Abstract
Importance
The Environmental Protection Agency (EPA) is required to re-examine its National Ambient Air Quality Standards (NAAQS) every 5 years, but evidence of mortality risk is lacking at air pollution levels below the current daily NAAQS, in unmonitored areas and for sensitive subgroups.
Objective
To estimate the association between short-term exposures to ambient PM2.5 and ozone and at levels below the current daily NAAQS and mortality in the continental US.
Design, Setting, and Participants
Case-crossover design and conditional logistic regression to estimate the association between short-term exposures to PM2.5 and ozone (mean of daily exposure on the same day of death and one day prior) and mortality in 2-pollutant models. The study included the entire Medicare population from January 1, 2000 to December 31, 2012 residing in 39,182 zip codes.
Exposures
Daily PM2.5 and ozone levels in a 1 km × 1 km grid were estimated using published and validated air pollution prediction models based on land use, chemical transport modeling, and satellite remote sensing data. From these gridded exposures, daily exposures were calculated for every zip code in the US. Warm-season ozone was defined as ozone levels for the months April to September of each year.
Main Outcome and Measure
All-cause mortality in the entire Medicare population from 2000 to 2012.
Results
During the study period, there were 22,433,862 million case days and 76,143,209 control days. Of all case and control days, 93.6% had PM2.5 levels below 25 μg/m3, during which 95% of deaths occurred (21,353,817 of 22,433,862), and 91.1% of days had ozone levels below 60 ppb, during which 93.4% of deaths occurred (20,955,387 of 22,433,862). The baseline daily mortality rate was 137.33 and 129.44 (per 1 million persons at risk per day) for the entire year and for the warm season, respectively. Each short-term increase of 10 μg/m3 in PM2.5 (adjusted by ozone) and 10 ppb (parts-per-billion, 10−9) in warm-season ozone (adjusted by PM2.5) were statistically significantly associated with a relative increase of 1.05% (95% confidence interval [CI]: 0.95%, 1.15%) and 0.51% (95% CI: 0.41%, 0.61%) in daily mortality rate, respectively. Absolute risk differences in daily mortality rate were 1.42 (95% CI: 1.29, 1.56) and 0.66 (95% CI: 0.53, 0.78) per 1 million persons at risk per day. There was no evidence of a threshold in the exposure-response relationship.
Conclusions and Relevance
In the US Medicare population from 2000-2012, short-term exposures to PM2.5 and warm-season ozone were significantly associated with increased risk of mortality. This risk occurred at levels below current national air quality standards, suggesting that these standards may need to be reevaluated.
Introduction
In the US, the Clean Air Act (42 U.S.C. §7401 et seq. [1970]) requires a review of National Ambient Air Quality Standards (NAAQS) for fine particulate matter (PM2.5) and ozone every 5 years.1 In 2012, the annual and 24-hour NAAQS for PM2.5 were set to 12 μg/m3 and 35 μg/m3, respectively. With no annual standard for ozone, the 8-hour NAAQS for ozone was set to 70 ppb. Currently, the review of these standards is ongoing with public comments expected in the Fall of 2017.2
Several studies have provided evidence that short-term exposures to PM2.5 and ozone were associated with mortality,3–7 but these studies primarily included large and well-monitored metropolitan areas. While the US Environmental Protection Agency (EPA) is considering more stringent NAAQS, evidence is needed to clarify the association between mortality risk and exposure levels below the daily NAAQS, and in rural and unmonitored areas.
The Clean Air Act also requires the US EPA to set standards to protect “sensitive subgroups.” To estimate the health risk of short-term exposure to air pollution for specific subgroups (e.g., underrepresented minorities and those with low socioeconomic status, such as persons eligible for Medicaid), a large population is necessary to achieve maximum accuracy and adequate statistical power.
A case-crossover study was conducted to examine all deaths of Medicare participants in the continental US from 2000 throughout 2012 and estimate the mortality risk associated with short-term exposures to PM2.5 and ozone in the general population as well as in subgroups. The study was designed to estimate the association between daily mortality and air pollution at levels below current daily NAAQS to evaluate the adequacy of the current air quality standards for PM2.5 and ozone.
Methods
This study was approved by the IRB at the Harvard T.H. Chan School of Public Health. As a study of previously collected administrative data, it was exempt from informed consent requirements.
Study population
Using claims data from the Centers for Medicare and Medicaid Services, all deaths among all Medicare beneficiaries were identified during the period 2000 to 2012, providing enough power to analyze the risk of mortality associated with PM2.5 and ozone concentrations much lower than the current standards. For each beneficiary, information was extracted on the date of death, age, sex, race, ethnicity, zip code of residence, and eligibility for Medicaid (a proxy for low income), to assess the associations of mortality with PM2.5 and ozone concentrations in potentially vulnerable subgroups Self-reported information on race and ethnicity was obtained from Medicare beneficiary files.
