference wire is in the front and will be soldered to the frontal reference screw. A – anterior, P - posterior. (E.) Microwires are pinned into the EIB and attached to an optic fiber for stability and accuracy of targeting, especially for deeper structures. (F.) The completed drive after all the wires have been covered with dental cement. Arrow shows a channel that was left open for an LFP wire that will be implanted and pinned into the drive during surgery. LFP channels added to the drive during surgery will also be covered up with dental cement. (G.) Additions to recording electrodes can be optic fibers or cannulae. Either of these can be customized to be implanted unilaterally or bilaterally. Arrowheads in 3E and 3F point to electrode bundle attached to an optic fiber.
Surgical Implantation
Electrodes should be implanted using standard approved stereotactic methods under aseptic conditions.
After inducing anesthesia (#16, #41,Isoflurane 2.5% mixed with oxygen), shave the mouse’s scalp using the clippers (#14). The animal should be maintained on continuous Isoflurane anesthesia throughout surgery, with anesthesia levels adjusted while monitoring for breathing rate and reflexes (0.6–1.5% mixed with oxygen).
Use dexamethasone (#31, 2 mg/kg) injected subcutaneously about 30 minutes prior to any drilling in order to minimize the impact of brain swelling on targeting.
Use local scalp injections of bupivacaine (#35, 0.1ml) at the site of the incision for local anesthesia (subcutaneous injection).
Maintain the mouse’s body temperature at 37 degrees Celsius with the temperature controller (#18) throughout the surgery.
Cover the eyes, whiskers and edges of the surgical incision with antibiotic ointment (#34) to prevent damage during surgery (e.g., from dripping dental cement).
After ensuring that the skull is level in all directions to within 30 microns, use a bone marker (#19) to mark Bregma and the stereotactic coordinates of the targeted brain regions.
Electrical muscle activity can interfere with neural recordings. Removing the muscular attachment at the intersection of parietal, temporal and occipital bones helps reduce that source of noise and does not appear to impair neck mobility.
Use a 0.7 mm burr (#21) to create holes for the ground screw (typically over the cerebellum) and reference screw (typically ~5–7mm rostral and ~3mm lateral of the frontal suture). Alternatively, you can short the reference and ground wires to the ground channel on the EIB (depending on what system you are using to record, this may negatively impact your signal, verify with the manufacturer first). To insert the Reference and Ground screws (Supplementary Figure 1) in the skull, hold the shaft of the screw above the hole with a student’s forceps and using a small jeweler’s screw driver (#22) turn the screw several times into the skull. The hole should be small so that the screw fully engages with skull and touches the cerebrospinal fluid without disturbing brain surface.
Dental cement the screw to the skull using the strongest dental cement you have available (e.g. Metabond). Make sure to cover the shaft and head of the screw with dental cement. Make sure no blood or cerebrospinal fluid is leaking from the screw as this can destabilize the screw and result in a noisy ground or reference signal.
-
Several precautions must be taken to avoid the skull screw and drive detaching from the skull during behavior.
The skull should be completely dry before applying any superglue or dental cement
Scrape the skull with a fine tipped forceps to provide additional surface area for bonding
Apply a layer of superglue to the skull before applying dental cement.
Consider adding machine screws (#10) on the skull for additional drive stability.
Drill a burr hole for the LFP wires. Using a dura pick (#27), fine forceps (#6), Kim wipes (#40), saline (#30), and small cotton tipped applicators (#37), clear the burr hole of any debris.
-
Implant each LFP wire by first fresh cutting the wire right before implantation, and slowly lowering it into position using the stereotactic arm (#15). Then dental cement it in place. To focally apply dental cement, use wooden applicator sticks (#38) broken or cut with a 45 degree bevel. Avoid obscuring Bregma with dental cement. Be sure that the dental cement has dried and the wire is securely in place before moving on to the next wire. The typical wait for dental cement will vary between 3–5min (depending on the cement and how liquid it is at the time of application).
Tip: 3-D printer resin can be cured with a UV laser pointer to rapidly secure light items such as LFP wires in place.
Thread the LFP wires into dedicated holes on the EIB as you lower the board over the head. Once it is about 10 mm above the head, place gold pins (Figure 1c6) into each hole. Use the long arm of the pinner to apply pressure to the head of the gold pin and bring it as deep into the hole as possible. Doing so removes the insulation from the electrode to provide a good electrical contact with the EIB. Remove excess LFP wire that protrudes above the EIB.
Solder the ground and reference wires emerging from the EIB to the respective wires attached to the skull screws. If using Isoflurane with oxygen, for safety briefly turn off the oxygen while using the soldering iron next to the mouse’s head.
Gently fold the excess wires under the EIB close to the skull and use them as scaffolding to further secure the EIB to the skull with dental cement. Avoid kinking the wire.
Cover all wire with dental cement. No wire should be visible. Protruding wire acts as an antenna for noise and can snag as the mouse walks around, breaking the wire or pulling the EIB off the head.
When the surgery is finished, give the mouse an analgesic (e.g. carpofen, 1mg/kg, i.p.), take the animal off anesthesia and place it in its home cage to recover from anesthesia. Place the cage such that half of it is on a heating pad (#17). Once the animal wakes up, it has the option of going to the unheated side of the cage.
Allow 1 week for the mouse to recover from surgery before beginning experiments.
Habituate the mouse to wearing the tether by plugging it in its home cage for 10–15 minutes for several days before beginning experiments.
