Abstract
Nanoparticles have attracted considerable interest as cancer vaccine delivery vehicles for inducing sufficient CD8+ T cell-mediated immune responses to overcome the low immunogenicity of tumor microenvironments. Our studies described here are the first to examine the effects of clinically-tested human cancer-testis (CT) peptide epitopes within a synthetic nanoparticle. Specifically, we focused on two significant clinical CT targets, the HLA-A2 restricted epitopes of NY-ESO-1 and MAGE-A3, using a viral-mimetic packaging strategy. Our data shows that simultaneous delivery of a NY-ESO-1 epitope (SLLMWITQV) and CpG using the E2 subunit assembly of pyruvate dehydrogenase (E2 nanoparticle), resulted in a 25-fold increase in specific IFN-γ secretion in HLA-A2 transgenic mice. This translated to a 15-fold increase in lytic activity toward target cancer cells expressing the antigen. Immunization with a MAGE-A3 epitope (FLWGPRALV) delivered with CpG in E2 nanoparticles yielded an increase in specific IFN-γ secretion and cell lysis by 6-fold and 9-fold, respectively. Furthermore, combined delivery of NY-ESO-1 and MAGE-A3 antigens in E2 nanoparticles yielded an additive effect that increased lytic activity towards cells bearing NY-ESO-1+ and MAGE-A3+. Our investigations demonstrate that formulation of CT antigens within a nanoparticle can significantly enhance antigen-specific cell-mediated responses, and the combination of the two antigens in a vaccine can preserve the increased individual responses that are observed for each antigen alone.
Keywords: Nanoparticle, Cancer-testis antigen (CT), NY-ESO-1, MAGE-A3, HLA-A2, Cancer vaccine
1. Introduction
Boosting a patient’s immune system by immunotherapy represents a promising approach in cancer treatment,1 and cancer vaccines in particular enable the recognition of tumor-associated antigens for targeted destruction.2 Although these cancer vaccines have been shown to elicit CD8 T cell immune responses, the typical response levels generated are usually clinically insufficient to overcome the low immunogenicity and immunosuppressive microenvironment of tumors.3,4 For example, in a review of clinical studies, an overall objective response rate (50% tumor size reduction) of only 2.6% was observed with peptide vaccines derived from gp100, MART-1, TRP-2, cancer testis NY-ESO-1, MAGE-A12, or HER2 antigens (alone or in combination with adjuvant); these results suggest the need for development of more effective strategies and therapies.1
The application of nanotechnology has shown an exceptional promise towards improvement of cancer diagnosis and treatment in recent years.5–7 Antigen uptake by dendritic cells (DCs) depends on the antigen properties such as geometry,8 surface charge,9 and importantly, size.10,11 Some nanoparticles have the advantage of being in the optimal size for DCs uptake and passive transport to the lymphatic system, with prior research demonstrating that particles between approximately 20–45 nm are taken up more effectively by the DCs residing in the lymph-nodes.8,12,13 Therefore, delivery of vaccine components with these nanoparticles may facilitate and increase DC interaction, resulting in a more effective immune responses.14–15
One nanoparticle with favorable DC uptake properties is the E2 nanoparticle.15–17 E2 is a 25-nm non-viral protein nanoparticle derived from the pyruvate dehydrogenase complex of Bacillus stearothermophilus.18 It is composed of 60 identical monomer subunits that self assemble into a highly thermostable dodecahedral caged structure with a hollow 12-nm cavity,19 and it can be engineered at the internal, external, and inter-subunit interfaces to change the properties and functionality of the nanoparticle.20–23 Our research group has also previously demonstrated that the viral-mimetic, simultaneous delivery of adjuvant (internally packaged) and MHC-I restricted antigens (bound on the surface) via E2 nanoparticles resulted in an increase in DCs activation and antigen cross-presentation.16 This was associated with a significant increase in antigen-specific IFN-γ secretion, tumor cell lysis, delayed B16-F10 tumor growth, and increased survival time in C57Bl/6 mice.15 Cell uptake by immune cells and biodistribution of E2 have also been reported.17
The target epitopes in this current study are HLA-A2 restricted peptide sequences from New York esophageal squamous cell carcinoma-1 (NY-ESO-1) and melanoma antigen family A, 3 (MAGE-A3).24 While other tumor-associated antigens (TAAs) are often expressed at low levels in healthy tissue, expression of CTs is restricted only to cancer cells and the immune-privileged cells in the testis. Furthermore, CT antigens exist in a high proportion of different human tumors such as melanoma, bladder, lung, prostate, and breast cancers.25,26 In particular, NY-ESO-1 is expressed in 82% of neuroblastomas and 46% melanomas27 while MAGE-A3 is also expressed in 76% of melanoma cancers.28 A phase II clinical trial of NY-ESO-1/ISCOMATRIX vaccine which was recently completed in June 2017 (NCT00518206, ClinicalTrials.gov) resulted in 4% partial response (based on a standard of 30% reduction in tumor size), 48% stable disease, and 48% progressive disease; this result highlights the generation of response to NY-ESO-1, but also the need and potential for development of alternative strategies that will yield more effective therapies.
