Abstract
Objective
Activity-based anorexia is a translational rodent model that results in severe weight loss, hyperactivity, and voluntary self-starvation. The goal of our investigation was to identify vulnerable and resistant phenotypes of activity-based anorexia in adolescent female rats.
Method
Sprague-Dawley rats were maintained under conditions of restricted access to food (N = 64; or unlimited access, N = 16) until experimental exit, predefined as a target weight loss of 30–35% or meeting predefined criteria for animal health. Nonlinear mixed effects statistical modeling was used to describe wheel running behavior, time to event analysis was used to assess experimental exit, and a regressive partitioning algorithm was used to classify phenotypes.
Results
Objective criteria were identified for distinguishing novel phenotypes of activity-based anorexia, including a vulnerable phenotype that conferred maximal hyperactivity, minimal food intake, and the shortest time to experimental exit, and a resistant phenotype that conferred minimal activity and the longest time to experimental exit.
Discussion
The identification of objective criteria for defining vulnerable and resistant phenotypes of activity-based anorexia in adolescent female rats provides an important framework for studying the neural mechanisms that promote vulnerability to or protection against the development of self-starvation and hyperactivity during adolescence. Ultimately, future studies using these novel phenotypes may provide important translational insights into the mechanisms that promote these maladaptive behaviors characteristic of anorexia nervosa.
Keywords: anorexia nervosa, activity-based anorexia, food restriction, animal model, vulnerability, hyperactivity, exercise, rat, adolescence, resistance
Introduction
Dieting and exercise are widely endorsed by adolescent girls, yet the progression to severe self-starvation and, in many cases, the excessive exercise that is characteristic of anorexia nervosa occurs in a much smaller subpopulation. 1 An understanding of pre-existing risk factors that drive these maladaptive behaviors could enhance early identification of the disorder and provide information useful to developing targeted pharmacological treatments. 2 Identifying pre-existing risk factors for self-starvation and hyperactivity in anorexia nervosa is a difficult task, given the inability to identify individuals prior to the onset of illness. In contrast, animals can be studied from birth in order to systematically identify factors that confer vulnerability or resistance to self-starvation and hyperactivity in response to food restriction.
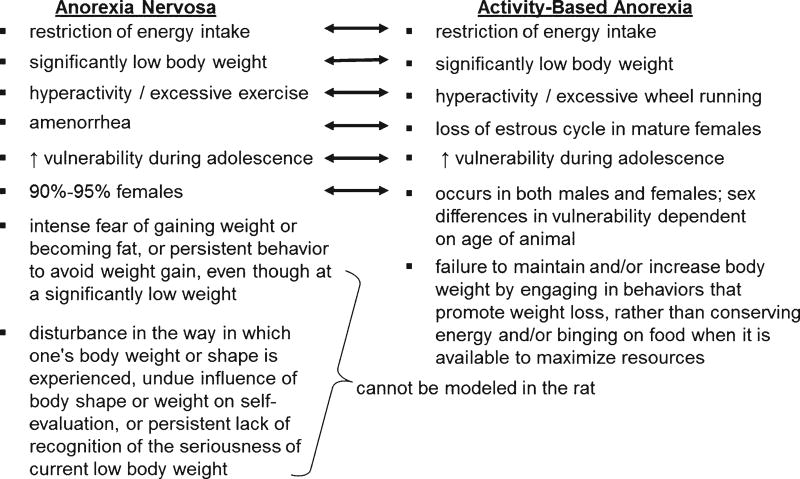
Activity-based anorexia is the most widely utilized rodent model of anorexia nervosa and was first identified over half a century ago.3–7 The model combines limited access to food (typically 1–1.5 h/day) and unlimited access to a running wheel, resulting in a rapidly escalating cycle of severe weight loss and hyperactivity (for example, see Refs. 3–10). Remarkably, many animals with activity-based anorexia continue to run throughout the period of food access, thereby promoting voluntary self-starvation and, if not stopped, death. Whereas it is clear that no animal model can mimic all aspects of a complex psychiatric disorder such as anorexia nervosa (Fig. 1), activity-based anorexia provides a rational approach for systematically studying the development and maintenance of self-starvation and hyperactivity in a controlled environment,2,7,10 and has the potential to provide useful insight into preexisting risk factors for these maladaptive behaviors.
FIGURE 1.
A comparison of features of anorexia nervosa and activity-based anorexia. Figure adapted from Ref. 2 to reflect proposed changes in terminology for diagnosing anorexia nervosa in DSM-5.
When we conducted a posthoc analysis combining several of our prior studies in female adolescent animals, we found a large variation in the between-subject response of animals to the activity-based anorexia rat paradigm. This suggested identifying phenotypes of vulnerability and resistance may provide an important mechanism for identifying risk factors for self-starvation and hyperactivity. This hypothesis was supported by early research by Epling and Pierce6 who identified four anorexia “effects” in adolescent male rats that were restricted to 1.5 h/day of food access with varying levels of wheel access (0, 2, 6, 12, 18, and 22 h/day). Animals were classified on the basis of individual regression curves for food intake and body weight that two independent investigators subjectively coded. At the two extremes, “strong anorexia” was defined as a decline in body weight, with some downward acceleration occurring during the last few days; food intake was low upon entry but increased over the first few days, indicating that the animal was no longer defending body weight. “Strong survival” was defined by a drop in body weight that leveled off and then increased; food intake increased linearly over time. This experiment provided preliminary information that confirmed a need to pursue the hypothesis of different phenotypes in the free-running “choice” paradigm with wheel running as a dependent variable and not an independent variable, and to extend this research to female rats given the predominately female nature of anorexia nervosa.