Basic Protocol 2
Incorporating single unit recordings
There are several different options for electrodes that record single cell activity, ranging from silicon probes (Rossant et. al., 2016) to carbon fibers (Guitchounts et al., 2013) to bundles of microwires. We will describe using electrodes that are twisted together into stereotrodes (two wires) or tetrodes (4 wires, for recording in structures with densely packed cell layers) to help isolate individual neurons by the characteristic waveforms on the different wires (see Figure 6C for an example). Several stereotrodes are generally lowered as a bundle to ensure the rigidity necessary to penetrate brain tissue. We will describe how to build a moveable drive, which allows the investigator to lower the drive after implantation in order to record from different populations of cells within a structure during behavioral paradigms that last several days. An alternative approach is to fix the electrodes to the skull, sacrificing mobility for greater recording stability. This latter approach is particularly well suited to obtaining cells during single trial behaviors (see Commentary). Keep in mind that there are many ways to construct a drive (Liang et al., 2017; Headley et. al., 2015; Brunetti et. al., 2014; Felix et al., 2013). Your choice of drive will depend on the needs of your experiment.
Figure 6.
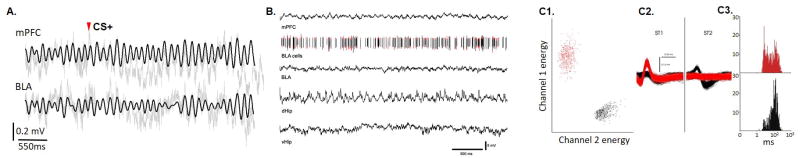
Physiological recordings of local field potentials at multiple sites with simultaneously recorded single units during aversive experience. (A.) Local field potentials recorded in two areas (the medial prefrontal cortex [mPFC] and the basolateral amygdala [BLA]) as a mouse is exposed to a previously trained aversive auditory conditioned stimulus (CS+, stimulus onset is shown with a red arrowhead). Raw recorded traces (grey) and theta range filtered traces (black, 4-12 Hz) are shown. (B.) Local field potential recordings from four areas (mPFC, BLA, dorsal hippocampus [dHip], and ventral hippocampus [vHip]) during exposure to an open field. Stereotrode microwires that were implanted in the BLA allowed for simultaneous acquisition of units and local field potentials. Lines representing spike times of two different units (red and black) are shown on the same time scale as the local field potential. (C.) Unit isolation for single unit analysis. (C1.) The clustering out in principal component space of the two units (red and black) shown in B (energy on Channel 1 and on Channel 2 were used as the first two dimensions for clustering). (C2.) The waveforms of the two units as they were recorded on each wire of the stereotrode (ST1 and ST2). (C3.) Inter-spike-interval histograms of the two clustered units. Cells were clustered out using SpikeSort3D.
Additional Materials
Microwires (12.5 micron Tungsten (California fine wire, #1000211) or Nichrome 80 (Sandvik, Kanthal, on Amazon # PX000004)
Twister for electrode construction (you can make your own, see the tutorial by Open Ephys listed in the Resources section or purchase a commercial one, e.g. Neuralynx)
Dissecting microscope (e.g. Leica S6 Stereomicroscope with Mountable Focus Arm S Series, #10447255)
Sharp scissors for cutting stereotrodes (e.g., Fine Science Tools #15006-09)
Dumont forceps (for making a drive, Fine Science Tools, #11294-00)
Student’s forceps (Fine Science Tools, #91110-10)
Teflon sheet/or 3-d printer (see Support Protocol 1, Formlabs)
Hex screws that will attach the EIB to the drive and will also be used to advance the drive (18-8 Stainless Steel Socket Cap Screw, Internal Hex Drive, Meets ASME B18.3/ASTM F837, ¼″)
Hex driver (Moody Tools, # 51-1572)
Metal cannulas (20 gauge; 16 gauge). These can be constructed from metal tubing or from syringe needles.
Alternatively, the wires can be attached to a fiberoptic instead of being fed through a cannula
Dremel (Dremel, #4000-6/50)
Dremel Cut-off Wheels .025″ thick (Dremel, #409)
Vet wrap bandaging, 2″ (Amazon)
Starrett digital calipers with 30 micron accuracy (Grainger, #2ZUG1)
Impedance tester (NanoZ, Neuralynx or White Matter)
Heat gun(Paladin, PA1873)
Hydraulic fine manipulator (Narishige, MO-10)
Preparatory steps: Making stereotrode electrodes and a moveable drive
-
Using the Open Ephys Twister (#2), spin a sufficient number of electrodes for your EIB (generally between 5–8 stereotrodes if using a 16-channel EIB). Apply 3–5 seconds of heat with a heat gun (#17, Additional Materials list) on three sides of the twisted wire (keeping the heat gun ~2 inches away from the wire) to ensure that the strands do not unravel. Cut the twisted wire with sharp surgical scissors (#4) to form two stereotrodes, each of which should have a pair of untwisted tails. Examine the electrodes and discard any electrodes with kinks or supercoiling.
Store the prepared electrodes next to one another on a Kim wipe so that they do not get tangled. You may store them for later use, preferably in a closed box.
Note: It’s useful to have separate sets of forceps and pinners that are used during surgery and for implant drive construction. Ideally, a separate workshop area is setup in the lab space for drive and optic fiber construction.
-
Using either a Teflon sheet or a 3-D printer, make the base of a drive (see Support Protocol 1 below) that has:
3 holes forming a right triangle with sides 7–8 mm long (for an example, see figure 3A,B). These are used for the 3/8″ hex screws that advance the drive (#8 from Additional Materials list).
Ensure ample room for implantation sites. The same guidelines described in Preparatory step 4 of Basic Protocol 1 apply here, with the additional consideration that there should be room on the skull for the 3 cuffs that anchor the drive to the skull (Figure 3B,C). To allow the mouse to comfortably navigate after implantation, the overall dimensions of a drive should not exceed 10 mm (wide) × 27 mm (long) and any part of the drive that protrudes past the skull should hang over the mouse’s back.
Using either a Teflon sheet or a 3-D printer, make 3 cuffs, 3 mm wide and 7 mm high (Figure 3B), with a center hole 0.5 mm radius wide and 3 mm deep (Support Protocol 1). Next, use a 3/8″ hex screw to tap the threading for the screws’ in the cuffs.