Given the wide range of tumors that express CT antigens, their relatively high expression level in cancer, their restricted expression, and their potential for vaccine improvement, the CT class of antigens is an important and significant clinical target. In this study, we examined the feasibility of using the E2 nanoparticle to induce cell-mediated immune responses against NY-ESO-1 and MAGE-A3 in a mouse model that is transgenic for the human major histocompatibility complex, HLA-A2. While our prior work examined gp100, an antigen that has mouse and human analogues, this current study focuses on antigens that are specifically expressed in humans.
We also investigate the extent of cell-mediated and cytolytic responses by simultaneous delivery of NY-ESO-1-and MAGE-A3-containing nanoparticles. Tumor escape after single-epitope vaccination is common since cancers often lose expression of the targeted antigen to evade the immune system.29 Immunization with combined antigens can possibly decrease the risk of tumor escape resulting from antigen loss.30,31 Furthermore, increasing the number of different antigen targets in a vaccine can induce a broader range of T cell responses simultaneously, which could be effective in a higher number of patients. Because there is a lack of immune-competent murine tumor models expressing these CT antigens to examine in vivo anti-tumor efficacy in the most physiologically relevant way possible, we examined lytic ability ex vivo using human cancer cell lines expressing both NY-ESO-1 and MAGE-A3. To our knowledge, our study is the first to test the efficacy of cell-mediated responses to clinically-relevant CT peptide epitopes formulated as a nanoparticle, and it examines these human epitopes in nanoparticles both individually and in combination.
2. Methods
2.1. Materials
Reagents were purchased from Fisher Scientific unless otherwise noted. Complete RPMI used in this study for splenocytes was compromised of RPMI 1640 (Mediatech) with 10% heat-inactivated FBS (Hyclone), 1mM sodium pyruvate (Hyclone), 100 µg/ml of streptomycin (Hyclone), 0.1 mM nonessential amino acids (Lonza), 2 mM L-glutamine (Lonza), and 100 units/ml penicillin. Cancer cell lines used in this study were cultured in DMEM media (Sigma) supplemented with 10% heat-inactivated FBS (Hyclone).
2.2. Peptides and CpG
CpG 1826, a bacterial DNA ligand for TLR9, was purchased from Invivogen, and 5' benzaldehyde-modified CpG 1826 with a phosphorothioated backbone was synthesized by Trilink. The NY-ESO-1 and MAGE-A3 peptide epitopes were synthesized by Genscript or Genemed Synthesis (Table 1). Peptides were synthesized both with and without an N-terminal cysteine; the functionalized thiol on the cysteine-modified peptides was used for conjugation to E2, whereas peptides with no cysteine were used as controls. In this study, the abbreviation (e.g., NYESO, MAGE) refers to the peptide, while the names NY-ESO-1 and MAGE-A3 refer to the whole protein.
