Abstract
Background
Although downregulation of caveolin-1 (Cav-1), which is a key constituent of membrane caveolae and a regulator of cellular processes, is associated with colorectal cancer (CRC), its involvement in the disease progression is largely unknown. This study aimed to explore the role of Cav-1 in CRC and the associated mechanisms.
Material/Methods
Fresh tissues from patients with CRC and human CRC SW480 cells were used to evaluate Cav-1 and Ki-67 expression using immunohistochemistry and Western blotting. The MTS and Transwell assays were performed to determine the effects of Cav-1 overexpression via pcDNA3.1/Cav-1 plasmid on cell proliferation and metastasis. The effect of Cav-1 on the epidermal growth factor receptor (EGFR) activation was evaluated by Western blotting. The correlation of Cav-1 expression with clinicopathological factors was statistically analyzed.
Results
Overexpression of Cav-1 significantly reduced proliferation, migration, and invasion of SW480 cancer cells in vitro. The EGF-induced phosphorylation of EGFR and activations of the RAF-MEK-ERK and PI3K-AKT pathways were adversely regulated by Cav-1 overexpression in vitro. In 76 cases of CRC patients with EGFR expression, a negative correlation was observed between the level of Cav-1 and tumor-node-metastasis stage, lymph node metastasis, and distant metastasis (All p<0.05). Finally, the expression level of Cav-1 was negatively correlated with that of Ki-67.
Conclusions
This report is the first to show that overexpression of Cav-1significantly inhibits the proliferation, migration, and invasion potential of SW480 cells, possibly through reducing EGF-induced EGFR activation. High Cav-1 expression level may be a predictor of positive outcomes in patients with colorectal cancer.
MeSH Keywords: Caveolin 1; Cell Proliferation; Colorectal Neoplasms; Receptor, Epidermal Growth Factor; Tumor Suppressor Proteins
Background
Colorectal cancer (CRC) accounts for most cancer-related deaths, with a predicted prevalence of nearly 1.4 million new diagnosed cases a year [1]. In China, the mortality and morbidity of CRC cases have shown a rapid annual increase over the past 20 years [2,3]. The initiation and progression of CRC have been associated with complicated multi-stage processes, including gene mutations and epigenetic modifications [4,5]. Despite the vast progress in CRC therapeutic approaches, the disease recurrence and survival rates remain alarming. Therefore, the identification of biomarkers for disease relapse and progression are essential for the detection and management of CRC.
Caveolin-1 (Cav-1) is a key constituent of caveolae at the cell membrane [6]. With multiple binding partners, this multifunctional scaffolding protein has been implicated in diverse cancer-associated processes, such as cellular transformation, tumor growth, metastasis, and multidrug resistance [7,8]. Previous studies showed that Cav-1 is involved in various signaling pathways including estrogen receptor, epidermal growth factor receptor (EGFR), Her2/neu, tumor growth factor-β (TGFβ), and mTOR[9], and serves as an oncogene in lung, breast, and ovarian cancers [10–12]. In contrast, Cav-1 plays the role of a tumor suppressor in prostate and bladder cancers [13,14]. Bender et al. [15] confirmed that Cav-1 was downregulated in patients with colon cancer, while other studies revealed that Cav-1 was overexpressed in patients with colon adenocarcinoma [16,17]. Thus, the regulation of Cav-1 in CRC remains controversial.
It has been reported that approximately 25~77% of CRC cases are associated with overexpression of EGFR, which correlates closely with CRC progression, metastatic spread, and poorer prognosis [18,19]. It is worth noting that dimerization of ligand-activated EGFR has been associated with the channeling of mitogenic signals, predominantly via the Ras-extracellular signal-regulated kinase (ERK), phosphatidylinositide 3-kinases (PI3K)-Protein kinase B (AKT), and c-Jun NH2-terminal kinase pathways [20].
The present study aimed to elucidate the effect of Cav-1 on the EGFR pathway in CRC cells. We found that overexpression of Cav-1 suppresses CRC proliferation and metastasis in vitro. Furthermore, the present findings provide definitive evidence that Cav-1 exerts its suppressive role in CRC through the regulation of EGFR function.
Material and Methods
Human tissue specimens
Seventy-six fresh primary CRC tissues and neighboring healthy tissues from patients who underwent surgery between January 2013 and December 2014 at Bethune International Peace Hospital were analyzed. The diagnoses of all patients were confirmed by postoperative pathological examinations. None of the patients received chemoradiotherapy before the operation. All samples were frozen in liquid nitrogen and stored at −80°C for further analysis. This study was approved by the Institutional Review Board of Hebei Medical University. All patients provided signed informed consents for their participations.
Cell culture and transfection
The human CRC SW480 cell line (spontaneous loss of cav-1 expression) cells were incubated in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, South Logan, UT, USA), 100 IU/mL penicillin, and 100 IU/mL streptomycin (both from Shenzhen Huayao Nanfang Pharmaceutical Co., Ltd, Guangdong China) at 37°C and 5% CO2.