Similar to the age-dependent risk for clinical anorexia nervosa,1 the maladaptive cycle of self-starvation and hyperactivity characteristic of activity-based anorexia escalates more rapidly and to a more severe extent during adolescence compared to adulthood in rats.11,12 While the majority of adolescent female rats require removal from the experimental conditions in less than one week in order to prevent mortality,10 other rats from the same litter remain resistant to self-starvation and hyperactivity suggesting the existence of reproducible phenotypes varying in vulnerability. The primary goals of the current study were to (1) characterize activity-based anorexia animals’ inherent propensity for wheel running and self-starvation in an ad libitum wheel access paradigm and to (2) develop a set of objective criteria for characterizing phenotypes of vulnerable and resistant animals for use in future studies that would examine the biological underpinnings of risk and protection against maladaptive behaviors of self-starvation and hyperactivity. Further, the stability, maintenance, and reproducibility of the within subject behavior during the experimental conditions were also analyzed so as to ensure the utility of these phenotypes over time. We hypothesized that if vulnerable and resistant phenotypes were identified and stable, the characterization of objective criteria for defining these phenotypes would provide an innovative framework for future studies aimed at identifying the neural mechanisms that promote vulnerability to and/or provide protection against self-starvation and hyperactivity in adolescent female animals under identical experimental conditions, with the ultimate goal of translating this knowledge to a better understanding of the mechanisms that promote clinical anorexia nervosa.
Method
This study was approved by the Institutional Animal Care and Use Committees of Columbia University and the New York State Psychiatric Institute.
Animals
Eighty Sprague-Dawley rats were obtained from Taconic Farms on postnatal day 21 and individually housed upon arrival. This was an initial descriptive study and the sample size was based on the idea that 10–20% of the population falling into the extreme subpopulations would yield ~eight animals per group. Adolescent female rats were used to mimic the age- and sex-specific onset of anorexia nervosa in adolescent girls.1
Procedure
Animals were placed on a reverse 12 h light/12 h dark cycle upon arrival and allowed to acclimate for 16 days prior to beginning baseline data collection. Water was available ad libitum throughout the study. At the start of baseline (postnatal day 37), animals were matched for an exact day of age, rather than a specific body weight (body weight range of 84.3–148.4 g); this body weight range is typical of 37-day-old adolescent female animals beginning baseline measurements in our lab. Previous research using a modified paradigm of activity-based anorexia (2 h/day of wheel access, 1.5 h/day of food access) indicated that an initial low body weight predicted greater vulnerability to activity-based anorexia in animals at the same day of age.13 Thus, one goal of the current study was to determine whether these previous findings would apply to a standard activity-based anorexia protocol with ad libitum wheel access and a shorter period of food access (1 h/day).
At the start of the dark cycle on postnatal day 37, all animals were transferred to a home cage with an activity wheel attached (ENV-046; Med Associates, St. Albans, VT). Running wheel access was available 24 h/day throughout the study. Body weight, food intake, and wheel running activity were recorded daily within 30 minutes prior to the start of the dark cycle. Baseline wheel running activity in the presence of ad libitum access to food was measured for 3 days. Beginning on postnatal day 40, animals were divided into two groups matched for baseline body weight and wheel running. In the activity-based anorexia group (N = 64), food access was restricted to the first hour of the dark cycle each day, in order to mimic the time of day when control animals typically begin food consumption. In the control group (N = 16), food access was available ad libitum throughout the study. A time-to-event experimental design endpoint was used, wherein animals in the activity-based anorexia group were maintained under conditions of restricted food access until the day of experimental exit. The day of experimental exit for the control group was determined by the day the last animal in the activity-based anorexia group exited the study. Day of experimental exit was the primary target for the analysis of vulnerability, with wheel running, weight loss, and food intake selected as secondary endpoints.
Criteria for Experimental Exit
Early research on activity-based anorexia utilized criteria for removal that focused on weight recovery (i.e., weight on day 4 had to be equal to or greater than weight on day 1 of any 4 day period) or on absolute food intake (i.e., less than 1 g of food intake during the feeding period). 4 More commonly, studies have utilized a maximum percent weight loss for removal (typically 20–35%), in order to minimize animal distress. In our lab, many adolescent female animals reach weight loss criteria of 25% within 2–4 days, yet hyperactivity does not peak until a 30–35% weight loss has occurred, typically within 3–6 days for more vulnerable animals. Thus, the current study utilized a target weight loss of 30–35% as previously reported (for example, see Refs. 5, 12, 14–16).
Several issues should be considered when utilizing a 30% weight loss criteria. For example, 30% weight loss has been shown to produce ulcers in a significant number of animals compared to 25% weight loss and this has led to the activity-based anorexia paradigm being conceptualized as an animal model of ulceration.5,15,17 Yet it is not clear whether ulceration itself, or rather the more severe levels of hyperactivity that accompany a 30% versus 25% weight loss increased the risk of mortality, as despite a higher incidence of ulceration in animals fed during the dark cycle compared to the light cycle, there was no difference between groups in mortality rate but there were significant differences in hyperactivity.14 Further, despite no significant difference in the incidence of ulcers in animals fed on a reverse light/dark cycle versus a normal light/dark cycle, animals on the former cycle died sooner.17 As animals were given 30 days to adapt to the circadian shift prior to beginning experiments, it is not clear that this increased mortality was solely because of the use of a reverse light/dark cycle, as 30 days should be sufficient for adaptation. More likely, the increased mortality was because of the increased levels of running in animals maintained on the reverse light/dark cycle who were fed during the dark phase compared to animals maintained on a normal light/dark cycle who were fed during the light phase. Timing of food access may have also increased risk of mortality as food access was delayed 3 hours into the dark cycle, whereas animals typically begin food consumption at the onset of the dark cycle. This delay in food access may have increased food anticipatory activity characteristic of activity-based anorexia animals, thereby driving the increased hyperactivity and promoting mortality. Taken together, it is clear that the experimental paradigm must be carefully designed in order to maximize the maladaptive behaviors of interest, whereas minimizing the rate of mortality. In order to minimize potential animal distress in the current study, animals were allowed to adapt to the reverse light/dark cycle for 16 days prior to beginning baseline data recording and were fed at the onset of the dark cycle during restricted food access. Any animal that displayed clear signs of distress (e.g., complete cessation of food consumption, vocalization, engagement in stereotypic behaviors, hypoactivity) was removed from the study immediately, even if the maximum weight loss had not been attained.