Outcome
The study outcome was all-cause mortality. Individuals with a verified date of death between January 1, 2000 and December 31, 2012 were included. Individuals with an unverified date of death, or still living after December 31, 2012, were excluded.
Study design
We estimated the association between short-term exposure to PM2.5 (adjusted by ozone) and short-term exposure to ozone (adjusted by PM2.5), and all-cause mortality using a case-crossover design.8 Specifically, “case day” was defined as the date of death. For the same person, we compared daily air pollution exposure on the case day vs daily air pollution exposure on “control days.” Control days were chosen (1) on the same day of week as the case day to control for potential confounding effect by day of week; (2) before and after the case day (bidirectional sampling) to control for time trend;9,10 and (3) only in the same month as the case day to control for seasonal and sub-seasonal patterns.9,11 Individual-level covariates and zip code-level covariates that did not vary day-to-day (e.g., age, sex, race, socioeconomic status, smoking, and other behavioral risk factors) were not considered to be confounders as they remain constant when comparing case days vs control days.
Environmental data
Daily ambient levels of PM2.5 and ozone were estimated from published and validated air pollution prediction models.12,13 Combining monitoring data from EPA, satellite-based measurements, and other data sets, neural networks were used to predict 24-hour PM2.5 and 8-hour maximum ozone concentrations at each 1 km × 1 km grid in the continental US, including locations with no monitoring sites. Cross-validation indicated good agreement between predicted values and monitoring values (R2 = 0.84 for PM2.5, R2 = 0.76 for ozone) and at low concentrations (R2 = 0.85 when constraining to 24-hour PM2.5 <25 μg/m3; R2 = 0.75 when constraining to daily 8-hour maximum ozone <60 ppb). Details have been published elsewhere.12,13 Warm season is defined to be from April 1 to September 30, which is the specific time window to examine the association between ozone and mortality. Meteorological variables including air and dew point temperatures were retrieved from North American Regional Reanalysis data and estimated daily mean values were determined for each 32 km × 32 km grid in the continental US.14
For each case day (date of death) and its control days, the daily 24-hour PM2.5, 8-hour maximum ozone, and daily air and dew point temperatures were assigned based on zip code of residence of the individual (Section 1, Supplementary Material). Since we estimated air pollution levels everywhere in the continental US, the number of zip codes included in this study was 39,182, resulting in a 33% increase compared to the number of zip codes with a centroid <50 km from a monitor (N = 26,115).
Statistical analysis
The relative risk (RR) of all-cause mortality associated with short-term exposures to PM2.5 (adjusted by ozone) and warm-season ozone (adjusted by PM2.5) was estimated by fitting a conditional logistic regression to all pairs of case days and matched control days.8 The regression model included both pollutants as main effects, and natural splines of air and dew point temperatures with 3 degrees of freedom to control for potential residual confounding by weather. For each case day, daily exposure to air pollution was defined as the mean of the same day of death (lag 0 day) and one day prior (lag 1 day), denoted as lag 01 day.4,15,16 The absolute risk difference (ARD) of all-cause mortality associated with air pollution was defined as ARD= α × (RR-1)/RR, where RR denotes the relative risk and α denotes the baseline daily mortality rate (Section 2, Supplementary Material).
The robustness of the analysis results was assessed with respect to (1) choosing the degrees of freedom used for the confounding adjustment for temperature, (2) using lag 01 day exposure as the exposure metric, (3) the definition of warm season, and (4) using only air pollution measurements from the nearest EPA monitoring sites. Splines on meteorological variables with 6 and 9 degrees of freedom yielded results with a difference of less than 5% of the standard error (Figure S1). The main analysis, which used the lag 01 day exposure, yielded the lowest values of the Akaike Information Criteria values, indicating better fit to the data (Table S1). Different definitions of warm season yielded similar risk estimates (Section 5, Supplementary Material), and using exposure measurements from the nearest monitors resulted in attenuated, but still significant, risk estimates (Table 2).
Table 2.
Relative Risk and Absolute Risk Difference of Daily Mortality Associated with Each 10 μg/m3 Increase in PM2.5 and Each 10 ppb Increase in Ozone
| Relative Risk (Percentage Change) | Absolute Risk Difference in Daily Mortality Rates (No. Per 1 Million Persons at Risk Per Day)a | |||
|---|---|---|---|---|
|
| ||||
| Air Pollutant | PM2.5 | Ozoneb | PM2.5 | Ozoneb |
| Main Analysisc | 1.05% (0.95%, 1.15%) | 0.51% (0.41%, 0.61%) | 1.42 (1.29, 1.56) | 0.66 (0.53, 0.78) |
| Low-exposure Analysisd | 1.61% (1.48%, 1.74%) | 0.58% (0.46%, 0.70%) | 2.17 (2.00, 2.34) | 0.74 (0.59, 0.90) |
| Single-pollutant Analysise | 1.18% (1.09%, 1.28%) | 0.55% (0.48%, 0.62%) | 1.61 (1.48, 1.73) | 0.71 (0.62, 0.79) |
| Nearest Monitors Analysisf | 0.83% (0.73%, 0.93%) | 0.35% (0.28%, 0.41%) | 1.13 (0.99, 1.26) | 0.45 (0.37, 0.53) |
The daily baseline mortality rate was 137.33 per 1 million persons at risk per day; the warm-season daily baseline mortality rate was 129.44 per 1 million persons at risk per day.