The Cav-1 vector (pcDNA3.1/Cav-1) was constructed by sub-cloning the coding sequence of wild-type Cav-1 into pc-DNA3.1. The empty vector pc-DNA3.1 was used in the control group. The cells were transfected using X-treme GENE DNA transfection reagent (Roche, Nutley, NJ, USA) with OPTI-MEM (Gibco) according to the manufacturer’s instructions.
Cell proliferation assay
MTS assay was used to determine the role of Cav-1 in cell growth and viability in vitro. SW480/Cav-1 and SW480/pcDNA3.1 cells were seeded at a density of 6×103 cells per well in a 96-well plate. After overnight incubation in complete medium, cells were serum-starved for 4 h and then were left untreated or treated with various concentrations of EGF (4, 20, and 100 nM). After 48 h of incubation, cell viability was determined using the MTS tetrazolium substrate (Cell Titer 96 Aqueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA) following the manufacturer’s instructions. The optical density (OD) was determined at 490 nm using a spectrophotometer. All experiments were performed in triplicate. In each MTS assay, cells were kept under the same culture conditions, plated at the same cell density, and treated with EGF for the same periods of time.
Scratch test
SW480/Cav-1 and SW480/pcDNA3.1 cells were seeded in 24-well plates at a concentration of 2×105 per well and allowed to form a confluent monolayer for 24 h [23]. The cells were starved for 4 h after adding 1 mL of serum-free RPMI-1640 medium, and the monolayer was scratched with a pipette tip. Floating cells were discarded by washing with PBS. Subsequently, cells were cultured with 1 mL of RPMI 1640 medium with or without 20 nM EGF (Miltenyi Biotec, Auburn, CA, USA) in triplicate. The width of the scratch was assessed under an inverted microscope (10×) and photographed at the same field every 2 h until closure of the scratch. Mobility was assessed using the following equation: (width of initial scratch–width of current scratch)/2/width of initial scratch×100%.
Transwell assay
The invasion ability of the SW480 cells was assessed using the Transwell assay by means of Matrigel-coated Transwell chambers (Corning, Albany, NY, USA). After starvation in serum-free RPMI 1640 medium for 4 h, the SW480/Cav-1 and SW480/pcDNA3.1 cells (2×105) were placed on Matrigel (BD Biosciences, San Jose, CA, USA)-coated Transwell chambers (Corning, Albany, NY, USA) in which serum-free RPMI 1640 medium was added to the upper compartment of the Transwell chamber, and RPMI 1640 medium containing 10% FBS was added to the lower compartment. After a 24-h incubation period in a humidified atmosphere of 5% CO2 at 37°C, noninvasive cells were removed from the upper surface with a cotton swab, and invasive cells on the lower membrane surface were fixed with methanol, stained with H&E, photographed, and counted under a microscope at 200× magnification in 5 fields. These experiments were performed in triplicate.
Western blot
After being serum-starved for 4 h, stably transfected SW480 cells were stimulated with 20 nM EGF for 0, 5, 10, and 30 min. Total cell protein extracts were obtained in RIPA lysis buffer. After the protein was quantified using the BCA method (Pierce Biotechnology, Rockford, IL, USA), equal amounts of proteins were separated on 10% SDS-PAGE and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). The membranes were blocked in 5% nonfat milk or 3% bovine serum albumin, incubated with various primary antibodies, followed by incubation with HRP-conjugated secondary antibodies and visualization with enhanced chemiluminescence (ECL) detection reagents (Beyotime, Haimen, Jiangsu, China). Signals were quantitated by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Immunoblotting was performed using the following primary antibodies: anti-phosphotyrosine (clone 4G10) (1: 2000; Millipore, Temecula, CA, USA); anti-EGFR (1: 1000), anti-phospho-AKT (1: 1000) anti-AKT (1: 1000), anti-phospho-ERK1/2 (1: 3000) (Cell Signaling Technology, Danvers, MA, USA); anti-ERK1/2 (1: 4000), anti-Cav-1 (1: 4000) and anti-β-actin (1: 5000) (Santa Cruz Biotechnology Inc, Dallas, Texas, USA).
Immunohistochemical tissue staining
Immunostaining was performed on 5-μm formalin-fixed, paraffin-embedded tissue sections using an immunoperoxidase method with rabbit anti-Cav-1 (1: 400; Santa Cruz, Dallas, Texas, USA); rabbit anti-EGFR (1: 100; Santa Cruz, Dallas, Texas, USA), and rabbit anti-Ki-67 (1: 100; Sunbiote, Shanghai, China) monoclonal antibodies. Protein was visualized using the PV and DAB chromogenic kits (Beijing Zhongshan Golden Bridge Biotechnology) following the manufacturer’s instructions.