Data Analysis
Analyses were focused on two objectives that supported the overall research goals: (1) to make mathematical models that describe wheel running and self-starvation response for the purpose of identifying phenotype patterns and (2) to examine statistical associations of variables and correlations which identified phenotypes based on measures of vulnerability. Mathematical models that examined the variance in the “time to experimental exit” variable with and without the co-factors of “wheel running”, “postnatal day of age”, “food intake”, and “baseline wheel running” were tested with four statistical software packages (R1.14.1; SAS/BASE® 9.2, SAS Institute, Cary, NC18; Orange19; NONMEM® 6.2, Globo-Max/ICON, Ellicott City, MD).
Survival Analysis of Activity-Based Anorexia-Induced Experimental Exit
The amount of time for individual animals to exit the experiment was not normally distributed, suggesting that analysis of the “time to experimental exit” variable should not be based on the group mean. A longitudinal mathematical modeling approach, rather than a cross-sectional analysis would not bias the measurement as a mean-based approach would, because it does not ignore “time” (as an odds-based approach for subgroup analysis would). The “time to experimental exit” variable was first modeled using the lifetable method (LIFETEST, SAS) to provide the distribution and frequency of the experimental exit at a given point in time during the experiment. We used the SAS LIFEREG procedure to statistically identify predictors of the day of experimental exit following the start of activity-based anorexia. The values of the predictors were log transformed because they were not normally distributed. Covariates in the model included “baseline wheel running”, “food intake”, and “body weight”.
Nonlinear mixed effects
Nonlinear mixed effects modeling was used to identify subpopulations of animals in their wheel running behavior for several reasons. The amount of time an animal spent in wheel running often increased with time and in some animals was nonlinear. There was also the need to mathematically model how wheel running changed with time while individual animals exited the study, for example, because of excessive weight loss. An approach based on the average total wheel running time would bias towards individual subjects that stayed in the study for a longer duration and a simple average would not identify subpopulations that started the experiment with low running behavior and then increased curvilinearly later in time. Therefore, an approach was needed that allows the identification of individual contributions to the population’s group variability in wheel running. A modeling approach was needed that would also allow for both a linear or nonlinear change in the measured variable. We hypothesized that wheel running would start low and increase to a maximum level at a rate that was different for each subject. The goal of the analyses in the present study was to identify animals in subpopulations based on their individual wheel running effort independent of different experimental exit times, therefore wheel running was analyzed using nonlinear mixed effects modeling to identify sub-populations based on their intersubject variability distributions of model parameters (e.g., baseline, maximum running plateau, and rate). The selection of the best descriptive and predictive models and covariates was guided by goodness of fit criteria including diagnostic scatter plots, the precision of parameter estimates, and the relationship of the estimated parameters to the number of degrees of freedom that were needed in the model to achieve a satisfactory description of the data.
Recursive Partitioning
Once it became clear from the analysis of the experimental exit and wheel running data that there were subpopulations of animals, the goal was to discriminate the subpopulations based on the vulnerability criteria: wheel running and food intake. These subpopulations of vulnerability and resilience to activity-based anorexia were identified using statistical methods utilizing recursive partitioning with the implementation of conditional inference trees (R Party; Ref. 20). A conditional inference (permutation) tree was used to select the experiment variable with the strongest association with the time to experimental exit. In the current data sample, the association was a regression. This technique followed procedure similar to linear regression, but also allowed inclusion of nonlinear and nonmonotone associations between variables. This was important because it allowed the use of both linear and nonlinear predictors as it was not clear a priori what the relative importance of each predictor would be in discriminating subpopulation differences in the experimental exit variable. A dendogram of tree-structured models was then used to create rules regarding group sensitivity to the procedure of splitting the data. Importantly, this algorithm stopped once the null hypothesis was rejected and the number of nodes created in the dendrogram was equal to the number of discernible subpopulations.
Analysis of Phenotypic Differences
Following the characterization of four distinct phenotypes, the phenotypes were analyzed for significant differences in body weight, food intake, and wheel running during baseline and the first 4 days of activity-based anorexia using ANOVAs, with post hoc Tukey multiple comparison tests to determine specific differences between groups. A Kaplan-Meier time to event analysis with a log-rank test for group differences was used to determine significance between experimental exit times of the different phenotypes.