Fasten the EIB onto the drive base using ¼″ hex screws (Figure 3C,D)
Drive the 3/8″ hex screws through the drive base and 1.5 mm into the cuffs (Figure 3C,E)
Carefully glue a fiberoptic into one of the holes on the EIB (without filling the hole with glue) or to the side of the board. This will serve as an attachment point, anchor and guide for the microwires (Figure 3C,D).
-
Alternatively, using a Dremel with the Cut-off wheels tool attached (#12 and #13), cut a guide cannula (~10 mm long) from a 20-gauge needle. Ream the center of the cannula with a 26 gauge needle and file away any rough edges. Use superglue to affix the guide cannula within a premade hole on the drive base. Note, that if you are using this approach, you will need to include this hole in the design of your drive base.
Note: An optional outer guide cannula can be made from a 16 gauge needle (~6 mm long). Slide the inner electrode guide cannula through this outer cannula until it is flush with the drive base and hold it in place with wax. The outer cannula can then be gently lowered and rest on the skull after the electrode bundle has been implanted and acts as a sheath to protect the parts of the electrode bundle that remain exposed.
-
Using the dissecting microscope as necessary (#3 from Additional Materials list), gently thread the twisted part of the stereotrode through the guide cannula or the hole on the EIB. Avoid bending/kinking the electrode. Using forceps, place each tail of the stereotrode into a dedicated hole on the EIB and place a gold pin through that hole. Pin in place as described in Basic Protocol 1 (Step 14). Leave open holes on the EIB for any LFP wires to be implanted in other brain regions during surgery (see Figure 3F for an example).
Note: Try to avoid applying any pressure to the shaft of the gold pins which are quite delicate. Also, be careful not to tear previously pinned electrodes while pinning subsequent electrodes or breaking the EIB with the pressure of the forceps.
Using a saline-filled 1 mL syringe equipped with a 26 gauge needs, push out a drop of saline that continues to be attached to the needle, and using this drop of saline gather the electrodes together into a bundle to gather all electrodes into a bundle that emerges straight from the cannula. If necessary, use a very small quantity of superglue on a portion of the electrodes that will not enter the brain to hold the bundle together or to attach it to the cannula/fiberoptic. If the planned analysis includes LFP recordings, consider including a low impedance tungsten wire in the bundle. The LFP wire can serve as a stabilizing and guiding wire instead of the fiberoptic described in Step 6.
Use dental cement to cover all wire connections to the EIB. Be sure to cover all loose wires (Figure 3E–F) on the EIB so they won't snag and break, or pick up noise from the environment. Ensure that the Hex screws do not get covered by dental cement and can be turned freely (Figure 3F).
Using an impedance tester (#16), measure the impedance of the electrodes in saline. Stereotrode wires should measure between 300 and 900 kOhms. Note any open or shorted channels, or any wire below 30 kOhms.
Optional: Electroplating the electrodes (generally silver, gold or platinum) lowers the electrode impedance and can improve single unit yield (see Ferguson et. al., 2009). To plate, apply current and measure impedance (#16, Additional Materials list) through the electrodes while they are in the plating solution. Set a goal for the desired impedance (e.g., 180 kOhms). Overly low impedance can result in cross-connected electrodes (see Resources for gold-plating instructions from Neuralynx). If the electrodes do become cross – connected, they can be salvaged by cutting off the plated ends.
Typically, electrode placement is confirmed at the end of experiments using electrolytic lesions prior to perfusion (see Support Protocol 3). However, an alternative method is to coat the electrode with DiI just prior to implantation which provides a fluorescent marker of the electrodes location (Montgomery and Buzsáki, 2007)
Surgical Implantation
Surgical implantation of the drive should be done as described above with the following additions. After balancing the skull, implant hardware that will be fixed in place, such as skull screws and LFP wires, as described in Basic Protocol 1. Try to keep the dental cement securing these elements at a low profile (~ 1 mm height, as at Step 13, Basic Protocol 1). Dental cement should never obscure Bregma until all the wires have been implanted.
Using a stereotactic arm, position the pre-fabricated drive above the skull.
Thread any LFP wires that have already been secured to the skull through the open holes on the EIB (Figure 3F).
After ensuring that a burr hole is clear of debris, and that the bundle will enter the brain straight, fresh cut the stereotrodes to their final length using the sharp scissors (#4), and slowly lower the drive to implant it. The length of bundle protruding below the end of the cuff should account for ~1mm of space between the top of the skull and the top of cortex in addition to the dorso-ventral stereotaxic coordinate of the bundle.
It is crucial to remove dura and blood clots from the top of cortex to prevent damage to the stereotrodes. Consider using a pneumatic arm (#18, Additional Materials list) to lower the electrodes, minimizing brain tissue damage. Stop lowering the bundle 200 microns above the final destination. Once the bundle is lowered into place, the cuffs have to be very close to or at the skull surface. While lowering, remember to gently fold the LFP wire, and the Reference and Ground wires under the EIB (as in Step #16 of the Surgical Implantation Protocol, Basic Protocol 1).
Gently dental cement the cuffs (Figure 1c5, Figure 3) to the skull without moving the implant. Secure the drive to the skull at multiple attachment points. Avoid getting dental cement on any of the movable parts (electrode bundle, advancing screws). If using the optional outer cannula to protect the stereotrode bundle (Note to Step #7 in Preparatory Steps), gently lower it to rest on the skull using a forceps once the drive is securely attached.
Build up a wall of dental cement around the implant using the cuffs, the Reference and Ground wires, and the LFP wires that have been folded under the EIB as scaffolding. The dental cement wall will go up roughly 2/3rds up the height of the cuffs and will act as protection against debris going into the craniotomy with the bundle.
Once the drive is firmly secured with dental cement (so that applying pressure will not move the position of the electrodes within the brain), pin the LFP wires in the holes that were left open on the EIB (Figure 3F).