Table 1.
List of peptide epitopes sequences and their respective abbreviations in this study. Conventional single-letter abbreviations for amino acids (aa) are used in the peptide epitope sequence.
2.3. E2 purification and characterization
In this study, we used the D381C mutant of the E2 nanoparticle, which has an aspartic acid-to-cysteine mutation at position 381 in the internal hollow cavity of the nanoparticle. The cysteine of D381C can be used for site-directed conjugation, and this nanoparticle is abbreviated as "E2" in this study. Expression, purification, and characterization of E2 (D381C mutant) were performed as previously described.19 In summary, E. coli strain BL21 (DE3) containing the E2 gene was cultured in Luria-Bertani medium containing 100 µg/ml ampicillin. Expression was induced by adding 1mM of IPTG when the culture reached the optical density of 0.7–0.9 measured at 600 nm. Cells were harvested and stored at −80°C. Cells were lysed using a French pressure cell (Thermo Scientific), and the insoluble fraction was removed by centrifugation. The soluble fraction heated at 70°C for 20 min. Denatured native E. coli protein aggregates were removed by centrifugation. The recovered supernatant was loaded to a HiPrep Q Sepharose anion exchange column followed by a Superose 6 size exclusion column.19 Purity and the molecular weight of purified E2 were confirmed with SDS-PAGE and electrospray ionization mass spectrometry. Dynamic light scattering and transmission electron microscopy were used to check the size, assembly, and monodispersity of the particles. As previously described, lipopolysaccharide was removed using Triton X-114 (Sigma) extraction, and endotoxin levels were evaluated using an LAL ToxinSensor kit (Genscript).16
2.4. CpG and peptides conjugation
CpG 1826 modified with a 5'-benzaldehyde was attached to the TCEP-reduced cysteines in the internal cavity of E2 nanoparticles using a N-β-maleimidopropionic acid hydrazide (BMPH) linker. The average number of CpG molecules conjugated to the internal cavity of E2 nanoparticle was estimated with intensity analysis in ImageJ software, using standardized concentrations.16 Peptides with N-terminal cysteines were conjugated to the native lysines on the surface of the E2 nanoparticle by mixing the nanoparticle with a sulfo-SMCC linker in the presence of a 10-fold excess of TCEP-reduced peptides (relative to E2 monomer), and incubating overnight at 4°C. HPLC was used for peptide quantification as previously described,15 and details are in Supplementary Materials.
2.5. Zeta potential measurements
To measure zeta potential, the diffusion barrier method with monomodal analysis were used because they are compatible with the amounts and physiological ionic strengths of our samples. Malvern capillary cells were filled with buffer (50 mM potassium phosphate at pH 7.4 with 100 mM NaCl), and 150 µl of nanoparticles (at 1.3 mg/ml) were gently injected to the bottom of the buffer-filled capillary cells. Zeta potential was measured with a Malvern Zetasizer (Nano ZS).
2.6. Mice
Transgenic mice expressing the human HLA-A2 gene were obtained from Jackson Laboratory. All animal studies were carried out in accordance with protocols approved by the Institute for Animal Care and Use Committee (IACUC) at the University of California, Irvine. Briefly, 6–8 week old female HLA-A2 transgenic C57BL/6 mice were immunized subcutaneously at the base of the tail at Day 0. A priming immunization was followed by a booster after 14 days. Injections were 120 µl and contained specific amounts of peptide, E2, and CpG, based on the formulations tested. Seven days after the last immunization, mice were sacrificed and spleens were isolated.
2.7. IFN-γ ELISpot
For IFN-γ ELISpot, we used the Ready-Set-Go kit (eBioscience). Single-cell suspensions in RPMI were prepared from the spleens isolated from immunized mice, and added at 5×105 and 106 cells/well to PVDF ELISpot plates that were pre-coated with an anti-mouse IFN-γ antibody. Cell were incubated with either 10 µg/ml of the relevant peptide or an irrelevant peptide (SIINFEKL) for 24 hrs at 37°C. U nstimulated cells in RPMI were plated and served as a negative control. Positive control wells contained 2% PHA-M (Gibco). IFN-γ spots were developed following the manufacturer's protocol. Plates were scanned and quantified using an ELISpot reader (Cellular Technology) and immunospot analysis software (Immunospot Analysis Pack).