RESULTS
Comparison of Control and Activity-Based Anorexia Groups
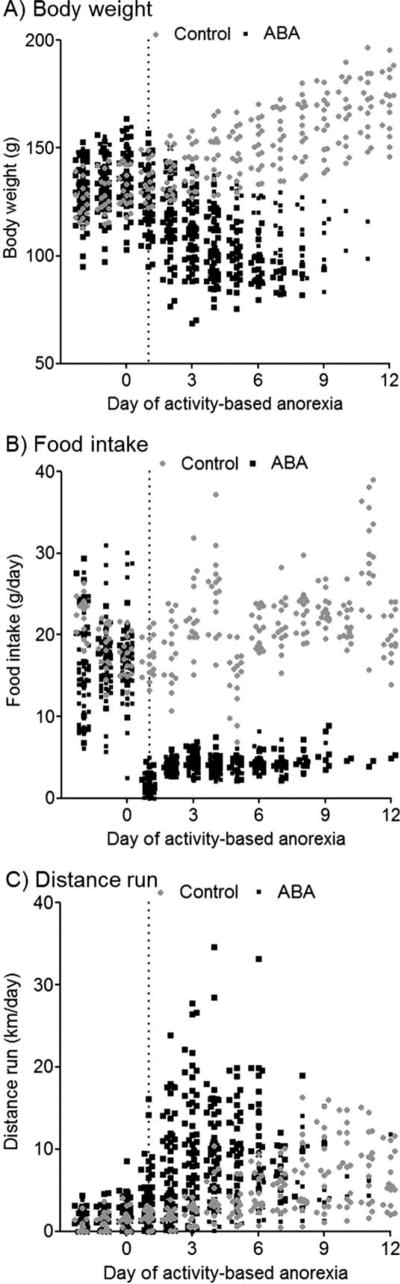
Within this population of adolescent female rats, control animals increased their body mass fairly linearly during the experimental time (Fig. 2A), maintained stable food intake (Fig. 2B), and gradually increased their wheel running over time (Fig. 2C). In contrast, the activity-based anorexia animals lost body mass (Fig. 2A), reduced their food intake (Fig. 2B), and rapidly increased wheel running (Fig. 2C). The distance run in the wheel increased 3.7 folds for the activity-based anorexia group as a whole, whereas the control group increased about 2 fold. The activity-based anorexia animals as a group exhibited a rapid increase in wheel running during the first 3 days of restricted food access that then plateaued and shifted down to converge with normal animals after approximately 7–8 days. This downward shift was due to high runners being removed from the experiment early (within the first 2–4 days) and low runners remaining in the experiment over time.
FIGURE 2.
Initial observations of the activity-based anorexia (ABA) model in adolescent female Sprague-Dawley rats indicated variability in the response to restricted food access. The sample included 16 control animals (24 h/day access to food and a running wheel) and 64 ABA animals (1 h/day access to food and 24 h/day access to a running wheel). Both groups received unlimited access to food and the wheel for three baseline days. Restricted food access at the onset of the dark cycle began on experimental day 1 for ABA animals. Overall, control animals increased their body mass (A), maintained food intake (B), and increased wheel running activity over time (C). ABA animals lost body mass (A), reduced food intake (B), and increased wheel running (C). As a group, the ABA animals had a rapid increase in wheel running during the first 3 days of restricted food access, which then plateaued and shifted down to converge with control animals after approximately 7–8 days. This effect was the result of early dropout by high wheel runners during days 2–4.
The body weight change of control animals was fit with a linear regression with a slope of ~4. This indicated control animals gained ~4 g per day of body weight from the ~20 g per day of food that was consumed during this time. At experimental day 5, which represented the approximate mean day of experimental exit for the activity-based anorexia group, there was a ~17% increase from the mean baseline body weight in the control group. Animals in the activity-based anorexia group immediately decreased the amount of food consumed by ~77% beginning on the first day of restricted food access. Food intake increased gradually over the next few days in the activity-based anorexia group, but remained significantly lower than the controls throughout the experiment.
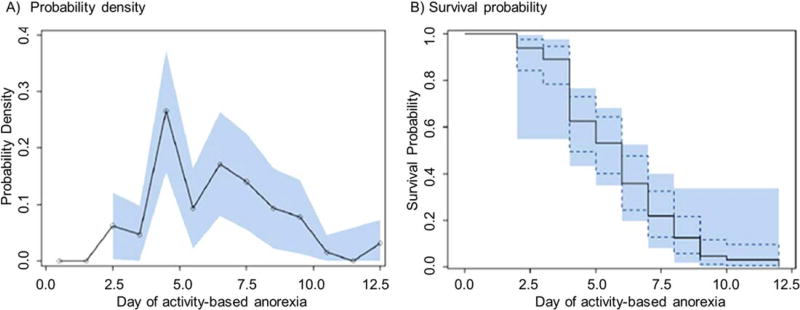
Survival Analysis and Phenotype Characterization of Activity-Based
Anorexia Survival analysis of experimental exit data of the activity-based anorexia group (Fig. 3A) demonstrated at least two subpopulations, one subpopulation with experimental exit occurring at day 4 and a second subpopulation at day 6; this first peak at day 4 could also be considered an early shoulder from the day 6 normal population. The survival probability graph (Fig. 3B) indicated ~40% of the animals exited the experiment by day 4 of activity-based anorexia, indicating a vulnerable subpopulation. Using the SAS LIFEREG procedure, maximum wheel running during the first 4 days followed by maximum food intake were both significant factors for influencing the time to experimental exit, with higher wheel running during the first 4 days decreasing survival and higher food intake during the first 4 days increasing survival.
FIGURE 3.
Day of experimental exit from the activity-based anorexia model indicated multiple sub-populations. (A) The probability density graph (95% confidence interval indicated by the shaded area) indicated a double peak with two sub-populations—the first peak occurred at day 4 and the second peak at day 6. (B) The survival probability graph (95% confidence interval indicated by the dotted line; 95% Hall-Wellner band indicated by the shaded area) indicated that 40% of the animals exited the experiment by day 4, representing a vulnerable phenotype. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Wheel running (km) was modeled using a nonlinear mixed effects model. A nonlinear sigmoidal shaped curve was a better fit than a linear regression. During model development, clusters of between subject parameter estimates indicated one or more unique subpopulations were observed that had relatively higher versus lower maximum wheel running estimates.