Two pathologists blinded to the experiments assessed the extent and intensity of immunostaining. The protein expression levels of Cav-1 and EGFR were observed under high-power magnification (×400); 5 different fields of view were randomly selected in each section, and 200 cells were counted. The degree of staining was scored between 0 and 3 points: 0 point=no staining, 1 point=weak staining, 2 points=moderate staining, and 3 points=strong staining. Cells scoring >2 points were defined as positive cells; <50% of positive cells within a field was defined as a negative expression, and ≥50% of positive cells was defined as a positive expression. We chose EGFR-positive expression tissues for the subsequent study. A total of 76 cases of colorectal cancer tissues were further tested for the expressions of Cav-1 and Ki-67.
Statistical analysis
All statistical evaluations were achieved using SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL, USA). The results were analyzed using Student’s t-test and chi-square test. Data were expressed as mean ± standard error of the mean (SEM), and statistical significance was indicated by p<0.05.
Results
Cav-1 inhibits the proliferation of human CRCSW480 cells in vitro
We used the pcDNA3.1/Cav-1 vector to induce Cav-1 overexpression in human CRC SW480 cells line and evaluate cell proliferation, migration, and invasion. Our results indicated a significant upregulation of Cav-1 in SW480/Cav-1 cells (p<0.01) compared with control SW480/pcDNA3.1 cells (Figure 1).
Figure 1.
Inducing caveolin-1 (Cav-1) overexpression in human colorectal cancer SW480 cells. (A) Transfection of SW480 cells was induced using Cav-1-pcDNA (SW480/Cav-1 cells) or pcDNA3.1 (SW480/pcDNA3.1 cells, i.e., controls) plasmid, and transfected cells were selected by G418. The Cav-1 overexpression was confirmed by cell lysates immunoblotting. (B) The ratio of Cav-1 signal to β-actin. ** p<0.01 versus SW480/Cav-1 cells.
We subsequently evaluate the proliferative rate of the Cav-1 overexpressing SW480 cells following EGF stimulation at different concentrations (Figure 2). Based on the results of the MTS assay, both SW480/Cav-1 and control cells exhibited increased proliferative rate (both p<0.01), which was endorsed by increasing EGF concentrations. However, the proliferative rate was lower in SW480/Cav-1 cells treated with EGF at all concentrations than in control cells (all p<0.01).
Figure 2.
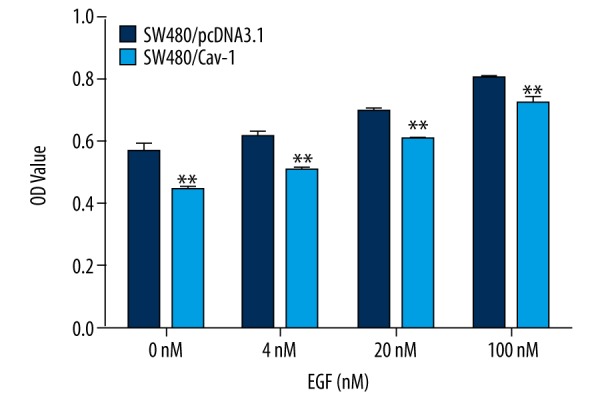
Effects of caveolin-1 (Cav-1) overexpression on SW480 cell proliferation. The MTS assay was performed on SW480/pcDNA3.1 (control) and SW480/Cav-1cells after 4-h serum-starvation followed by incubation with epidermal growth factor (EGF) at 0, 4, 20, or 100 nM for 48 h. ** p< 0.01 versus SW480/pcDNA3.1 cells.
Cav-1 inhibits the migration and invasion potential of SW480 cells in vitro
Cell migration and invasion were assessed in Cav-1-overexpressing cells by using the scratch and Transwell assays, respectively. Although cell stimulation with EGF increased the migration rates in both SW480/Cav-1 and SW480/pcDNA3.1 cells (Figure 3), the effect was less apparent in SW480/Cav-1 cells (p<0.05). Moreover, the migration rate in untreated SW480/Cav-1 cells was lower than in untreated control cells (p<0.05).
Figure 3.
Effects of caveolin-1 (Cav-1) overexpression on SW480 cell migration. (A) The scratch assay was conducted using SW480/pcDNA3.1 (control) and SW480/Cav-1 cells with or without EGF (20 nM). (B) The rate of cell migration was measured and plotted at different time points. Note that cell migration is decreased in SW480/Cav-1 cells. *p< 0.05 versus SW480/pcDNA3.1 cells with EGF. # p<0.05 versus SW480/pcDNA3.1 cells without EGF.
Tumor cell invasion to tissues adjacent to the cancer site is elemental in cancer metastasis. Based on the results of the Transwell assay (Figure 4), the rate of cell invasion in SW480/Cav-1 cells was significantly lower than in controls (p<0.05).
Figure 4.