Ozone analyses included days from the warm season only (April 1 to September 30).
The main analysis used mean of daily exposure on the same day of death and one day prior (lag 01 day) as the exposure metric for both PM2.5 and ozone, and controlled for natural splines of air and dew point temperatures with 3 degrees of freedom. The main analysis considered the 2 pollutants jointly included into the regression model and estimated the percentage increase in the daily mortality rate associated with a 10 μg/m3 increase in PM2.5 exposure adjusted for ozone and the percentage increase in daily mortality rate associated with a 10 ppb increase in warm-season ozone exposure adjusted for PM2.5.
The low-exposure analysis had the same model specifications as the 2-pollutant analysis and was constrained for days when PM2.5 was below 25 μg/m3 or ozone below 60 ppb.
The single-pollutant analysis estimated the percentage increase in the daily mortality rate associated with a 10 μg/m3 increase in PM2.5 exposure without adjusting for ozone and the percentage increase in the daily mortality rate associated with a 10 ppb increase in ozone exposure without adjusting for PM2.5.
PM2.5 and ozone monitoring data were retrieved from the US EPA Air Quality System (AQS). AQS provides the daily mean of PM2.5 and daily 8-hour maximum ozone levels at each monitoring site. Daily ozone concentrations were averaged from April 1 to September 30. Individuals were assigned to the PM2.5 and ozone levels from the nearest monitor site within 50 kilometers. Those living 50 kilometers from any monitoring site were excluded.
The subgroup analyses were conducted by sex (male and female), race (White, non-White, and others), age (≤69, 70 to 74, 75 to 84, and ≥85 years), eligibility for Medicaid, and population density (quartiles). We fitted separate conditional logistic regressions to the data for each subgroup and obtained subgroup-specific estimates of RR and ARD. We implemented a two-sample test for assessing statistically significant differences in the estimated RR and ARD between categories within each subgroup (e.g., female vs. male), based on the point estimate and standard error (se): (Section 3, Supplementary Material).
The goal was to estimate mortality rate increases (both RR and ARD) at air pollution levels well below the current daily NAAQS. The analysis was restricted to days with daily air pollution concentrations below 25 μg/m3 for PM2.5 and 60 ppb for ozone. We chose 25 μg/m3 and 60 ppb instead of the current daily NAAQS (35 μg/m3 for daily PM2.5 and 70 ppb for 8-hour maximum ozone) because levels of PM2.5 and ozone on most of the days included in the analysis were already below the current safety standards.
Exposure-response curves were estimated between PM2.5 or ozone and mortality by replacing linear terms for the 2 pollutants with penalized splines for both PM2.5 and ozone.
All analyses were performed in R software, version 3.3.2. Computations were run on (1) the Odyssey cluster supported by the FAS Division of Science, Research Computing Group at Harvard University; and (2) the Research Computing Environment supported by the Institute for Quantitative Social Science in the Faculty of Arts and Sciences at Harvard University.
Results
During the study period, there were more than 22 million case days (deaths) and more than 76 million control days (Table 1). Of all case and control days, 93.6% had PM2.5 levels below 25 μg/m3, during which 95% of deaths occurred (21,353,817 out of 22,433,862), and 91.1% of days had ozone levels below 60 ppb, during which 93.4% of deaths occurred (20,955,387 out of 22,433,862). The baseline daily mortality rate was 137.33 and 129.44 (per 1 million persons at risk per day, [per 1M per day]) for the entire year and for the warm season, respectively. The mean time between case and control days was 12.55 days (range 7-28 days), with minimal differences in air and dew point temperatures between case and control days (0.003°C and 0.01°C, respectively). During the study period, the mean concentrations of PM2.5 and ozone were 11.6 μg/m3 and 37.8 ppb, respectively. Figure 1 shows the daily PM2.5 and ozone time series by state.
Table 1.
Baseline Characteristics of Study Population (2000-2012)
| Baseline Characteristics | |
|---|---|
| Case days (No.) | 22,433,862 |
| Control days (No.) | 76,143,209 |
|
| |
| Among All Cases | |
|
| |
| Age at death | |
| ≤69 years | 10.38% |
| 70 to 74 years | 13.37% |
| 75 to 84 years | 38.48% |
| ≥85 years | 37.78% |
| Sex | |
| Male | 44.73% |
| Female | 55.27% |
| Race/ethnicity | |
| White | 87.34% |
| Black | 8.87% |
| Asian | 1.03% |
| Hispanic | 1.51% |
| Native American | 0.31% |
| Medicaid eligibility | |
| Ineligible | 77.36% |
| Eligible | 22.64% |
Figure 1. Daily Air Pollution Concentrations in the Continental United States, 2000-2012.