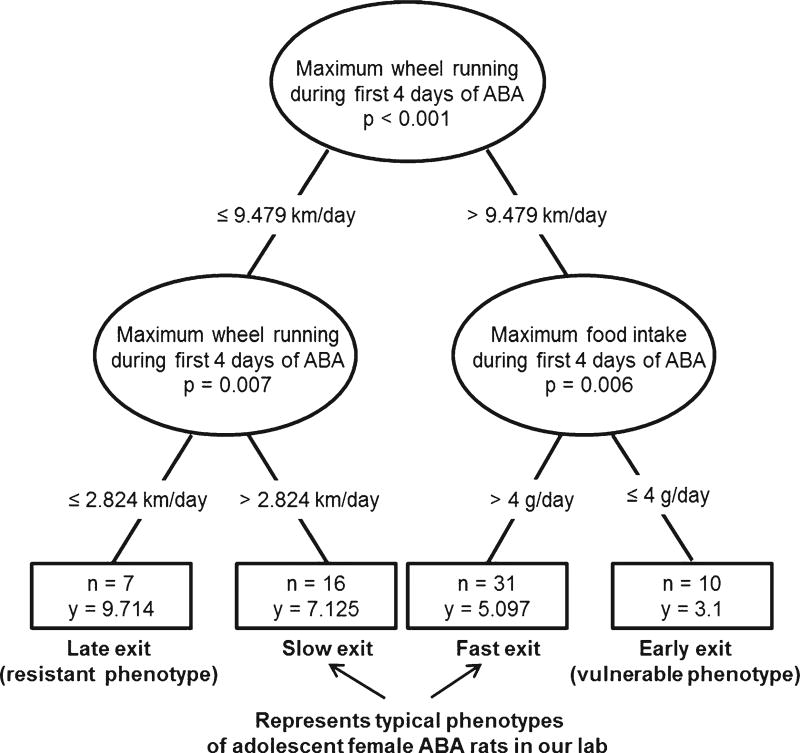
Recursive partitioning resulted in a four-group cluster that split from the total population sampled by the maximum wheel running variable (first 4 days of activity-based anorexia; km > 9.479 n = 41; km ≤ 9.479, n = 23, Fig. 4). In the animals that had equal to or less than 9.479 km/day of running during the first 4 days of activity-based anorexia, the slow exit phenotype and late exit resistant phenotype were separated by a wheel running measure of 2.824 km/day. In the animals that had greater than 9.479 km/day of running during the first 4 days of activity-based anorexia, the fast exit phenotype and the early exit, vulnerable phenotype were separated by the maximum food intake during the first 4 days of activity-based anorexia (early exit ≤4 g). These cutoffs were used as a set of rules to derive the classification of groups. For example, if an animal ran >9.479 km/day and also consumed ≤4 g/day of food during the first 4 days of restricted food access, then the animal was assigned to the vulnerable phenotype (~16% of population), with dropout occurring ~day 3. At the opposite extreme, if an animal never ran greater than 2.824 km/day, the animal was assigned to the resistant phenotype (~11% of population), with dropout occurring ~day 10. Animals that fell in between these two extremes (~73% of population) were characterized as one of two “typical” phenotypes that represent the behavioral response of most animals typically seen in our laboratory. Moreover, important to our central hypothesis of the existence of different phenotypes was that phenotypes were stable over time. In this respect, animals in the resistant phenotype did not develop the same severity of hyperactivity as the vulnerable phenotype, even when maintained in the experimental conditions for up to 12 days.
FIGURE 4.
A targeted regression analysis was used to generate classifiers of activity-based anorexia. The R package “party” implementation of recursive partitioning is shown as a regression tree graph. The different splits were ultimately used to define the classification of the activity-based anorexia data into groups with sample size (n) and day of experimental exit (y) represented on the nodes of the figure. Wheel running and food intake drove the classification of smaller subgroups. Main subpopulations of interest include the animals at the extremes – the early exit, vulnerable phenotype and the late exit, resistant phenotype; the majority of animals were identified as one of two intermediate phenotypes (fast exit or slow exit).
Differences Between Phenotypes: Post Hoc within Sample Activity-Based Anorexia Measures
An overall analysis of variance of the first 4 days of activity-based anorexia indicated relevant differences between groups in mean body weight (F = 15.40, R2 = .44, p < .0001), food intake (F = 22.45, R2= .53, p < .0001), and distance run per day (F = 42.20, R2= .68, p < .0001) (Table 1). During the first 4 days of activity-based anorexia, a post hoc tukey multiple comparison test indicated that the vulnerable phenotype weighed less than the fast exit (p < .05), slow exit (p < .001), and resistant (p < .001) phenotypes. The resistant phenotype weighed more than the slow exit (p <.05), fast exit (p < .001), and vulnerable (p < .001) phenotypes. There was no difference in mean body weight between the slow exit and fast exit phenotypes. During the first 4 days of activity-based anorexia, a post hoc tukey multiple comparison test indicated that the vulnerable phenotype consumed significantly less food than all other groups (p < .001). The resistant phenotype consumed significantly more food than the slow exit (p < .01), fast exit (p < .05), and vulnerable (p < .001) phenotypes. There was no difference in mean food intake between the slow exit and fast exit phenotypes. During the first 4 days of activity-based anorexia, a post-hoc tukey multiple comparison test indicated that the vulnerable phenotype ran more than the fast exit (p < .05), slow exit (p < .001), and resistant (p < .001) phenotypes. The resistant phenotype ran less than the fast exit (p < .001) and vulnerable (p < .001) phenotypes. The slow exit phenotype ran significantly less than the fast exit (p < .001) phenotype, providing an important distinction between these intermediate phenotypes.
TABLE 1.