2.8. Cell lines
A375, a human malignant melanoma cell line, and MCF-7, a human breast cancer cell line, were purchased from ATCC. A375 was cultured in DMEM supplemented with 10% FBS, and MCF-7 was cultured in DMEM supplemented with 10% FBS and 0.01 mg/ml human recombinant insulin. Cells were incubated at 37°C, under 5% CO2, and were passaged 2–3 times a week.
2.9. Cell lysis assay
Single cell suspensions prepared from splenocytes isolated from immunized mice were cultured in RPMI at 5×106 cell/ml and incubated overnight at 37°C. On day 1, 10 µg/ml of target peptide was added to the cells, incubated for 24 hrs at 37°C, and washed twice with PBS to remove the unbound peptide. On day 3, culture was supplemented with 0.4 ng/ml of IL-2. Peptide stimulated splenocytes were collected on day 5 to perform the cytotoxicity assay. LDH release was measured with a calorimetric assay, CytoTox 96 (Promega), to examine the specific lysis of target cancer cell lines expressing NY-ESO-1 and MAGE-A3 antigens. A375 expresses both NY-ESO-137 and MAGE-A3,38 while MCF7 has low expression of these antigens.39,40 Splenocytes were counted with a hemocytometer, co-cultured with the target cancer cell lines at effector-to-target ratios of 100:1, 50:1, and 25:1, and evaluated for LDH release following the manufacturer's protocol. Data is reported as "% Lysis", calculated as
where "experimental LDH release" is the LDH release from cancer cells co-cultured with splenocytes minus the sum of LDH release from the cancer cells and the splenocytes cultured separately. "Maximum LDH release" is from lysed cancer cells using the kit's lysis buffer minus background LDH release from cancer cells.
2.10. Statistical analysis
Statistical analysis was carried out using GraphPad Prism. Data are presented as mean ± standard error of the mean (S.E.M.) of at least three independent experiments (n ≥ 3). Statistical analysis was determined by a two-way ANOVA over all groups followed by a Tukey's multiple comparison test, unless otherwise noted. P-values less than 0.05 were considered significant.
3. Results and Discussion
3.1. Conjugation of CpG and cancer-testis peptides to E2 nanoparticle yielded intact nanoparticles
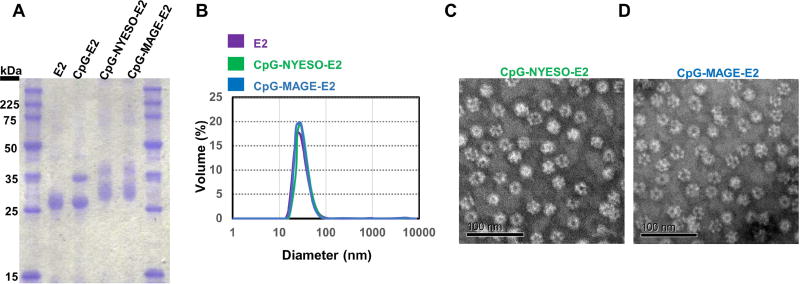
Both CpG and peptides were successfully conjugated to the E2 nanoparticle, and the results are consistent with those previously described for conjugation of other epitopes to the E2 nanoparticle.15,16 The CpG-conjugated nanoparticle (CpG-E2) displayed two distinct bands on SDS-PAGE [Fig. 1A]; the lower band at ~28 kDa is the unconjugated E2 monomer and coincides with 28118 ± 13 Da measured by mass spectrometry, and the band at ~ 35 kDa is indicative of the conjugation of one CpG molecule attached to one 28-kDa E2 monomer. By analyzing band intensities, we estimate a conjugation ratio of 21 ± 4 CpG molecules encapsulated per (60-mer) protein particle, similar to prior studies.16
Figure 1. Characterization of functionalized nanoparticles.