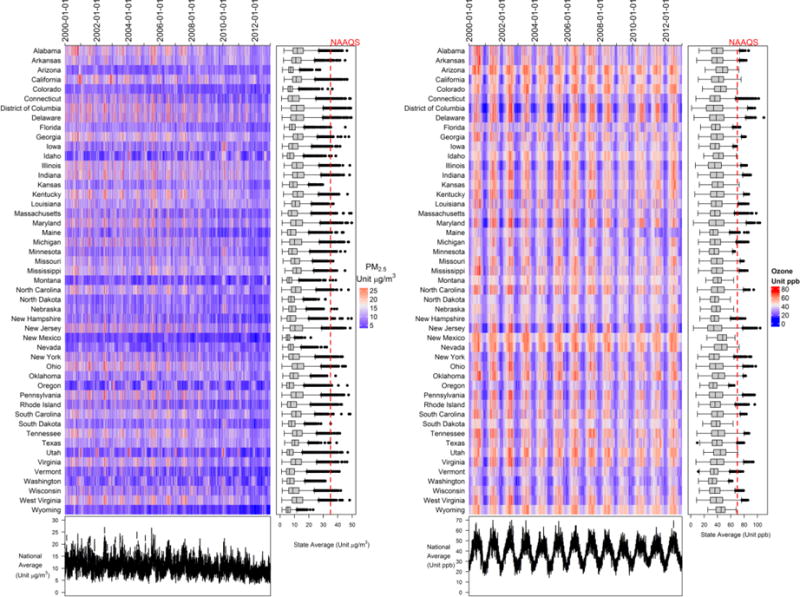
Daily mean of PM2.5 (left panel) and 8-hour maximum ozone (right panel) concentrations were calculated and plotted by state. The time-series plots at the bottom indicate the national daily mean values across all locations. Red dashed lines indicate the daily NAAQS for PM2.5 (35 μg/m3) and ozone (70 ppb). Boxplots show the distribution of daily PM2.5 and ozone levels for each state. The line across the box, upper hinge, and lower hinge represent the median value, 75th percentile (Q3), and 25th percentile (Q1), respectively. The upper whisker is located at the smaller of the maximal value and Q3+1.5*interquartile (IQR); the lower whisker is located at the larger of the minimal value and Q1 – 1.5*IQR. Any values that lie beyond upper and lower whiskers are outliers.
Each 10 μg/m3 and 10 ppb increase in the lag 01 day exposure for PM2.5 and warm-season ozone was associated with an increase of 1.05% (95% confidence interval [CI]: 0.95%, 1.15%) and 0.51% (0.41%, 0.61%) in the daily mortality rate. The ARD was 1.42 (95% CI: 1.29, 1.56) and 0.66 (95% CI: 0.53, 0.78) per 1M per day. These associations remained significant when examining days below 25 μg/m3 for PM2.5 and below 60 ppb for ozone, with larger effect size estimates for both PM2.5 and ozone (RR: 1.61% [95% CI: 1.48%, 1.74%] and 0.58% [95% CI: 0.46%, 0.70%]; ARD: 2.17 [95% CI: 2.00, 2.34] and 0.74 [95% CI: 0.59, 0.90] per 1M per day) (Table 2). PM2.5 was associated with higher mortality rate in some subgroups, including Medicaid-eligible individuals (RR: 1.49% [95% CI: 1.29%, 1.70%]; ARD: 3.59 [95% CI: 3.11, 4.08] per 1M per day, interaction: p<0.001), individuals above 70 years of age (e.g., for ≥85 years, RR: 1.38% [95% CI: 1.23%, 1.54%]; ARD: 5.35 [95% CI: 4.75, 5.95] per 1M per day, interaction: p<0.001), and females (RR: 1.20% [95% CI: 1.07%, 1.33%]; ARD: 1.56 [95% CI: 1.39, 1.72] per 1M per day, interaction: p=0.019) (Figure 2). The effect estimates for PM2.5 increased with age. The effect estimate for Blacks was higher than that for Whites (p=0.001, Figure S2). For ozone, similar patterns were observed, but with less contrast between groups. No significant differences were found in the short-term associations between air pollution exposure (PM2.5 and ozone) and mortality across areas with different population density levels (Figure 2).
Figure 2. Absolute Risk Difference and Relative Risk of Daily Mortality Associated with 10 μg/m3 Increase in PM2.5 and 10 ppb Increase in Ozone.