Mean body weight, food intake, and wheel running during baseline and activity-based anorexia across four phenotypes
| Variable | Vulnerable | Fast exit | Slow exit | Resistant | ANOVA | Between group differences |
|---|---|---|---|---|---|---|
| Mean baseline body weight | 122.4 ± 13.6 | 132.8 ± 11.3 | 135.9 ± 9.0 | 148.6 ± 6.6 | F = 8.43, R2 = .30, p < .0001 | V < S*, R***; F < R** |
| Mean baseline food intake | 15.9 ± 1.6 | 16.2 ± 3.3 | 16.7 ± 4.4 | 19.7 ± 2.9 | F = 2.20, R2 = .10, p = 0.0977 | none |
| Mean baseline distance run | 2.1 ± 1.3 | 1.8 ± 1.0 | 1.0 ± 0.6 | 0.03 ± 0.04 | F = 10.32, R2 = .34, p < .0001 | V > S*; R***; F > S*, R*** |
| Mean ABA body weight | 100.0 ± 12.0 | 113.0 ± 11.9 | 121.2 ± 12.2 | 136.6 ± 7.1 | F = 15.40, R2 = .44, p < .0001 | V < F*, S***, R***; S*, F*** < R |
| Mean ABA food intake | 2.6 ± 0.2 | 3.7 ± 0.5 | 3.5 ± 0.5 | 4.2 ± 0.4 | F = 22.45, R2 = .53, p < .0001 | V < F***, S***, R***; S**, F* < R |
| Mean ABA distance run | 12.1 ± 4.4 | 9.5 ± 2.7 | 3.5 ± 1.2 | 0.8 ± 0.8 | F = 42.20, R2 = .68, p < .0001 | V > F*, S***, R***; F > S***, R*** |
Baseline data was averaged for each individual animal over 3 days of ad libitum food and wheel access. Activity-based anorexia data was averaged over the first 4 days of restricted food access. Results are presented as mean ± standard deviation. Abbreviations include: Vulnerable (V), Fast exit (F), Slow exit (S), Resistant (R),
p < .05,
p < .01,
p < .001
Day of experimental exit was predefined as reaching a target weight loss of 30–35% or meeting predefined criteria for animal health (see methods). There were no significant differences in mean % weight loss at the time of experimental exit between phenotypes (Table 1). Both Log-Rank (ChiSquare = 62.66, DF = 3, p < .0001) and Wilcoxon (ChiSquare = 56.80, DF53, p < .001) tests between groups indicated a significant difference in survival time between phenotypes, with the median and range of days to experimental exit as follows: vulnerable (median = 3 days; range = 2–5), fast exit (median = 5 days; range = 3–8), slow exit (median = 7 days; range = 6–10), resistant (median = 9 days; range = 8–12).
Differences Between Phenotypes: Post Hoc Analysis of Within Sample Baseline Measures
An overall analysis of variance indicated important differences between groups based on mean baseline body weight (F = 8.43, R2 = .30, p< .0001) and distance run per day (F = 10.32, R2 = .34, p < .0001), but no significant differences between groups in baseline food intake (F = 2.20, R2 = .10, p = ns) (Table 1). For mean baseline body weight, a post hoc tukey multiple comparison test indicated that the vulnerable phenotype weighed less than the slow exit (p < .05) and resistant (p < .001) phenotypes. The resistant phenotype weighed more than the fast exit (p < .01) and vulnerable (p < .001) phenotypes. There was no difference between the slow exit and fast exit phenotypes. For mean baseline distance run per day, a post hoc tukey multiple comparison test indicated that the vulnerable phenotype ran more than the slow exit (p < .05) and resistant (p < .001) phenotypes. The fast exit phenotype ran more than the slow exit (p < .05) and resistant (p < .001) phenotypes. There was no difference between vulnerable and fast exit phenotypes or between resistant and slow exit phenotypes.
DISCUSSION
The current study identified four novel phenotypes of activity-based anorexia in adolescent female rats ranging from vulnerable to resistant. As previously discussed, activity-based anorexia is a maladaptive behavioral response wherein the investigator controls food access and an animal continues to engage in exercise behavior rather than consume food, resulting in voluntary self-starvation. Wheel running in activity-based anorexia is a highly variable feature of the model, with some individuals responding very differently from one another. While most experimental designs compare activity-based anorexia animals to other control groups, the knowledge to be gained from understanding the between-subject variability of animals within the activity-based anorexia group where animals are under identical experimental conditions and are often littermates should provide a more experimentally clean elucidation of phenotypic information. Since the genetic and environmental conditions are very similar, this more focused design could drive better resolution of individual contributions to phenotypic differences that a broad genetic*environment interaction experiment may miss. The current study is the first to provide objective criteria for defining stable phenotypes of activity-based anorexia in adolescent female rats that differed in vulnerability to self-starvation and hyperactivity using both univariate and multivariate approaches.
Phenotypic differences in the response to the activity-based anorexia paradigm were driven primarily by maximum wheel running in the first 4 days of restricted food access, with animals running the most and, secondarily consuming the least amount of food, requiring the earliest removal from experimental conditions. These findings corroborate and extend earlier work that demonstrated hyperactivity was a crucial aspect of the model that determined survival (for example, Refs. 4, 5, 17). Further, early work by Epling and Pierce that examined the effects of experimental intervention of the duration of wheel running access by varying wheel running as the independent variable demonstrated that when activity increased exponentially during the first few days of restricted food access, survival in the model was zero.6 Although this paper attempted to characterize animals into four different groups based on anorexia “effects”,6 it did not focus on capturing the animals’ inherent propensity for wheel running in a 24 h/day, ad libitum access paradigm and the inference from this study would only be comparable to our results if the investigators explored phenotypes within the different levels of the wheel running independent variable, which they did not. Further, in line with many initial studies on activity-based anorexia, male animals were studied, whereas research over the past 20 years has more commonly utilized female animals, in order to provide a more translationally relevant model for the clinical anorexia nervosa population, which is 90–95% female.1 Finally, the regression curves were subjective and open to experimenter bias, and did not attempt to supply objective criteria for the characterization of anorexia “effects” as phenotypes for use in future studies, such as we present here. What would be of interest for future study would be to go back to a similar paradigm as in the initial Epling and Pierce study6) and determine with an appropriate sampling strategy if there is a critical point in the energy access curve where phenotypes emerge. An initial hypothesis is that the phenotypes do not exist at the lower levels of wheel access, but begin to emerge at the 6–12 hours of wheel access that the investigators previously reported as having the increased variance with one or two animals represented as outliers.