A) SDS-PAGE shows successful conjugation of CpG and peptides (NYESO, MAGE) to E2 nanoparticles. B) DLS reveals nanoparticle sizes of about 30 nm, before and after conjugation. C) TEM of CpG-NYESO-E2 nanoparticles. D) TEM of CpG-MAGE-E2 nanoparticles. Scale bars are 100 nm.
Simultaneous conjugation of CpG to the internal cavity and peptides to external surface of E2 resulted in two distinct broad bands [Fig. 1A]. The broad band between 30–35 kDa shows the heterogeneous conjugation of peptides (from Table 1) to the surface of E2 monomers (peptide-E2), and the band between 35–40 kDa confirms the simultaneous conjugation of peptide and CpG to the E2 monomers (CpG-peptide-E2). We quantified the number of peptides conjugated to the E2 nanoparticle with HPLC,15 and found that on average, 140 ± 16 NYESO or 155 ± 21 MAGE peptides were attached on each protein nanoparticle. Conjugation of CpG and peptides to the E2 nanoparticle resulted in a 1:1 mass ratio. This ratio of CpG and peptide has also been used in other studies to successfully induce CD8 T cell responses.41,42
Dynamic light scattering revealed a hydrodynamic diameter of 28.4 ± 0.7, 30 ± 1.3, and 30 ± 0.9 nm for E2, CpG-NYESO-E2, and CpG-MAGE-E2 respectively [Fig. 1B]. DLS data confirmed that particles remained unaggregated and within the optimal reported size for lymphatic drainage (20–45 nm),5,12,13 even after conjugation. This data verifies that attachment of short peptides (9 amino acid length) on the surface of the nanoparticle does not result in a dramatic change in size. TEM analysis further confirmed intact, non-aggregated CpG-NYESO-E2 [Fig. 1C] and CpG-MAGE-E2 [Fig. 1D] nanoparticles. The zeta potential of E2, CpG-NYESO-E2, and CpG-MAGE-E2 nanoparticles were −11.7 ± 1 mV, −12.8 ± 1 mV, and −11.1 ± 1.8 mV, respectively. This data confirmed that conjugation of NYESO or MAGE peptides on the surface and CpG inside of the nanoparticles did not change the overall surface charge compared to the E2 nanoparticle itself.
3.2. Immunization with CpG-NYESO-E2 nanoparticles yielded increased antigen-specific IFN-γ secretion
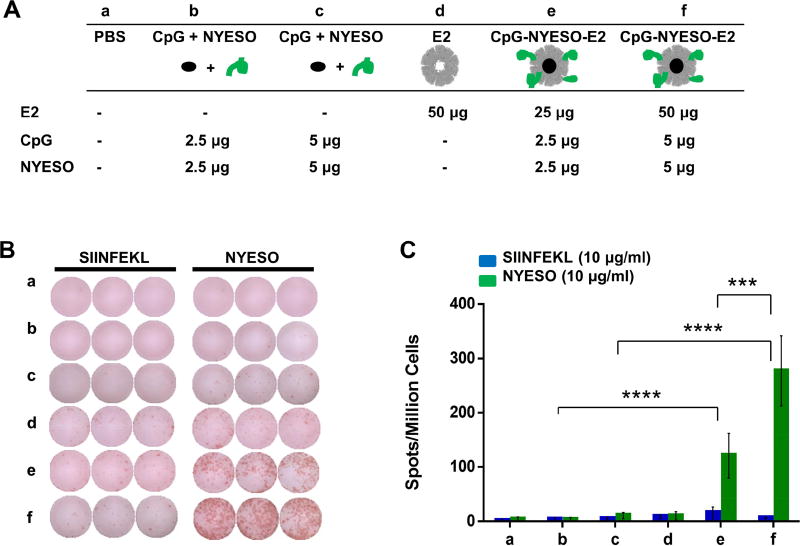
Different vaccine formulations of NY-ESO-1 antigen peptide, CpG, and E2 were investigated, and ELISpot results are presented in Figure 2. Immunization with the CpG-NYESO-E2 nanoparticle significantly increased the NY-ESO-1 epitope-specific IFN-γ secretion by 25-fold, compared to immunization with unbound CpG and NYESO, at an equivalent amount of antigen and adjuvant [Fig. 2B,C]. In contrast, we observed negligible IFN-γ response by the cells pulsed with an irrelevant SIINFEKL peptide [Fig. 2C], confirming that the immune response generated from immunization was specific to the NYESO epitope. Splenocytes isolated from mice immunized with bare E2 nanoparticle also lacked significant amounts of NYESO-specific IFN-γ secretion [Fig. 2C], confirming that higher IFN-γ secretion resulting from CpG-NYESO-E2 immunization was not a result of non-specific immune responses to the E2 delivery platform itself.