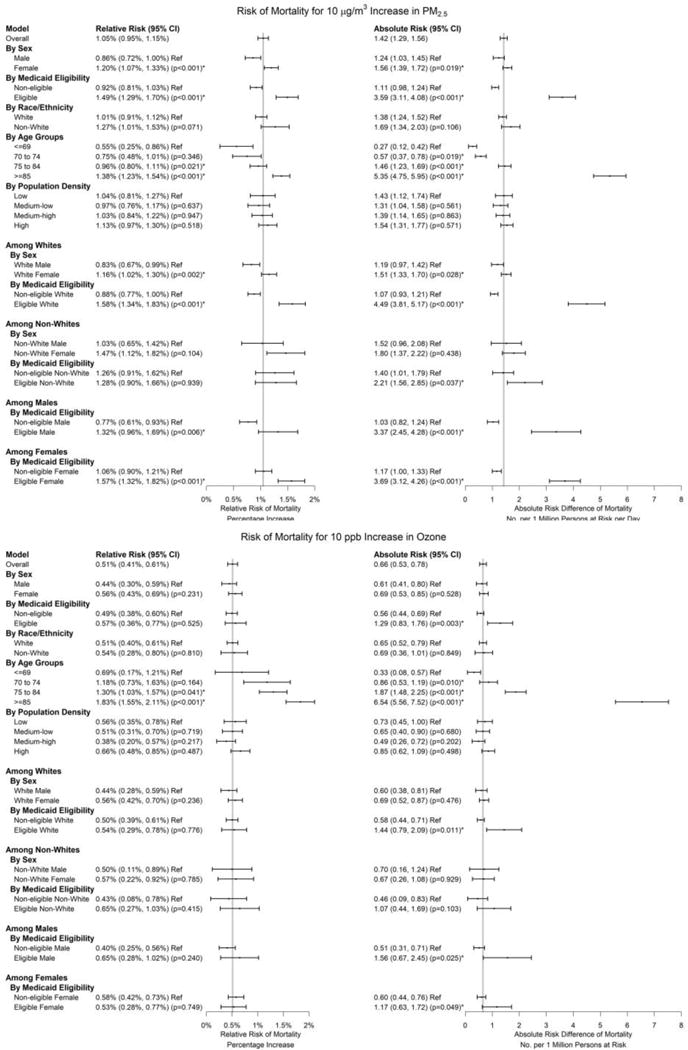
As for the main analysis, subgroup analyses used a 2-pollutant analysis (with both PM2.5 and ozone), based on the mean of daily exposure on the same day of death and one day prior (lag 01 day) as the exposure metric for PM2.5 and ozone, and controlled for natural splines of air and dew point temperatures (each with 3 degrees of freedom). Vertical lines indicate effects for the entire study population. Subgroup analyses were conducted for each subgroup (e.g., male or female, White or non-White, Medicare-eligible or Medicare-ineligible, age groups, quartiles of population density). For the main analysis and each subgroup, we ran conditional logistic regressions to obtain RR, and calculated ARD based on baseline mortality rates (See Section 2, supplementary material). For ozone, analyses were restricted to the warm season (April to September). Numbers in the figure represent point estimates, 95% confidence intervals, and p-values for effect modifications. “Ref” indicates reference group when assessing effect modification; asterisks indicate a statistically significant effect estimate (at 5% level) compared with the reference group.
Figure 3 shows the estimated exposure-response curves for PM2.5 and ozone. The slope was steeper at PM2.5 levels below 25 μg/m3 (p<0.001), consistent with the low-exposure analysis (Table 2). Both PM2.5 and ozone exposure-responses were almost linear, with no indication of a mortality risk threshold at very low concentrations.
Figure 3. Estimated Exposure-response Curves for Short-term Exposures to PM2.5 and Ozone.
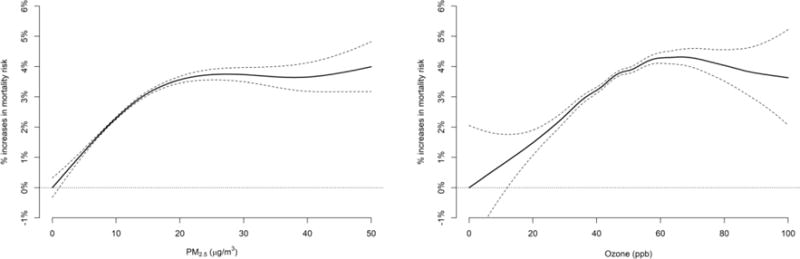
A 2-pollutant analysis with separate penalized splines on PM2.5 and ozone was conducted to assess the percentage increase in daily mortality at various pollution levels. Dashed lines indicate 95% confidence intervals. The mean of daily exposure on the same day of death and one day prior (lag 01 day) was used as metrics of exposure to PM2.5 and ozone. Analysis for ozone was restricted to the warm season (April to September).