In future studies, the phenotypes at the extremes of vulnerability and resistance would appear to offer the most potential for teasing apart metabolic or neurochemical mechanisms driving these inherent differences in self-starvation and hyperactivity. Nonetheless, it is important to recognize that while the intermediate phenotypes (fast exit and slow exit) did not differ in body weight or food intake, the fast exit phenotype ran significantly more than the slow exit phenotype during both baseline and activity-based anorexia and exited the experiment earlier, providing additional evidence for hyperactivity as the main factor driving survival. This discrete or continuous way to view the individual contributions will ultimately depend on the hypothesis to be tested and the design of the experiment. At this point it is unclear if 1–2 days of difference in survival is driven by significant differences in the underlying biology of the phenotypes or represents a numerical nuisance driven by statistics. However, the adolescent female rats in this study displayed remarkable differences in behavioral response to the model, even when dropout differed by 1– 2 days. In general, animals that required removal by day 3–4 displayed more signs of distress than animals removed on day 5–6 (and the same was generally true for animals removed on day 5–6 compared to day 7–8). This marked increase in behavioral distress occurred despite a similar % body weight loss between phenotypes at the time of experimental exit. In general, animals that reached weight loss criteria the fastest displayed the most behavioral signs of distress, whereas animals that took closer to a week or more to reach the same weight loss criteria often did not display the same severity in behavioral signs of distress and were removed from experimental conditions because they reached maximum weight loss criteria. Additional studies are necessary to understand the potential biological differences between phenotypes, such as whether fundamental changes in energy expenditure are driven by differences in baseline metabolism of carbohydrates, lipids, and proteins, and if the intermediate phenotypes truly differ from each other and from the more extreme phenotypes of vulnerability and resistance.
In the post hoc analysis of baseline characteristics of the four phenotypes to determine predictors for risk of early exit, there was strong evidence that animals most vulnerable to activity-based anorexia weighed less and ran more during ad libitum food access. The identification of high baseline wheel running as a risk factor for activity-based anorexia supports previous work predicting vulnerability to the development of activity-based anorexia and gastric lesions when introduced to restricted food access paradigms in male rats.17 Baseline wheel running activity levels have also been shown to predict activity-based anorexia susceptibility in 11 inbred mouse strains and outbred Wistar rats suggesting cross-genome validity given these diverse backgrounds.21 The identification of low baseline body weight as a risk factor confirms previous research using a modified paradigm of activity-based anorexia (2 h/day of wheel access, 1.5 h/day of food access) that demonstrated an initial low body weight predicted greater vulnerability to activity-based anorexia in animals at the same day of age.13 Thus, this study confirms previous research in other populations that both baseline hyperactivity and low body weight are significant risk factors for the development of severe self-starvation and hyperactivity in adolescent female activity-based anorexia rats. It is a curiosity that food intake did not predict experimental exit given the importance of energy intake to the equation of balance, but perhaps this also gives better insight into the potential opportunities for mechanisms for intervention and drives the important point of this paradigm as a model representing some facets of anorexia nervosa, but not the disease itself. The variability in the baseline body weight also provides support for the hypothesis of energy reserve based on weight as a mechanism for survival, with evidence of this in studies where age differences were tested. A follow-up would be to identify animals with a lower base rate of metabolism and/or measures of energy reserve (body fat/protein levels) and determine if these lead to different survival curves.
Ultimately, careful consideration needs to be undertaken when interpreting this post hoc analysis of the observational data as inference should not be done without an intervention. It would be of great interest to design a future study attempting to intervene during the baseline wheel adaption period and measure the effect on time to experimental exit rather than continue to use this as an observational period. Alternatively or in addition, discovering a network of small molecules to distinguish the different phenotypes would also be very interesting and something that should be further pursued.
Taken together, phenotypic differences in the extremes of activity-based anorexia—those that engage in severe self-starvation and hyperactivity and those that do not, despite identical experimental conditions—provide a critical framework for identifying factors that increase risk or provide protection against the development of these maladaptive behaviors. Importantly, these differences in vulnerability are reminiscent of the clinical population wherein only a small subpopulation of adolescent girls develops anorexia nervosa despite the nearly universal prevalence of dieting and exercise in this population. Furthermore, establishing these individual differences in the animal model enables the question of risk factors to be addressed from a wide range of basic science approaches that are not possible in the clinical field. Along these lines, our group recently identified pre-existing differences in metabolic pathways that predict individual differences in vulnerability to activity-based anorexia using metabolomics (Barbarich-Marsteller et al., in preparation) and our next step is to systematically test these metabolic pathways for causality using dietary, pharmacological, and genetic manipulations within these novel phenotypes of vulnerability and resistance. Further, evidence from mouse studies has begun to identify genetic factors involved in hyperactivity, including the identification of chromosomes that contribute to the development of intense running wheel activity during the habitual rest phase and subsequently an accelerated body weight loss in activity-based anorexia mice. Importantly, these chromosomes display homology with regions on human chromosomes linked to anxiety and obsessionality in individuals with anorexia nervosa22 and thus underscore the translational utility of animal models to study risk factors for self-starvation and hyperactivity.