Figure 2. ELISpot analysis of splenocytes from immunization with NY-ESO-1 formulations.
A) Vaccine components per dose of different formulation groups (a–f). B) Representative ELISpot data from splenocytes of immunized group, pulsed with irrelevant peptide (SIINFEKL) or relevant epitope peptide (NYESO). C) Summary of averaged ELISpot data, which evaluated antigen-specific IFN-γ secretion. HLA-A2 mice were immunized with different formulations (a–f), and splenocytes were pulsed ex vivo in the presence of relevant peptide (NYESO) or irrelevant peptide (SIINFEKL) and analyzed for specific IFN-γ secretion. Higher NY-ESO-1 epitope-specific IFN-γ secretion was observed for the group that received CpG-NYESO-E2. Data is presented as average ± S.E.M. of at least 3 independent experiments (n ≥ 3). Statistical significance was determined by two-way ANOVA followed by a Tukey's test (***p < 0.001; ****p < 0.0001).
We previously observed higher DC activation towards CpG-E2 nanoparticles and also an elevated antigen cross-presentation when a model ovalbumin peptide and CpG were delivered within an E2 nanoparticle, relative to unbound antigen and adjuvant.16 This suggests that our observed increase in IFN-γ secretion in this experiment results from higher DCs activation and more efficient cross-presentation of the NYESO epitope. This mechanism of improving T cell immunity by increasing DC uptake and activation is supported by Dhodapkar et al., who observed higher antigen-specific CD8 T cell responses with a vaccine that targeted NYESO-1 antigen to the DEC205 receptor of DCs.43 The high IFN-γ secretion specific to NYESO epitope (aa 157–165) in our study is consistent with ELISpot/IFN-γ data previously reported on a NY-ESO-1 protein with ISCOMATRIX vaccine44 and a lentiviral vector encoding the NY-ESO-1 gene.45 However, in contrast to those cases, our vaccine formulation contains only a single NYESO-1 epitope rather than the whole protein or gene, respectively, used in the previous studies.
We also observed higher peptide-specific IFN-γ secretion for the group receiving immunizations of 50 µg CpG-NYESO-E2 compared to 25 µg CpG-NYESO-E2 [Fig. 2C; groups e, f]. This demonstrates dose dependency of the generated cell-mediated immune response to the NYESO epitope. However, immunization with 100 µg of CpG-NYESO-E2 did not increase the IFN-γ secretion compared to 50 µg, and in fact showed a significant decreased across the cohort of mice [Fig. S1]. This could be due to increased levels of suppressive T cells,46 T cell exhaustion,47 or high antigen doses leading to increase tolerance.48
In addition to the NY-ESO-1 peptide epitope examined in Figure 2 (NYESO), we examined another epitope [NYESO(p2)] (see Table 1) that had been previously reported to yield a relatively low lysis of NYESO(p2)-pulsed T2 cells when incubated with T lymphocytes isolated from a patient.34 In contrast to that study, however, our data showed that immunization with nanoparticle formulations of the NYESO(p2) epitope [CpG-NYESO(p2)-E2] did not result in any significant increase in the IFN-γ secretion [Fig. S2-B]. To our knowledge, there have been no additional reports regarding the immunogenicity of this peptide. One explanation for this apparent discrepancy is that the in vitro data using T2 cells34 may not recapitulate what happens in vivo.