Effects of Caveolin-1 (Cav-1) overexpression on SW480 cell invasion. The Transwell assay was used to determine the invasive capacity of SW480/pcDNA3.1 (control) and SW480/Cav-1 cells. (A) Cells were cultured for 24 h and subsequently fixed by 95% alcohol for 30 min, stained with hematoxylin, and microscopically examined at high magnification (200× objective). (B) The mean number of invaded cells was assessed in 5 fields. Note that cell invasion is decreased in SW480/Cav-1 cells. * p<0.05 versus SW480/pcDNA3.1 cells.
Effect of Cav-1 on ligand-induced EGFR phosphorylation
Several intracellular substrates are recruited upon EGFR activation, thus stimulating cell proliferation, survival, invasion, adhesion, migration, and repair [24]. Based on this, we assumed that EGFR phosphorylation and the consequent activation of downstream proteins in the Raf-MEK-ERK and PI3K-AKT cascades lead to the Cav-1-associated inhibition of human CRC cell motility and growth.
To confirm this assumption, we used Western blotting to detect the phosphorylated form of EGFR (pY-EGFR), ERK1/2 (p-ERK1/2), and AKT (p-AKT). The results indicated increased pY-EGFR, p-ERK1/2, and p-AKT levels following EGF stimulation in both SW480/Cav-1 and SW480/pcDNA3.1 cells. The expression levels peaked at 5 min and 10 min in SW480/Cav-1 and SW480/pcDNA3.1 cells, respectively, and waned thereafter (Figure 5A). However, although the amount of EGFR was comparable between the 2 cell groups, the response of SW480/Cav-1cells to EGF stimulation was weaker than that of control cells (Figure 5A), resulting in a lower pY-EGFR-to-total EGFR ratio in SW480/Cav-1 cells (Figure 5B). Similarly, we observed lower levels of p-ERK1/2 and p-AKT in SW480/Cav-1 cells than in SW480/pcDNA3.1 cells upon EGF stimulation (Figure 5C, 5D). These results indicated that Cav-1 inhibits EGFR phosphorylation and activation of the Raf-MEK-ERK and PI3K-AKT cascades following EGF stimulation.
Figure 5.
Ectopic expression of Cav-1 promotes ligand-induced phosphorylation of the epidermal growth factor receptor (EGFR) in SW480/pcDNA3.1 (control) and SW480/Cav-1 cells. (A) After 4-h serum starvation, cells were treated with EGF (20 nM) for 0, 5, 10, or 30 min, and evaluated by Western blotting using the following primary antibodies: anti-Cav-1, anti-phosphotyrosine (4G10 clone), anti-EGFR, anti-AKT, anti-phosphorylated AKT (p-AKT), anti-ERK1/2, anti-p-ERK1/2, and anti-β-actin (loading control). (B) The index of pY-EGFR to total EGFR in SW480/pcDNA3.1 and SW480/Cav-1 cells was calculated. (C) The index of p-ERK to total ERK in SW480/pcDNA3.1 and SW480/Cav-1 cells was calculated. (D) The index of p-AKT to total AKT in SW480/pcDNA3.1 and SW480/Cav-1 cells was calculated. * p<0.05 or ** p<0.01 versus SW480/pcDNA3.1.
Association of Cav-1 expression with clinicopathological features of patients with CRC
The expression of EGFR, Cav-1, and Ki-67 in CRC tissues was analyzed by IHC (Figure 6, Table 1). In total, tissues from 76 patients with CRC with EGFR expression were evaluated. Of these, 43 samples were Cav-1+, while 33 were Cav-1−. Furthermore, the majority of Cav-1+ tumors had a tumor-node-metastasis (TNM) stage of I to II (26/43), compared with only 9 of the 33 Cav-1− tumors (p=0.004). Moreover, the number of Cav-1+ cases with metastasis (lymph node, p=0.006; distant, p=0.017) was higher than Cav-1− cases (Table 1). In contrast, Ki-67+ tumors were associated with TNM stage III–IV (p=0.003) and metastasis (lymph node, p=0.024; distant, p=0.016). Table 2 summarizes the correlation results between Cav-1 and Ki-67 expression levels, and reveals a significant negative correlation (r=0.283; p=0.024).
Figure 6.
Immunohistochemical staining of colorectal cancer tissue sections against epidermal growth factor receptor (EGFR), Cav-1, and Ki-67 from colorectal cancer tissues (×400). Examples of negative (A) and positive (B) colorectal cancer tissues.
Table 1.