After the dental cement has dried, cover the drive with Vet wrap (#14, Additional Materials list) bandaging for protection against cage bedding, etc.
Post-surgical recovery and recording
Allow the mouse to recover and habituate as described in Basic Protocol 1 (Steps 18–20).
Plug the drive in to the recording apparatus and use the hex screws to advance in 80 micron increments (1/4 turn) until electrodes reach desired depth.
Support Protocol 1
3-D printing small parts for moveable drives
Materials
design software (e.g. Blender, RRID: SCR_008606)
3D printer (Formlabs, Form 1+)
resin for 3D printing (FormLabs, Black resin,#GPBK02)
isopropyl alcohol (VWR, #470301-474)
small UV oven (e.g. USpicy, #USND-3603)
Steps
Design a stage that will carry the EIB and the attached electrodes (Figure 3A, Supplmentary Figure 2), as well as the cuffs (Fig. 3B) using design software (e.g. Blender). Design files for the illustrated stage are provided as a Resource, and are available for download at http://likhtiklab.com/tools/
Transfer the design file to software that interfaces with a 3D printer (e.g. PreForm software for Formlabs printers)
Make appropriately sized supports for your design to print correctly (Supplementary Figure 2)
Print the design. Specifics will depend on the type of printer used. Ideally, the printer should be able to resolve details up to 30–50 μm.
Depending on the company you are using, after the printing is complete, you may need to soak the print in isopropyl alcohol (#4) for about 20 minutes.
Place your item in the UV oven (#5) to cure for at least 30 minutes.
After it has finished curing you can take the item off the supports and use for the drive.
If using Formlabs, it is important to adjust the placement of where each item (e.g. drive base, cuff) will be printed on the printer platform based on the dimensions of the item. For instance, smaller items may print more efficiently in the center of the platform, whereas larger items may print more efficiently further from the center. For a proper print, consider rotating the figure and printing it on an angle (Supplementary Figure 2). If a print fails, use a scraper to clean out any hardened residues on the bottom of the ink layer. When scraping, take care to gently smooth out any bubbles that appear along the resin layer. If your design contains holes, it is crucial to spend more time cleaning the print in isopropyl alcohol and shaking it to ensure that the resin does not cure within the hole. Keep in mind that a single print run can print many items at once. The Blender designs for the stage and cuff shown in figures 3A and 3B are available as Supplementary Material and can be downloaded from http://likhtiklab.com/tools/
Support Protocol 2
Incorporating optogenetic and pharmacological circuit manipulation
Experiments combining neural recordings with interventions that modulate neural activity can provide a more detailed cell-type specific circuit dissection during anxiety. These manipulations include pharmacological infusions of neurotransmitter agonists and antagonists into nodes of a circuit via a cannula, and more recently, optogenetics, or the expression of light activated proteins that increase or decrease neural activity in genetically defined populations. A full description of these methods is beyond the scope of this paper (for more details on optogenetics see Allen et. al., 2015; Grosenick et. al., 2015). Here we briefly describe how to integrate these methods into multi-site in vivo recordings during behavioral testing of anxiety.
If the planned experiments include optogenetic illumination, you can use the optic fiber attached to the electrode bundle for light delivery to the same area where recordings are performed (see Figures 3 and 4 for differently constructed examples). Use superglue to attach the electrodes to the optic fiber and cut the electrodes 300–500 μm past the end of the fiber (detail shown in Figure 4).
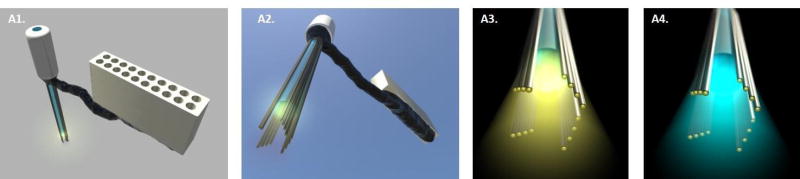
Figure 4.
Example design for the integration of microelectrodes with an optic fiber. (A1.) Top view of the assembly. Microelectrode wires are glued around the optic fiber with methylacrylate glue and connected to an Omnetics connector directly (without an EIB) with silver paint. For protection the wires running between the optic fiber and the connector are protected with silicone. (A2.) Bottom view of the optrode. The wires extend 300 -500 μm beyond the end of the optic fiber to record neural activity. (A3.) Detail of the bottom of the optrode that is shining yellow light (~589nm) to drive the inhibitory channel halorhodopsin with 16 microwires surrounding the fiber. (A4.) In this example, the same optrode is shining blue light (~465 nm) to activate the excitatory opsin channel rhodopsin. Arrowheads in 4A and 4B point to electrode bundle attached to the optic fiber. Images courtesy of Drs. Cyril Herry and Cyril Dejean (Neurocentre Magendie, INSERM, Université Bordeaux, France).
Materials
infusion cannulas (custom built; Plastics One)
optic fibers (200 μm; Thor Labs, #FT200UMT)
ceramic ferrules (230 μm, Thorlabs, #CFLC230-10)
silicon (Dow Corning, #3140 RTV)
Preparation
Polish optic fibers according to the Thor Lab guide (https://www.openoptogenetics.org/images/5/59/Guide_to_Connectorization_and_Polishing_of_Optical_Fibers.pdf).
Attach the fiber to the EIB (Figure 3) or if you are not using an EIB, the fiberoptic can be implanted with wires glued to it and attached directly to the Omnetics connector with silver paint (Figure 4). The wire that will be connected via silver paint has to first be stripped with a razor blade to deinsulate the part that will make contact. The wires are then covered with silicon (#4) for protection (Figure 4A–B).
Use superglue to attach optic fiber to the microwires or LFP wires and cut the electrodes 300 – 500 microns past the end of the fiber.