3.3. Higher lysis activity toward NY-ESO-1+ cancer cells was observed for the group immunized with CpG-NYESO-E2 nanoparticles
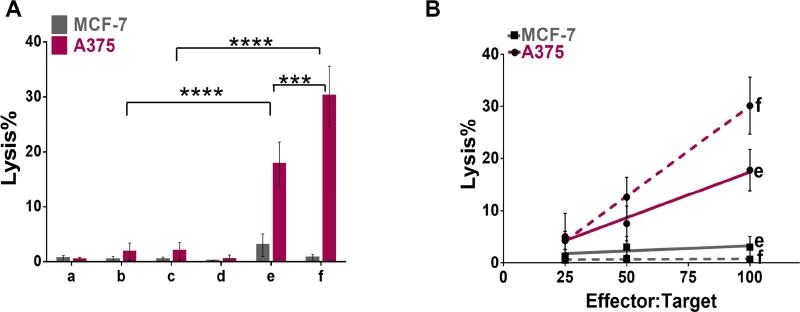
Lytic capacity of splenocytes isolated from immunized mice was tested on a human melanoma cell line expressing NY-ESO-1 (A375)49–50 and on a human breast cancer cell line negative for NY-ESO-1 expression (MCF-7)39, both positive for HLA-A2.39,49 The increase in antigen-specific IFN-γ levels that resulted from CpG-NYESO-E2 immunization [Fig. 2] translated to a 15-fold increase in the lysis activity towards A375, compared to unbound peptide and CpG [Fig. 3A]. In contrast, no significant lysis was observed for the control cell line, MCF-7 [Fig. 3A]. This data supports the specificity of the immune response for the NY-ESO-1 antigen.
Figure 3. Results of cell lysis assays using splenocytes from immunization with NY-ESO-1 formulations.
A) Summary of average lysis data at 100:1 effector:target ratio. HLA-A2 mice were immunized with different formulations (a–f; see Fig. 2A for schematic), and splenocytes were pulsed ex vivo in the presence of NYESO peptide. Splenocytes isolated from mice immunized with CpG-NYESO-E2 nanoparticles enhanced lysis activity toward A375, relative to free peptide and CpG. B) Cell lysis results at different effector:target ratios. HLA-A2 mice were immunized with different formulations (a–f; see Fig. 2A for schematic), and splenocytes were pulsed ex vivo in the presence of NYESO peptide. Lytic ability toward A375 cell line resulted from CpG-NYESO-E2 nanoparticles immunization is dose dependent. Data is presented as average ± S.E.M. of at least 3 independent experiments. Statistical significance was determined by two-way ANOVA followed by a Tukey's test (***p < 0.001; ****p <0.0001).
Our data also confirms that lysis activity towards the target cancer cell line is dose-dependent; higher activity toward A375 can be achieved by increasing the effector-to-target ratio [Fig. 3B], where the effector cells are the splenocytes isolated from the immunized mice and the target cells are the cancer cells. Our lysis data is comparable to those previously reported on the NY-ESO-1/ISCOMATRIX vaccine by which the entire NY-ESO-1 protein was delivered.44 Remarkably, we observed almost the same extent of lysis by delivering a single NYESO-1 epitope rather than the whole antigen with multiple epitopes, albeit toward a different target cell line.