Baseline featuresof the patients.
| N (%) | Cav-1 | p | Ki-67 | p | |||
|---|---|---|---|---|---|---|---|
| Negative (%) | Positive (%) | Negative (%) | Positive (%) | ||||
| 76 (100) | 33 (43.42) | 43 (56.58) | 36 (47.37) | 40 (52.63) | |||
| Age, years | 0.565 | 0.679 | |||||
| ≤60 years | 34 (44.74) | 16 (48.48) | 18 (41.86) | 17 (47.22) | 17 (42.5) | ||
| >60 years | 42 (55.26) | 17 (51.52) | 25 (58.14) | 19 (52.78) | 23 (57.5) | ||
| Gender | 0.526 | 0.981 | |||||
| Female | 40 (52.63) | 16 (48.48) | 24 (55.81) | 19 (52.78) | 21 (52.5) | ||
| Male | 36 (47.37) | 17 (51.52) | 19 (44.19) | 17 (47.22) | 19 (47.5) | ||
| Degree of differentiation | 0.412 | 0.961 | |||||
| Low | 42 (55.26) | 20 (60.61) | 22 (51.16) | 20 (55.56) | 22 (55) | ||
| Moderate or high | 34 (44.74) | 13 (39.39) | 21 (48.84) | 16 (44.44) | 18 (45) | ||
| TNM stage | 0.004 | 0.003 | |||||
| I–II | 35 (46.05) | 9 (27.27) | 26 (60.47) | 23 (63.89) | 12 (30) | ||
| III–IV | 41 (53.95) | 24 (72.73) | 17 (39.53) | 13 (36.11) | 28 (70) | ||
| Location | 0.056 | 0.377 | |||||
| Right | 18 (23.67) | 12 (36.36) | 6 (13.95) | 6 (16.67) | 12 (30) | ||
| Left | 26 (34.21) | 8 (24.24) | 18 (41.86) | 14 (38.89) | 12 (30) | ||
| Rectum | 32 (42.11) | 13 (39.39) | 19 (44.19) | 16 (44.44) | 16 (40) | ||
| Lymph node metastasis | 0.006 | 0.024 | |||||
| Yes | 44 (57.89) | 25 (75.76) | 19 (44.19) | 16 (44.44) | 28 (70) | ||
| No | 32 (42.11) | 8 (24.24) | 24 (55.81) | 20 (55.56) | 12 (30) | ||
| Distant metastasis | 0.017 | 0.016 | |||||
| Yes | 32 (42.11) | 19 (57.58) | 13 (30.23) | 10 (27.78) | 22 (55) | ||
| No | 44 (57.89) | 14 (42.42) | 30 (69.77) | 26 (72.22) | 18 (45) | ||
Results were analyzed by using the Chi-square test. TMN – tumor-node-metastasis.
Table 2.
Correlation between Cav-1 and Ki-67 expression.
| Cav-1 | Ki-67 | rs | P | |
|---|---|---|---|---|
| Negative (n=36) | Positive (n=40) | |||
| Negative (n=33) | 8 (22.22) | 25 (62.5) | 0.283 | 0.024 |
| Positive (n=43) | 28 (77.78) | 15 (37.5) | ||
Discussion
Although Cav-1 has been well established as a tumor suppressor in CRC, the mechanism remains unclear. The most accepted idea is that Cav-1 inhibits key signaling pathways that favor cell proliferation and reduce cell death, such as heterotrimeric G proteins, H-Ras, c-Src, endothelial nitric oxide synthase, AKT, MAPK, and tyrosine kinase receptors [21–26]. The present study aimed to elucidate the role of Cav-1 in CRC cell proliferation, migration, and invasion in vitro, both under normal conditions and following EGFR phosphorylation. Furthermore, the link between Cav-1 and clinicopathological features of patients with CRC and EGFR expression was also evaluated.
To induce Cav-1 overexpression, we transfected human SW480 cells with wild-type Cav-1 gene, and subsequently evaluated cell proliferation, migration, and invasion using the MTS, scratch, and Transwell assays, respectively. Compared with control cells, the SW480 cells overexpressing Cav-1 exhibited a slower rate of growth, lower migration rate, and decreased invasion capacity. These results indicated that the overexpression of Cav-1 could reduce cell proliferation, migration, and invasion capacities in vitro, which is in line with previous findings. For example, Bender et al. [15] demonstrated a reduction in cell tumorigenicity following Cav-1 re-expression in colon carcinoma cell lines, indicating that the expression of Cav-1 can retard tumor formation in nude mice. Furthermore, an interesting study by Lili et al. [27] provided evidence of decreased motility of cells expressing Cav-1, which exhibited less filopodia or lamellipodia. These findings indicated that Cav-1 not only is a colon cancer suppressor that regulates tumorigenicity, but it also downregulates colon cancer metastasis. Friedrich et al. [28] reported that Cav-1 deficiency promoted colorectal tumorigenesis in mice. Moreover, Erdemli et al. [29] demonstrated lower Cav-1 serum levels in patients with CRC with disease progression than in those without disease progression. They also found a higher mean progression-free survival in patients with higher Cav-1 levels than in those with lower Cav-1 levels.