Discussion
This large case-crossover study of all Medicare deaths in the continental US found that 10 μg/m3 daily increase in PM2.5 and 10 ppb daily increase in warm-season ozone exposures are associated with a statistically significant increase of 1.42 and 0.66 deaths per 1 million persons at risk per day, respectively. The risk of mortality remained statistically significant when restricting the analysis to days with PM2.5 and ozone levels much lower than the current daily NAAQS.17 This study included individuals living in smaller cities, towns, and rural areas that were unmonitored and thus excluded from previous time series studies. There were no significant differences in the mortality risk associated with air pollution among individuals living in urban versus rural areas. Taken together, these results provide evidence that short-term exposures to PM2.5 and ozone, even at levels much lower than the current daily standards, are associated with increased mortality, particularly for susceptible populations.
The Clean Air Act requires the administrator of the US EPA to set NAAQS at levels that provide “protection for at-risk populations, with an adequate margin of safety.”18 In this study, Medicaid-eligible individuals, females, and the elderly had higher mortality rate increases associated with PM2.5 than other groups. Previous studies have found similar results in some subgroups.19,20 Poverty, unhealthy lifestyle, poor access to healthcare, and other factors may make some subgroups more vulnerable to air pollution. The exact mechanism is worth exploring in future studies.
The current daily NAAQS for daily PM2.5 is 35 μg/m3. When restricting the analysis to daily PM2.5 levels below 25 μg/m3, the association between short-term PM2.5 exposure and mortality remained, but was elevated. The current daily NAAQS for ozone is 70 ppb; when restricting the analysis to daily warm-season ozone concentrations below 60 ppb, the effect size also increased slightly. The exposure-response curves revealed a similar pattern. These results indicate that air pollution is associated with an increase in daily mortality rates, even at levels well below the current standards.
The exposure-response relationship between PM2.5 exposure and mortality was consistent with findings of previous studies. One study combined exposure-response curves from 22 European cities and reported an almost linear relationship between PM2.5 and mortality.21 Another multi-city study reported a linear relationship down to 2 μg/m3 PM2.5.22 The present study found a similarly linear exposure-response relationship below 15 μg/m3 PM2.5 and a less steep slope above this level.
For ozone, the linear exposure-response curve with no threshold described in this study is consistent with earlier research. An almost linear exposure-response curve for ozone was previously reported with no threshold or a threshold at very low concentrations.23 A study from the Netherlands also concluded that if an ozone threshold exists, it does so at very low levels.24
Findings from this study are also consistent with the literature regarding the observed effect sizes of both PM2.54,7,15,25–27 and ozone.6,19,28,29 This study further demonstrates that in more recent years, during which air pollution concentrations have fallen, statistically significant associations between mortality and exposures to PM2.5 and ozone persisted.
The association of mortality and PM2.5 exposure is supported by a large number of published experimental studies in animals30–32 and in humans exposed to traffic air pollution,33,34 diesel particles,35 and unfiltered urban air.36 Similarly, a review of toxicological studies and a recent panel study found that ozone exposure was associated with multiple adverse health outcomes.37,38
This study has several strengths. First, to the best of our knowledge, this is the largest analysis of daily air pollution exposure and mortality to date, with approximately 4 times the number of deaths included in a previous large study.4 Second, this study assessed daily exposures using air pollution prediction models that provide accurate estimates of daily levels PM2.5 and ozone for most of the US, including previously unmonitored areas. An analysis that relied only on exposure data from monitoring stations was found to result in a downward bias in estimates (Table 2). Third, the inclusion of more than 22 million deaths from 2000 to 2012 from the entire Medicare population provided large statistical power to detect differences in mortality rates in potentially vulnerable populations and to estimate mortality rates at very low PM2.5 and ozone concentrations. Fourth, this study estimated the air pollution‒mortality association well below the current daily NAAQS and in unmonitored areas, and did not identify significant differences in the mortality rate increase between urban and rural areas. Fifth, this study used a case-crossover design that individually matched potential confounding factors by month, year, and other time-invariant variables and controlled for time-varying patterns, as demonstrated by the minimal differences in meteorological variables between case and control days.
This study also has several limitations. First, the case-crossover design does not allow estimation of mortality rate increase associated with long-term exposure to air pollution. Long-term risks in the same study population have been estimated elsewhere.39 Second, because this study used residential zip code to ascertain exposure level rather than exact home address or place of death, some measurement error is expected. Third, the Medicare population primarily consists of individuals older than 65 years, which limits the generalizability of findings to younger populations. However, because more than two-thirds of deaths in the US occur in people older than 65 years of age, and air pollution-related health risk rises with age, the Medicare population in this study includes most cases of air pollution-induced mortality. Fourth, Medicare files do not report cause-specific mortality. Fifth, the most recent data used in this study are nearly 5 years old, and it is uncertain whether exposures and outcomes would be the same with more current data.
Conclusions
In the US Medicare population from 2000-2012, short-term exposures to PM2.5 and warm-season ozone were significantly associated with increased risk of mortality. This risk occurred at levels below current national air quality standards, suggesting that these standards may need to be reevaluated. (Word Count: 2824)
Supplementary Material
Key Points.