Surgical Implantation
Proceed with Step 1 as in Basic Protocol 2, but plan additional room on the skull for slightly larger burr holes (~1 mm radius) when incorporating cannulas or optic fibers.
As with the above case for LFPs, implant any cannulas or non-moving optic fibers before implanting a moveable drive.
If necessary to make room, after affixing optic fibers to the skull, the ferrules can be slightly angled away from the EIB to make room for the drive.
Support Protocol 3
Making an electrolytic lesion to identify electrode placement
In order to identify the placement of electrodes within brain tissue, you will need to perform a transcardial perfusion, section and stain the brain for soma or nuclei. Prior to perfusion, the final location of the tip of the recording electrodes has to be marked by a small electrolytic lesion for easy identification during histological processing. To do so, a small current is passed through the electrodes, burning the tissue next to the tips (see example in Figure 5, Histological Confirmation). Lesion localization allows one to see the cell layer or nucleus where the electrodes were located at the time of the lesion.
Figure 5.
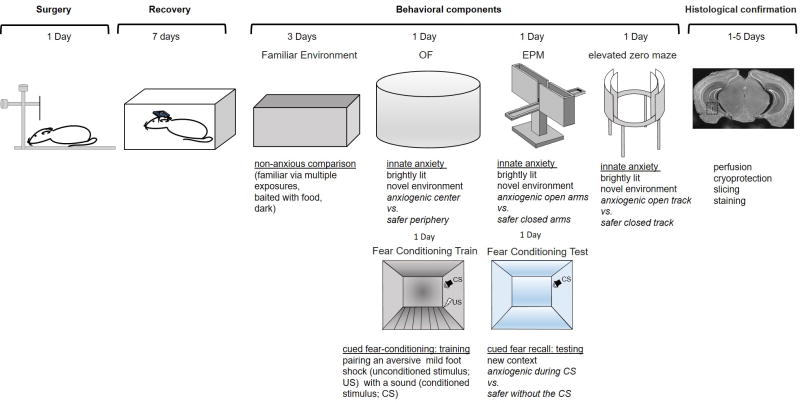
Suggested timeline of experiments. Top Row: Allot 1 day for surgery and 7 days for recovery before beginning behavioral experiments. To record neural activity during a non-anxious environment, habituate the mice to a neutral environment for 3 days and record neural activity on the third day. Mice can then be run through a battery of anxiety-related tasks, each of which has components that are anxiogenic and can be compared to the safer parts of the task. The suggested comparisons for behavior and physiology are highlighted in italics below each task. For example, in the open field (OF) test, mice tend to avoid the anxiogenic center of the brightly-lit novel environment and spend time on the safer periphery. Similarly, mice avoid the open areas of the elevated plus maze (EPM) and elevated zero maze. Each anxiety test should be separated by at least 1 day to avoid influencing performance on subsequent anxiety tests (Paylor et. al., 2006). After completing all recording sessions, passing current through the electrodes leaves a lesion (example outlined in box) that allows confirmation of electrode placement. Bottom Row: Learned fear paradigms, such as threat conditioning in which a mouse is trained to fear a conditioned stimulus (CS) such as an auditory stimulus by pairing it with an unconditioned stimulus (US) such as a mild footshock to the paws. The mouse can then be tested for learned fear by presenting the CS in a new context in the absence of the US and assaying the conditioned response (cue-dependent freezing or avoidance).
Materials
Ketamine (Henry Schein Vet, Ketathesia (100 mg/ml), #11695-0701-1)
Xylazine (Henry Schein, Anased (20mg/ml), #59399-110-20)
Lesion maker (e.g. Ugo Basile, Stoelting, #53500)
Specialized connector that fits the connector on the EIB with wires connected to each channel (Plexon, #CON/16f-VA8858-001) for passing current from the lesion maker
Lesioning
Deeply anesthetize the mouse with a mixture of ketamine and xylazine
Connect the implant to the specialized connector (#4) that will connect with the lesion maker input
Connect the wire carrying current from the lesion maker (#3) to the channel you would like to mark with a lesion on the specialized connector (#4).
Connect the ground to the mouse
-
Pass .05mA current for 20 seconds
Wait for about 10 minutes from the last lesion prior to perfusing
Commentary
Background Information
Multisite extracellular electrophysiology during freely-moving behavior was first primarily used in the hippocampus, and other midbrain structures to study learning and spatial encoding (Adey and Walter, 1963; O’Keefe and Dostrovsky, 1971; Ranck, 1973). Although extracellular physiology had been used in anesthetized and head fixed preparations prior to its application during behavior, most notably by Hubel and Wiesel for the study of single cell processing of visual stimuli (Hubel, 1959;Hubel and Wiesel, 1959), this new expansion into learning was moving into uncharted territory. The study of neural activity during aversive experience gained attention shortly thereafter with early amygdala recordings during paradigms such as aversive eye-blink conditioning (Pascoe and Kapp, 1985). As technology progressed, allowing for larger numbers of electrodes across more brain regions and lighter drives, exploration of neural activity during anxiety-related behavior has become more widespread. This protocol outlines an approach that will allow for simultaneous recordings from LFP and microwires in different brain regions during behaviors that test anxiety and anxiety- related learning. We provide a flexible design that can be used with a moveable or immobile drive, with or without additional parts for manipulation of neural activity. We provide a list of resources where other drive designs can be found, and these can be easily adapted to the general behavioral setup that we describe (Figure 1).
Critical Parameters and Troubleshooting
When planning for electrophysiology and behavior, consider the setup of the home cage where animals spend the majority of their time. The head mounted implants will need anywhere from 2–7cm of clearance above the head, thus cages with wire tops that do not leave room for the implant have to be changed for cages where the animal has plenty of head room. Furthermore, consider the setup of water delivery. Whereas some animal facilities have housing systems that allow for water delivery through spouts connected to the water supply in the room (e.g. OptiMice racks), others use water bottles in each cage. Make sure that the water delivery system is setup in such a way that it there are no spaces or protrusions where the implant can get caught, and ripped off the head. Consider water bottles that hang off the side of the cage (e.g. BioServ, #9019).