Our present results also indicated that overexpression of Cav-1 decreased EGFR, ERK, and AKT phosphorylation, which is consistent with previous reports [30]. Furthermore, EGFR activation promoted cell motility and growth, which corroborates earlier findings [31]. Several lines of evidence indicated that EGFR is increased and activated in CRC, and promotes cell proliferation, migration, and metastasis, while mitigating apoptosis and angiogenesis [32,33]. Similar to Cav-1, EGFR is also an actin-binding protein that undergoes dimerization and autophosphorylation upon EGF binding. The phosphorylation of EGFR activates MAPK/ERK and PI3K/AKT downstream signaling pathways [34–37], and promotes tumor growth through cell proliferation and migration.
The Ras/Raf/MEK/ERK pathway is involved in human neoplasia, and is a key player in the regulation of gene expression via growth factor receptors, thus preventing apoptosis [38]. Furthermore, ERK promotes cell proliferation, survival, and metastasis, and is activated by EGFR and Ras small guanosine triphosphatases [39]. Miao et al. [40] demonstrated that ERK1/2 downregulation inhibits CRC invasion partly due to ERK1/2-dependent downregulation of matrix metalloproteinases (MMPs). The PI3K/AKT pathway transduces the activated EGFR signals, leading to cell proliferation, survival, and motility [41], and plays an important role in cell survival during various stages of colon cancer. Indeed, the increased activity of PI3K/AKT is associated with a more malignant phenotype of differentiated colon carcinoma [42]. Furthermore, activated AKT prevents calcium release from mitochondria, which is essential for apoptosis [38]. The PI3K/AKT pathway is activated following interaction with the Ras protein, indicating cross-talk between Ras/Raf/MAP/MEK/ERK and PI3K/AKT pathways [43].
Taken together, these findings suggest that the Cav-1-induced inhibition of CRC proliferation and metastasis may occur through the downregulation of Ras/Raf/MEK/ERK and PI3K/AKT pathways by reducing EGFR phosphorylation. It is also worth noting that Cav-1 mediates the endocytosis of cholesterol-enriched membrane microdomains (CEMMs), which may be associated with the suppression of various signaling pathways and subsequent cell-cycle arrest. Indeed, in the absence of Cav-1, CEMMs cannot be internalized from the plasma membrane, thus resulting in the activation of Rac-, PI3K/AKT-, and ERK-mediated growth signals [44]. Ahmed et al. [45] reported that Cav-1 inhibited tumor growth and suppressed the activation of AKT and ERK pathways, through increasing E-cadherin and β-catenin levels and promoting their localization at the cell membrane.
We also investigated the role of Cav-1 in EGFR+CRC tissues and found that more Cav-1+ cases had a TNM stage I to II than Cav-1− cases (p=0.004). Moreover, we observed a higher rate of lymph node metastasis (p=0.006) and distant metastasis (p=0.017) in the Cav-1+ cases than in the Cav-1− cases. In addition, we observed a significantly negative correlation between Cav-1 and Ki-67 immunoreactivity (p=0.024). Garouniatis et al. [46] reported lower levels of Cav-1 in the ascending colon carcinomas than within tumors of the left colon and the rectum; they also reported a higher survival rate in patients expressing Cav-1. In contrast, Fine et al. [17] reported an elevated expression Cav-1 in colon adenocarcinomas. In the present study, we observed a higher expression of Cav-1 in the left-sided colon and rectal cancer tissues than in the right-sided colon cancers; however, the difference was not statistically significant. In line with this, previous reports have indicated that right-sided colon cancers are more aggressive and are associated with poorer clinical outcomes than left-sided colon cancers [47,48]. These findings may indicate different mechanisms in the right and left colon carcinogenesis.
Several limitations are worth noting in the present study. First, the number of patients with colorectal cancer included in our analysis was relatively small and selected from a single hospital. Second, no follow-up analysis to assess the impact of Cav-1 expression on the patients’ outcomes was included. Therefore, the present findings need further validation in multiple-center studies with larger samples and including follow-up analyses.