Question
What is the association between short-term exposure to air pollution below current air quality standards and all-cause mortality?
Finding
In a case-crossover study of more than 22 million deaths, each 10 μg/m3 daily increase in PM2.5 and 10 ppb daily increase in warm-season ozone exposures were associated with a statistically significant increase of 1.42 and 0.66 deaths per 1 million persons at risk per day, respectively.
Meaning
Day-to-day changes in PM2.5 and ozone exposures were significantly associated with higher risk of all-cause mortality at levels below current air quality standards, suggesting that those standards may need to be reevaluated.
Acknowledgments
Funding/Sponsor:
This study was supported by NIH grants R01 ES024332-01A1, ES-000002, ES024012, R01ES026217, 4953-RFA14-3/16-4; HEI grant 4953-RFA14-3/16-4, and USEPA grants 83587201-0 and RD-83479801.
Role of the Funder/Sponsor:
The funding organizations and sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The contents are solely the responsibility of the grantee and do not necessarily represent the official views of the funding agencies. Further, the funding agencies do not endorse the purchase of any commercial products or services related to this publication.
Footnotes
Author Contributions:
Qian Di, M.S., had full access to the data in the study and was responsible for the integrity of the data and the accuracy of the data analysis.
Analysis and interpretation of data: Di and Dai
Acquisition and preparation of data: Wang, Choirat, and Dominici
Drafting of the manuscript: Di, Dai, Schwartz, and Dominici
Critical revision of the manuscript: Di, Dai, Schwartz, and Dominici
Statistical analysis: Di, Dai, Zanobetti, Schwartz, and Dominici
Administrative and technical support: Wang, Zanobetti, Choirat, Schwartz, and Dominici
Conflict of Interest Disclosures:
Dr. Joel Schwartz received funding from the US Department of Justice. The authors have completed ICMJE forms and do not have any additional potential conflicts of interest.
Non-author Contributions:
We thank Stacey C. Tobin, Ph.D., and Kathy L. Brenner, M.A.T., from Harvard T.H. Chan School of Public Health, for editorial assistance on the manuscript; Sarah L. Duncan, M.Div., and William J. Horka, B.S., at the Institute for Quantitative Social Science, Harvard University, for their support with the Research Computing Environment; and Ista Zahn, M.S., at the Institute for Quantitative Social Science, Harvard University, for programming support. Stacey C. Tobin, Ph.D., received compensation for editorial assistance.
References
- 1.U.S. Environmental Protection Agency. Process of Reviewing the National Ambient Air Quality Standards. 2017 https://www.epa.gov/criteria-air-pollutants/process-reviewing-national-ambient-air-quality-standards. Accessed Nov 1, 2017.
- 2.U.S. Environmental Protection Agency. Integrated Review Plan for the National Ambient Air Quality Standards for Particulate Matter. 2016 https://www3.epa.gov/ttn/naaqs/standards/pm/data/201612-final-integrated-review-plan.pdf. Accessed May 30, 2017.
- 3.Krall JR, Anderson GB, Dominici F, Bell ML, Peng RD. Short-term exposure to particulate matter constituents and mortality in a national study of U.S. urban communities. Environ Health Perspect. 2013;121(10):1148–1153. doi: 10.1289/ehp.1206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environmental health perspectives. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA. 2004;292(19):2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J Air Waste Manag Assoc. 1996;46(10):927–939. [PubMed] [Google Scholar]
- 8.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 9.Bateson TF, Schwartz J. Control for seasonal variation and time trend in case-crossover studies of acute effects of environmental exposures. Epidemiology. 1999;10(5):539–544. [PubMed] [Google Scholar]
- 10.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 11.Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001;12(2):186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Di Q, Kloog I, Koutrakis P, Lyapustin A, Wang Y, Schwartz J. Assessing PM2.5 exposures with high spatiotemporal resolution across the continental United States. Environ Sci Technol. 2016;50(9):4712–4721. doi: 10.1021/acs.est.5b06121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Q, Rowland S, Koutrakis P, Schwartz J. A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. J Air Waste Manag Assoc. 2017;67(1):39–52. doi: 10.1080/10962247.2016.1200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalnay E, Kanamitsu M, Kistler R, et al. The NCEP/NCAR 40-Year Reanalysis Project. Bulletin of the American Meteorological Society. 1996;77(3):437–471. [Google Scholar]
- 15.Dai L, Zanobetti A, Koutrakis P, Schwartz JD. Associations of fine particulate matter species with mortality in the United States: a multicity time-series analysis. Environmental health perspectives. 2014;122(8):837–842. doi: 10.1289/ehp.1307568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostro B, Broadwin R, Green S, Feng WY, Lipsett M. Fine particulate air pollution and mortality in nine California counties: results from CALFINE. Environ Health Perspect. 2006;114(1):29–33. doi: 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Environmental Protection Agency. NAAQS Table. 2016 https://www.epa.gov/criteria-air-pollutants/naaqs-table. Accessed May 30, 2017. [PubMed]
- 18.US Environmental Protection Agency. Criteria Air Pollutants. 2015 https://www.epa.gov/sites/production/files/2015-10/documents/ace3_criteria_air_pollutants.pdf. Accessed May 30, 2017.