To obtain clean recordings during behavior it is imperative to control electrical and mechanical noise at multiple levels. Consider the various sources of noise: the building where you are conducting experiments, the equipment and/or lights in the room (60 cycle noise), noise due to malfunctioning implanted wires, noise due to the animal’s behavior, and noise due to the experiment (shock delivery through a grid floor). Therefore, noise control occurs at all stages, from planning the space where experiments will take place, to precautions taken during preparation of the drive, surgical implantation, and finally during recordings.
Prior to running experiments, it is important to make sure that there will be no noise carried through to the recording by the behavioral or recording equipment. Thus, it is important to make sure that your equipment is well-grounded. If possible, find out how the building where you are performing recordings is grounded. Grounding noisy equipment to the ground of an electrical outlet in the room where you are recording can be helpful. However, if the building contains ground loops, this approach may be limited. If there is a water pipe or a sink that connects to a water pipe in the room where recordings are taking place, you can also ground your equipment to the pipe (which is grounded inside the building).
After the animal’s implant is connected to the headstage, look at the quality of the signal in relation to the ground and the reference screws to check if one or both signals are contaminated by noise. If only one of them is noisy, this suggests that the noisy signal is coming from an improperly cemented skull screw. Cerebrospinal fluid that leaks from under the cement and out of the burr hole can destabilize the skull screw by dislodging the dental cement during and after surgery. To avoid leaks, make sure that the burr hole is completely dry when implanting the Reference and Ground screws.
Quality control for electrode and drive-related noise occurs during drive fabrication and implantation. Precautions include making sure that spun electrodes remain straight all throughout drive construction and surgery. When spinning electrodes, discard those that are supercoiled or contain sharply angled kinks, as both will introduce noise to the recording. Fresh-cutting LFP wires and microwires during implantation is important for getting rid of dirt build-up at the tip of the electrode, to prevent increased resistance and occlusion of signal. Decreasing impedance of microwires by plating electrodes (Ferguson et al., 2009) can be a helpful means for increasing cell yields during recording. During surgery, take utmost care to prevent bending the electrode bundle. When implanting the bundle, follow steps outlined in the Surgery Implantation Basic Protocols 1 and 2 to ensure that the bundle does not encounter blood clots, dust or other debris during insertion into cortex. Furthermore, lower the bundle to its final recording destination slowly over the course of several days (80–100 μm a day) after the animal has recovered.
When completing surgery, make sure to cover all parts of the drive that conduct electricity or that can act as antennae for noise with dental cement. Cover Ground and Reference wires, LFP wires and any skull screws with dental cement. Furthermore, on top of the EIB, cover all gold pins with dental cement as well. Proper coverage will strengthen the stability of the drive, and will protect electrodes from the mouse scratching at the skin surrounding the drive, or the drive hitting any obstacles in the home cage or during behavioral testing. If you encounter a drive that feels loose when you are connecting it to the headstage during habituation, briefly re-anesthetize the animal, and secure the drive to the skull with dental cement and glue.
Intermittent noise during recordings may be related to animal movement, food consumption, or a build-up of static noise due to the material used for the behavioral setup. One possible source of the noise is muscle movement conducted to the electrodes from the neck muscles that are touching the dental cement (known as electromyography or EMG noise). To avoid neck muscle related noise, it is important to remove neck muscles near the Ground screw during surgery, so they do not to touch the drive or the dental cement (see Basic Protocol 1, Surgical Implantation, Step 8). You can also implant a small silver electrode in the neck muscles, record EMG activity and then subtract it from your recording during analysis. Another possible source of noise is chewing artifact introduced via bone conduction when the animal is eating hard food. If you are interested in recording data during food consumption, introduce a soft food pellet or a liquid reward (e.g. condensed milk) instead of a hard food pellet. Alternatively, you can remove sections of data that are contaminated by chewing artifacts. Another source of occasional noise is the build-up of static electricity in the box where the animal is located during recordings. Consider using wooden (rather than plastic) behavioral apparatuses to avoid static noise.
LFP wires should be referenced to the skull screws while microwires that record local cellular activity should be referenced to other microwires (with the same impedance) that are quiet enough to serve as the reference. If spikes are collected by thresholding the amplitude of recorded events (e.g. all events above 40 μV), make sure that the input range is large enough to capture all the spikes, and the amplitude threshold is optimal to capture some of the noise and all of spikes above the noise. Alternatively, all channels may be collected with open filters, and spikes may then be isolated by filtering and amplitude thresholding during the analysis phase. When recording many channels during behavior, make sure that there is enough hard drive space on the computer that is storing the data during the session. Depending on the number of simultaneously recorded channels and sampling rate, drive space fill up quickly.
The general guidelines for planning and executing experiments are outlined in figure 5. After surgery, the animals should have at least a week to recover and regain pre-surgery weight and alertness prior to starting any experiments. The mice are then habituated to being plugged into the headstage and moving around with a tether (10–20 minutes a day, for 3 days in the home cage or another environment that becomes familiar with repeated exposure, Figure 5, Familiar Environment). The time line will then depend on the needs of your experiment. When designing anxiety-related behavioral experiments for the purpose of analyzing neural activity in mice, it is important to consider whether the behavior readily allows for several minutes of recordings during anxious and non-anxious portions of the task. For instance, the Elevated Plus maze and the Elevated Zero maze provide data from both innately anxiogenic open areas and the relatively safe closed areas (Figure 5, Top Row). Thus, in one task, comparisons of behavior and physiology as the animal explores each part of the maze will provide insight into neural processing during high and low anxiety states. When integrating intensely stressful paradigms such as fear conditioning into a behavioral battery, test order effects become important (Andreatini et al. 1999). Thus, fear conditioning should be done last unless the purpose of the experiment is to examine the effect of fear conditioning on the subsequent anxiety tests.