Conclusions
In summary, our present findings suggest a key role of Cav-1 in CRC proliferation, migration, and invasion in vitro, with a negative correlation of Cav-1 and Ki-67 immunoreactivity. Furthermore, the results indicated a plausible mediating role of EGFR phosphorylation as well as ERK/AKT pathways. We found a significant correlation between the expression of Cav-1 and the TNM stage, lymph node metastasis, and distant metastasis in CRC tissues expressing EGFR. Nevertheless, previous findings demonstrated that Cav-1 acts as a tumor promoter in CRC, indicating discrepancies in the reported effects, which may be due to differences in the activation status of the Cav-1 domains or the effect of other associated molecules [17]. Indeed, Cav-1 may be a “conditional” tumor suppressor that is influenced by its microenvironment and the tumor progression. Further studies are warranted to disclose the role Cav-1 in CRC and its prospective therapeutic value.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81071846, to T.N.Z); the Natural Science Foundation of Hebei Province of China (No. H2013505059, to T.N.Z); the Department of Science and Technology of Hebei Province of China (No.12396107D, to R.J.Z, No. 14397707D, No. 09966114D, and No. 092461102D, to T.N.Z); and the Wu Jieping Medical Foundation (No. 320.6750.12604, No. 320.6750.14063, and No. 320.6799.15005 to T.N.Z)
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W-Q, Zheng R-S, Zhang S-W, et al. The incidences and mortalities of major cancers in China, 2010. Chin J Cancer. 2024;33:402–5. doi: 10.5732/cjc.014.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Q, Li K, Lin G-Z, et al. Incidence trends and age distribution of colorectal cancer by subsite in Guangzhou, 2000–2011. Chin J Cancer. 2015;34(8):358–64. doi: 10.1186/s40880-015-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Network CGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–37. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia Y, Guo M. Epigenetic changes in colorectal cancer. Chin J Cancer. 2013;32:21–30. doi: 10.5732/cjc.011.10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Wang N, Liu P, et al. Caveolin-1, a stress-related oncotarget, in drug resistance. Oncotarget. 2015;6:37135–50. doi: 10.18632/oncotarget.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anwar SL, Wahyono A, Aryandono T, Haryono SJ. Caveolin-1 in breast cancer: Single molecule regulation of multiple key signaling pathways. Asian Pac J Cancer Prev. 2015;16:6803–12. doi: 10.7314/apjcp.2015.16.16.6803. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Che G. Value of caveolin-1 in cancer progression and prognosis: Emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression. Oncol Lett. 2014;8:1409–21. doi: 10.3892/ol.2014.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trane AE, Hiob MA, Uy T, et al. Caveolin-1 scaffolding domain residue phenylalanine 92 modulates Akt signaling. Eur J Pharmacol. 2015;766:46–55. doi: 10.1016/j.ejphar.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Chanvorachote P, Pongrakhananon V, Halim H. Caveolin-1 regulates metastatic behaviors of anoikis resistant lung cancer cells. Mol Cell Biochem. 2015;399:291–302. doi: 10.1007/s11010-014-2255-4. [DOI] [PubMed] [Google Scholar]
- 11.Salis O, Bedir A, Ozdemir T, et al. The relationship between anticancer effect of metformin and the transcriptional regulation of certain genes (CHOP, CAV-1, HO-1, SGK-1 and Par-4) on MCF-7 cell line. Eur Rev Med Pharmacol Sci. 2014;18:1602–9. [PubMed] [Google Scholar]
- 12.Sayhan S, Diniz G, Karadeniz T, et al. Expression of caveolin-1 in peritumoral stroma is associated with histological grade in ovarian serous tumors. Ginekol Pol. 2015;86(6):424–28. doi: 10.17772/gp/2398. [DOI] [PubMed] [Google Scholar]
- 13.Bennett NC, Hooper JD, Johnson DW, Gobe GC. Expression profiles and functional associations of endogenous androgen receptor and caveolin-1 in prostate cancer cell lines. Prostate. 2014;74:478–87. doi: 10.1002/pros.22767. [DOI] [PubMed] [Google Scholar]
- 14.Liang W, Hao Z, Han J-L, et al. CAV-1 contributes to bladder cancer progression by inducing epithelial-to-mesenchymal transition. Urol Oncol. 2014;32:855–63. doi: 10.1016/j.urolonc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Bender FC, Reymond MA, Bron C, Quest AF. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res. 2000;60:5870–78. [PubMed] [Google Scholar]
- 16.Patlolla JM, Swamy MV, Raju J, Rao CV. Overexpression of caveolin-1 in experimental colon adenocarcinomas and human colon cancer cell lines. Oncol Rep. 2004;11:957–63. [PubMed] [Google Scholar]
- 17.Fine SW, Lisanti MP, Galbiati F, Li M. Elevated expression of caveolin-1 in adenocarcinoma of the colon. American journal of clinical pathology. 2001;115:719–24. doi: 10.1309/YL54-CCU7-4V0P-FDUT. [DOI] [PubMed] [Google Scholar]
- 18.Peeters M, Price T, Van Laethem J-L. Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: Where are we today? Oncologist. 2009;14:29–39. doi: 10.1634/theoncologist.2008-0167. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang N, Ma J, et al. Expression profile analysis of new candidate genes for the therapy of primary osteoporosis. Eur Rev Med Pharmacol Sci. 2016;20:433–40. [PubMed] [Google Scholar]