- 19.Zanobetti A, Schwartz J. Is there adaptation in the ozone mortality relationship: a multi-city case-crossover analysis. Environ Health. 2008;7:22. doi: 10.1186/1476-069X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baccini M, Mattei A, Mealli F, Bertazzi PA, Carugno M. Assessing the short term impact of air pollution on mortality: a matching approach. Environ Health. 2017;16(1):7. doi: 10.1186/s12940-017-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samoli E, Analitis A, Touloumi G, et al. Estimating the exposure-response relationships between particulate matter and mortality within the APHEA multicity project. Environ Health Perspect. 2005;113(1):88–95. doi: 10.1289/ehp.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz J, Laden F, Zanobetti A. The concentration-response relation between PM(2.5) and daily deaths. Environmental health perspectives. 2002;110(10):1025–1029. doi: 10.1289/ehp.021101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell ML, Peng RD, Dominici F. The exposure-response curve for ozone and risk of mortality and the adequacy of current ozone regulations. Environmental health perspectives. 2006;114(4):532–536. doi: 10.1289/ehp.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoek G, Schwartz JD, Groot B, Eilers P. Effects of ambient particulate matter and ozone on daily mortality in Rotterdam, The Netherlands. Archives of environmental health. 1997;52(6):455–463. doi: 10.1080/00039899709602224. [DOI] [PubMed] [Google Scholar]
- 25.Alessandrini ER, Stafoggia M, Faustini A, et al. Association Between Short-Term Exposure to PM2.5 and PM10 and Mortality in Susceptible Subgroups: A Multisite Case-Crossover Analysis of Individual Effect Modifiers. Am J Epidemiol. 2016;184(10):744–754. doi: 10.1093/aje/kww078. [DOI] [PubMed] [Google Scholar]
- 26.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. Journal of exposure science & environmental epidemiology. 2007;17(3):279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 27.Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19:680–689. doi: 10.1097/ede.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy JI, Chemerynski SM, Sarnat JA. Ozone exposure and mortality: an empiric bayes metaregression analysis. Epidemiology. 2005;16(4):458–468. doi: 10.1097/01.ede.0000165820.08301.b3. [DOI] [PubMed] [Google Scholar]
- 29.Peng RD, Samoli E, Pham L, et al. Acute effects of ambient ozone on mortality in Europe and North America: results from the APHENA study. Air quality, atmosphere, & health. 2013;6(2):445–453. doi: 10.1007/s11869-012-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamagawa E, Bai N, Morimoto K, et al. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. American journal of physiology. 2008;295(1):L79–85. doi: 10.1152/ajplung.00048.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartoli CR, Wellenius GA, Coull BA, et al. Concentrated ambient particles alter myocardial blood flow during acute ischemia in conscious canines. Environ Health Perspect. 2009;117(3):333–337. doi: 10.1289/ehp.11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemmar A, Hoet PH, Vermylen J, Nemery B, Hoylaerts MF. Pharmacological stabilization of mast cells abrogates late thrombotic events induced by diesel exhaust particles in hamsters. Circulation. 2004;110(12):1670–1677. doi: 10.1161/01.CIR.0000142053.13921.21. [DOI] [PubMed] [Google Scholar]
- 33.Hemmingsen JG, Rissler J, Lykkesfeldt J, et al. Controlled exposure to particulate matter from urban street air is associated with decreased vasodilation and heart rate variability in overweight and older adults. Part Fibre Toxicol. 2015;12:6. doi: 10.1186/s12989-015-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langrish JP, Mills NL, Chan JK, et al. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part Fibre Toxicol. 2009;6:8. doi: 10.1186/1743-8977-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills NL, Tornqvist H, Gonzalez MC, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. The New England journal of medicine. 2007;357(11):1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 36.Brauner EV, Forchhammer L, Moller P, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 2008;177(4):419–425. doi: 10.1164/rccm.200704-632OC. [DOI] [PubMed] [Google Scholar]
- 37.Watkinson WP, Campen MJ, Nolan JP, Costa DL. Cardiovascular and systemic responses to inhaled pollutants in rodents: effects of ozone and particulate matter. Environ Health Perspect. 2001;109(Suppl 4):539–546. doi: 10.1289/ehp.01109s4539. Suppl 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176(4):370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 39.Di Q, Wang Y, Zanobetti A, et al. Air pollution and mortality in the Medicare population. N Engl J Med. 2017;376(26):2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