Other behavioral paradigms that easily combine with neural recordings include the open field test (Figure 5, Top Row), light-dark box, predator odor, conditioned place preference, immobilization stress, cued fear recall (Figure 5, Bottom Row) and extinction. Note that in all of these paradigms the tether that is attached to the animal’s head via the headstage (Figure 1B,C) must not be impeded to move freely by any boundaries (e.g. enclosure covers, Faraday Cages, etc). The advent of wireless recordings systems will take care of many (although not all) of these problems. However, wireless devices currently available are not yet light and small enough for mice and don’t transmit sufficiently large number of channels for high density recordings with the appropriate resolution (sampling rate). Several stress and anxiety tests present technical challenges for recording. For instance, during fear conditioning the electrical shock can interfere with neural recordings unless it is deflected from the headstage via a relay. Furthermore, shock delivery often causes the animal to jump and if the fear-conditioning box is not large, to hits its head and the implant on the ceiling, introducing noise to the recording (fear conditioning boxes will typically have a ceiling with a cutout line for the tether). Likewise, the implant can be damaged by water during a forced swim test and by physical contact during resident-intruder tests during paradigms such as social defeat.
Anticipated Results
The information that can be extracted from an in-vivo extracellular recording is affected by the type of electrode that is used (the material, electrode thickness, its impedance, whether it is gold plated, etc), the sampling rate of the recording, and the post-processing during the analysis phase of the experiment. In general, oscillatory activity in the range of <1 to about 400 Hz can be reliably obtained from an LFP wire, which is well within the physiological range of extracellular oscillations. Microwires will allow for the sampling of higher frequency activity, and therefore neural firing. Examples of recorded neural activity during behavior are shown in figure 6. Raw recordings (unfiltered recordings), or recordings obtained with a wide filter, such as LFP recordings (1 – 1000 Hz) can be filtered for the desired LFP frequency bands in the analysis phase (Figure 6a,b). Single cell neural activity is extracted by applying a different filter (600 – 6000Hz) to open channel recordings, or by collecting thresholded spikes during recording (Figure 6b, c). Spikes collected with multiple wires (stereotrodes, tetrodes) can be sorted by using principal component analysis techniques (Rossant et al., 2016) to identify single units.
A range of analyses of single cell firing characteristics (e.g. firing rate) and their relationship to oscillatory activity in different brain regions and frequency bands will give insight into neural synchrony between regions at different stages of behavior. Furthermore, adding area specific, cell-specific or input specific control over a region enriches our understanding of circuit function in behavior. Behavioral paradigms with precise temporal boundaries, particularly those that have repeated trials within a task, such as cued fear recall, are helpful for defining equal time windows for analysis of neural activity during anxiogenic (conditioned stimulus presentation) and anxiolytic (period of time immediately preceding the conditioned stimulus) phases of a task. Tasks without this inherent structure, such as the open field test, can either be artificially organized into trials (e.g., entries into the center third of the open field represent anxiogenic periods, while entries into the outer third represent safe periods). Alternatively, such data can be treated as a continuous variable, for instance by correlating neural activity with distance from the anxiogenic center of the open field. Finally, paradigms that can be repeated in the same subject over several days allow the experimenter to advance the electrodes through a brain structure and collect more single units for each subject. However, the anxiogenic quality of many paradigms attenuates with repeated exposure, and the physiological recordings will reflect this change. Thus, recordings for most anxiety tasks should be designed in such a way as to obtain sufficient neural activity from a single session. This limitation can occasionally be overcome by slightly varying the task. For example, one could record in the elevated plus maze followed by the elevated zero maze.
Time Considerations
Planning an experiment should take into account the time it takes to construct an implant, perform an implantation surgery, the recovery period (typically 1 week), and the length of the behavioral paradigm. The drive design described in this protocol should take an experienced individual anywhere between 1.5–4 hours to complete (depending on the number of channels). Surgery can take anywhere from 2 – 8 hours, depending on the number of items that are being implanted. For experiments that use virally delivered opsins, the time it takes for the virus to incorporate and express the various channels either at the cell bodies or at the terminals, also needs to be taken into consideration (on the order of weeks). Furthermore, one should consider that time it takes to analyze the obtained recordings during different phases of the task. Large datasets can lead to months of analysis.
Supplementary Material
Figure 2.
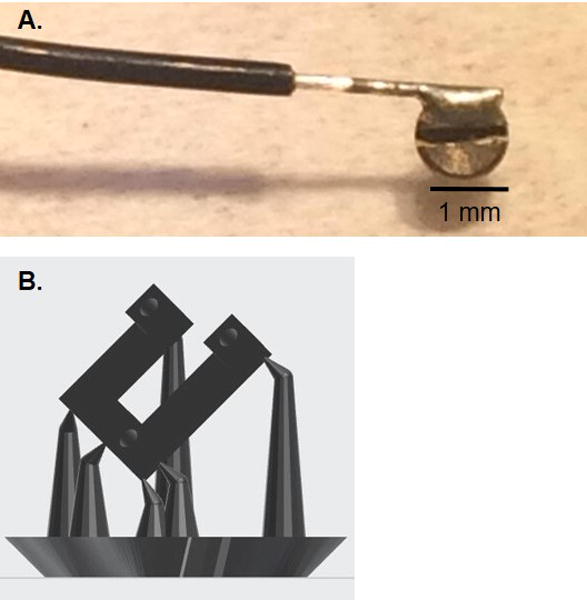
Details of Reference/Ground skull screw and 3D-print orientation. (A.) Head of machine screw with silver wire soldered to half of the head of the screw. This screw can be used as either a Reference or a Ground screw. Note that the slot remains accessible and is not covered by solder. (B.) Preform-generated supports for 3D printing a drive base. The supports place the object at an angle to generate better resolution during the print.