- 20.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–84. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–90. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Okamoto T, Chun M, et al. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 23.Scherer PE, Okamoto T, Chun M, et al. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996;93:131–35. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song KS, Li S, Okamoto T, et al. Co-purification and direct interaction of Ras with Caveolin, an integral membrane protein of Caveolae microdomains detergent-free purification of caveolae membranes. J Biol Chem. 1996;271:9690–97. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 25.Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–35. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271:3863–68. [PubMed] [Google Scholar]
- 27.Nimri L, Barak H, Graeve L, Schwartz B. Restoration of caveolin-1 expression suppresses growth, membrane-type-4 metalloproteinase expression and metastasis-associated activities in colon cancer cells. Mol Carcinog. 2013;52:859–70. doi: 10.1002/mc.21927. [DOI] [PubMed] [Google Scholar]
- 28.Friedrich T, Richter B, Gaiser T, et al. Deficiency of caveolin-1 in Apc min/+ mice promotes colorectal tumorigenesis. Carcinogenesis. 2013;34:2109–18. doi: 10.1093/carcin/bgt142. [DOI] [PubMed] [Google Scholar]
- 29.Erdemli HK, Kocabas R, Salis O, et al. Is serum Caveolin-1 a useful biomarker for progression in patients with colorectal cancer? Clin Lab. 2016;62:401–8. doi: 10.7754/clin.lab.2015.150719. [DOI] [PubMed] [Google Scholar]
- 30.Han F, Zhu H. Over-expression of caveolin-1 inhibits proliferation and invasion of pancreatic carcinoma cells in vitro. Zhonghua Zhong Liu Za Zhi. 2009;31:732–37. [in Chinese] [PubMed] [Google Scholar]
- 31.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spano J, Fagard R, Soria J-C, et al. Epidermal growth factor receptor signaling in colorectal cancer: Preclinical data and therapeutic perspectives. Ann Oncol. 2005;16:189–94. doi: 10.1093/annonc/mdi057. [DOI] [PubMed] [Google Scholar]
- 33.Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322–27. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- 34.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 35.Morgillo F, Martinelli E, Troiani T, et al. Antitumor activity of sorafenib in human cancer cell lines with acquired resistance to EGFR and VEGFR tyrosine kinase inhibitors. PLoS One. 2011;6:e28841. doi: 10.1371/journal.pone.0028841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Yi YW, Hong W, Kang HJ, et al. Inhibition of the PI3K/AKT pathway potentiates cytotoxicity of EGFR kinase inhibitors in triple-negative breast cancer cells. J Cell Mol Med. 2013;17:648–56. doi: 10.1111/jcmm.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiori JL, Zhu T-N, O’Connell MP, et al. Filamin A modulates kinase activation and intracellular trafficking of epidermal growth factor receptors in human melanoma cells. Endocrinology. 2009;150:2551–60. doi: 10.1210/en.2008-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 40.Miao Y, Zhang Y, Wan H, et al. GABA-receptor agonist, propofol inhibits invasion of colon carcinoma cells. Biomed Pharmacother. 2010;64:583–88. doi: 10.1016/j.biopha.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Meng F, Wang F, Wang L, et al. MiR-30a-5p overexpression may overcome EGFR-inhibitor resistance through regulating PI3K/AKT signaling pathway in non-small cell lung cancer cell lines. Front Genet. 2016;7:197. doi: 10.3389/fgene.2016.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piplani H, Rana C, Vaish V, et al. Dolastatin, along with Celecoxib, stimulates apoptosis by a mechanism involving oxidative stress, membrane potential change and PI3-K/AKT pathway down regulation. Biochim Biophys Acta. 2013;1830:5142–56. doi: 10.1016/j.bbagen.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 44.Cerezo A, Guadamillas MC, Goetz JG, et al. The absence of caveolin-1 increases proliferation and anchorage-independent growth by a Rac-dependent, Erk-independent mechanism. Mol Cell Biol. 2009;29:5046–59. doi: 10.1128/MCB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salem AF, Bonuccelli G, Bevilacqua G, et al. Caveolin-1 promotes pancreatic cancer cell differentiation and restores membranous E-cadherin via suppression of the epithelial-mesenchymal transition. Cell Cycle. 2011;10:3692–700. doi: 10.4161/cc.10.21.17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garouniatis A, Zizi-Sermpetzoglou A, Rizos S, et al. Vascular endothelial growth factor receptors 1, 3 and caveolin-1 are implicated in colorectal cancer aggressiveness and prognosis – correlations with epidermal growth factor receptor, CD44v6, focal adhesion kinase, and c-Met. Tumor Biol. 2013;34:2109–17. doi: 10.1007/s13277-013-0776-1. [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Zhou Z, Liang J, et al. [Analysis of clinicopathologic and survival characteristics in patients with right-or left-sided colon cancer]. Zhonghua Yi Xue Za Zhi. 2015;95:2268–71. [in Chinese] [PubMed] [Google Scholar]
- 48.Hussain M, Waqas O, Hassan U, et al. Right-Sided and Left-Sided colon cancers are two distinct disease entities: An analysis of 200 cases in Pakistan. Asian Pac J Cancer Prev. 2016;17:2545–48. [PubMed] [Google Scholar]