Ultimately, this is the first study with the purpose, design, and power to focus on the identification of objective criteria for defining phenotypes of self-starvation and hyperactivity in adolescent female activity-based anorexia rats and offers tremendous potential for future studies aimed at identifying the biological, metabolic, neurochemical, and environmental factors driving self-starvation and hyperactivity. The stability of these newly defined phenotypes and their potential utility across multiple studies will enable the more rapid identification of sources of risk versus resilience to maladaptive behaviors characteristic of anorexia nervosa.
We would like to acknowledge the effort of Anna Rita Colacino and Kevin Laurino for their instrumental help in collecting the data.
Acknowledgments
Supported by K01 DA024115 (to NCBM).
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 2.Barbarich-Marsteller NC, Walsh BT. A translational approach to understanding anorexia nervosa. In: Barbarich-Marsteller NC, editor. Anorexia Nervosa: Symptoms, Treatment, and Neurobiology. New York: Nova Science Publishers, Inc; 2012. pp. 161–165. [Google Scholar]
- 3.Hall JF, Hanford PV. Activity as a function of a restricted feeding schedule. J Comp Physiol Psychol. 1954;47:362–363. doi: 10.1037/h0060276. [DOI] [PubMed] [Google Scholar]
- 4.Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol. 1967;64:414–421. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- 5.Pare WP. Activity-stress ulcer in the rat: frequency and chronicity. Physiol Behav. 1976;16:699–704. doi: 10.1016/0031-9384(76)90239-0. [DOI] [PubMed] [Google Scholar]
- 6.Epling WF, Pierce WD. Activity-based anorexia in rats a a funciton of opportunity to run on an activity wheel. Nutr Behav. 1984;2:37–49. [Google Scholar]
- 7.Epling WF, Pierce WD, editors. Theory, Research, and Treatment. Mahwah: Lawrence Erlbaum Associates, Publishers; 1996. Activity Anorexia. [Google Scholar]
- 8.Dixon DP, Ackert AM, Eckel LA. Development of, recovery from, activity-based anorexia in female rats. Physiol Behav. 2003;80:273–279. doi: 10.1016/j.physbeh.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Adan RA, Hillebrand JJ, Danner UN, Cano SC, Kas MJ, Verhagen LA. Neurobiology driving hyperactivity in activity-based anorexia. Curr Top Behav Neurosci. 2011;6:229–250. doi: 10.1007/7854_2010_77. [DOI] [PubMed] [Google Scholar]
- 10.Barbarich-Marsteller NC. Activity-based anorexia in the rat. In: Avena NM, editor. Animal Models of Eating Disorders. New York: Humana Press; 2012. pp. 281–290. [Google Scholar]
- 11.Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav Neurosci. 1999;113:1080–1089. [PubMed] [Google Scholar]
- 12.Barbarich-Marsteller NC, Aoki C, Laurino K, Colacino AR, Marsteller DA. Vulnerability to activity-based anorexia varies with age and sex in Sprague-Dawley rats. In preparation. [Google Scholar]
- 13.Boakes RA, Dwyer DM. Weight loss in rats produced by running: Effects of prior experience and individual housing. Q J Exp Psychol B. 1997;50:129–148. doi: 10.1080/713932647. [DOI] [PubMed] [Google Scholar]
- 14.Hara C, Manabe K, Ogawa N. Influence of activity-stress on thymus, spleen and adrenal weights of rats: Possibility for an immunodeficiency model. Physiol Behav. 1981;27:243–248. doi: 10.1016/0031-9384(81)90264-x. [DOI] [PubMed] [Google Scholar]
- 15.Doerries LE, Stanley EZ, Aravich PF. Activity-based anorexia: Relationship to gender and activity-stress ulcers. Physiol Behav. 1991;50:945–949. doi: 10.1016/0031-9384(91)90419-o. [DOI] [PubMed] [Google Scholar]
- 16.Russell JC, Morse AD. The induction and maintenance of hyperactivity during food restriciton in rats. In: Epling WF, Pierce WD, editors. Activity Anorexia Theory, Research, and Treatment. Mahwah: Lawrence Erlbaum Associates; 1996. pp. 113–121. [Google Scholar]
- 17.Pare WP. The influence of food consumption and running activity on the activity-stress ulcer in the rat. Am J Dig Dis. 1975;20:262–273. doi: 10.1007/BF01070729. [DOI] [PubMed] [Google Scholar]
- 18.SAS 9.2. The [output/code/data analysis] for this paper was generated using [SAS/STAT] software, Version [9.2] of the SAS System for [Windows] SAS Institute Inc.; Cary, NC: Copyright © [2009] [Google Scholar]
- 19.Curk T, Demsar J, Xu Q, Leban G, Petrovic U, Bratko I, et al. Microarray data mining with visual programming. Bioinformatics. 2005;21:396–398. doi: 10.1093/bioinformatics/bth474. [DOI] [PubMed] [Google Scholar]
- 20.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partioning: A conditional inference framework. J Comput Graph Stat. 2006;15:651–674. [Google Scholar]
- 21.Pjetri E, de Haas R, de Jong S, Gelegen C, Oppelaar H, Verhagen LA, et al. Identifying predictors of activity based anorexia susceptibility in diverse genetic rodent populations. PLoS One. 2012;7:e50453. doi: 10.1371/journal.pone.0050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelegen C, Pjetri E, Campbell IC, Collier DA, Oppelaar H, Kas MJ. Chromosomal mapping of excessive physical activity in mice in response to a restricted feeding schedule. Eur Neuropsychopharmacol. 2010;20:317–326. doi: 10.1016/j.euroneuro.2009.10.001. [DOI] [PubMed] [Google Scholar]