Significance Statement.
Long-range projection neurons connect distant brain regions creating the neural circuits that govern complex behavior. Communication in these pathways fluctuates dynamically with changes in thoughts, feelings and behavior. To understand the neural activity underlying anxiety we need to chart the dynamic changes of circuit-level communication as we move from neutral to anxiety-provoking situations. To do so, researchers can simultaneously record and modulate electrical activity in multiple brain regions in mice as they engage in tasks that have distinct neutral and anxiogenic components. In this unit we provide instructions for integrating neural recordings from multiple brain regions during anxiety tasks with contemporary methods for modulating brain activity.
Acknowledgments
A.Z.H. is supported by The Hope for Depression Foundation (HDRF). E.L. is supported by the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH) under award numbers K01MH105731 and P50MH096891, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (Grant#24006).
Footnotes
Conflicts of Interest
The authors have declared no conflicts of interest for this article.
Literature Cited
- Adey WR, Walter DO. Application of phase detection and averaging techniques in computer analysis of EEG records in the cat. Exp Neurol. 1963;7:186–209. doi: 10.1016/0014-4886(63)90054-2. [DOI] [PubMed] [Google Scholar]
- Allen BD, Singer AC, Boyden ES. Principles of designing interpretable optogenetic behavior experiments. Learn Mem. 2015;22:232–238. doi: 10.1101/lm.038026.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci. 2014;8:241. doi: 10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreatini R, Bacellar LFS. The relationship between anxiety and depression in animal models: a study using the forced swimming test and elevated plus-maze. Brazilian Journal of Medical and Biological Research. 1999;32(9):1121–1126. doi: 10.1590/s0100-879x1999000900011. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Ressler KJ. An overview of translationally informed treatments for posttraumatic stress disorder: animal models of Pavlovian fear conditioning to human clinical trials. Biol Psychiatry. 2015;78:E15–27. doi: 10.1016/j.biopsych.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti PM, Wimmer RD, Liang L, Siegle JH, Voigts J, Wilson M, Halassa MM. Design and fabrication of ultralight weight, adjustable multi-electrode probes for electrophysiological recordings in mice. J Vis Exp. 2014;91:e51675. doi: 10.3791/51675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Roth BL. Pharmacosynthetics: Reimagining the pharmacogenetics approach. Brain Res. 2013;1511:6–20. doi: 10.1016/j.brainres.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix SH, Shah KG, Tolosa VM, Sheth HJ, Tooker AC, Delima TL, Jadhav SP, Frank LM, Pannu SS. Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable adhesive. J Vis Exp. 2013;79:50609. doi: 10.3791/50609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JE, Boldt C, Redish AD. Creating low-impedance tetrodes by electroplating with additives. Sens Actuators A Phys. 2009;156:388–393. doi: 10.1016/j.sna.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L, Marshel JH, Deisseroth K. Closed-loop and activity guided optogenetic control. Neuron. 2015;86:106–139. doi: 10.1016/j.neuron.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Kreitzer AC. Cortico-basal ganglia circuit function in psychiatric disease. Annu Rev Physiol. 2016;78:327–50. doi: 10.1146/annurev-physiol-021115-105355. [DOI] [PubMed] [Google Scholar]
- Guitchounts G, Markowitz JE, Liberti WA, Gardner TJ. A carbon-fiber electrode array for long-term neural recording. J Neural Eng. 2013;10:046016. doi: 10.1088/1741-2560/10/4/046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Gordon JA. Long-range neural synchrony in behavior. Annu Rev Neurosci. 2015;38:171–194. doi: 10.1146/annurev-neuro-071714-034111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsáki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol. 2000;84:401–4014. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- Headley DB, DeLucca MV, Haufler D, Paré D. Incorporating 3D-printing technology in the design of head-caps and electrode drives for recording neurons in multiple brain regions. J Neurophysiol. 2015;113:2721–2732. doi: 10.1152/jn.00955.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsáki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol. 2000;84:390–400. doi: 10.1152/jn.2000.84.1.390. [DOI] [PubMed] [Google Scholar]
- Hubel DH. Single unit activity in striate cortex of unrestrained cats. J Physiol. 1959;147:226–238. doi: 10.1113/jphysiol.1959.sp006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurons in the cat’s striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Wolff SB, Lüthi A, LeDoux JE. Controlling the elements: an optogenetic approach to understanding the neural circuits of fear. Biol Psychiatry. 2012;71:1053–1060. doi: 10.1016/j.biopsych.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale RP, Kouzani AZ, Walder K, Berk M, Tye SJ. Evolution of optogenetic microdevices. Neurophotonics. 2015;2:031206. doi: 10.1117/1.NPh.2.3.031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis N, Dejean C, Chaudun F, Khoder S, Rozeske RR, Wurtz H, Bagur S, Bencheanane K, Sirota A, Courtin J, Herry C. Nat Neurosci. 2016;19:605–612. doi: 10.1038/nn.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Oline SN, Kirk JC, Schmitt LI, Komorowski RW, Remondes M, Halassa MM. Scalable, lightweight, integrated and quick-to-assemble (SLIQ) hyperdrives for functional circuit dissection. Front Neural Circuits. 2017 doi: 10.3389/fncir.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala M, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Buzsáki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Bran Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiology & Behavior. 2006;87(1):95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Pascoe JP, Kapp BS. Electrophysioloogical characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res. 1985;16:117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Rank JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. 1973. Ex Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Rossant C, Kadir SN, Goodman DF, Schulman J, Hunter ML, Saleem AB, Grosmark A, Belluscio M, Denfield GH, Ecker AS, Tolias AS, Solomon S, Buzsáki G, Carandini M, Harris KD. Spike sorting for large, dense electrode arrays. Nat Neurosci. 2016;19:634–641. doi: 10.1038/nn.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.