Splenocytes isolated from mice immunized with bare E2 nanoparticles or antigen/CpG alone did not increase specific lysis [Fig. 3A]; this confirms that the significant increase in lysis resulting from the CpG-NYESO-E2 immunization is due to the complete nanoparticle-adjuvant-antigen delivery system, and not the effect of E2 or antigen alone. The E2 data is consistent with prior work by Perham and De Berardinis, which previously demonstrated a negligible lysis activity towards the mouse lymphoma RMA-S cell line by splenocytes isolated from mice immunized with bare E2 nanoparticle.51 Taken together, our data show that conjugation of the NY-ESO-1 epitope and CpG adjuvant to the nanoparticle is an effective delivery strategy for increasing INF-γ response, and it results in a functional lysis activity that is specific towards the NY-ESO-1 epitope.
3.4. Immunization with CpG-MAGE-E2 nanoparticles yielded increased antigen specific IFN-γ secretion
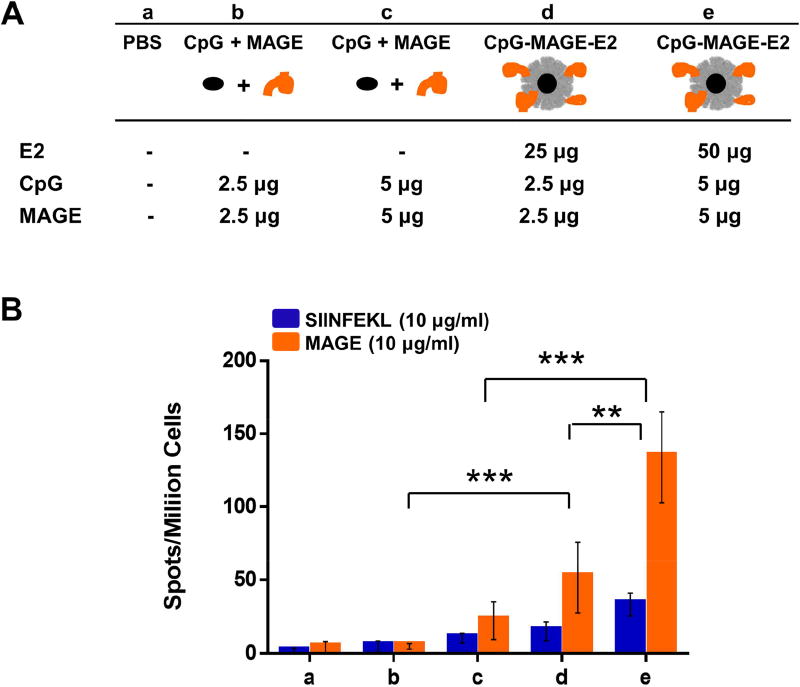
Peptide epitopes for MAGE-A3 were conjugated to the E2 nanoparticle bearing the CpG adjuvant. The ELISpot results of immunizations with the different MAGE formulations are presented in Figure 4. Vaccination with the CpG-MAGE-E2 nanoparticles increased the number of IFN-γ spots, relative to free CpG and MAGE [Fig. 4B]. We also observed a dose-dependent response, where significantly higher IFN-γ secretion was obtained for mice immunized with the 50 µg dose compared to the 25 µg dose. Statistical analyses show that IFN-γ levels resulting from the irrelevant SIINFEKL control peptide for mice immunized with 50 µg CpG-MAGE-E2 (group e) is not significantly different compared to the other vaccine formulations in Figure 4B. Similar to CpG-NYESO-E2, CpG-MAGE-E2 increased the specific IFN-γ secretion compared to the free peptide/CpG control group, but to a lower extent (6-fold for MAGE vs. 25-fold for NYESO for the comparable 50 µg dose). High IFN-γ secretion to this MAGE epitope was also observed in a previous study, where HLA-A2 mice were immunized with DNA vaccine encoding 5 epitopes, followed by a booster of peptide epitopes in solution plus a hepatitis B virus core containing a T helper peptide.35 In contrast to the previous study, however, we obtained high IFN-γ secretion to the MAGE epitope without a need for T helper peptides or a separate DNA vaccine.
Figure 4. ELISpot analysis of splenocytes from immunization with MAGE-A3 formulations.
A) Vaccine components per dose of different formulation groups (a–e). B) Summary of averaged ELISpot data, which evaluated antigen-specific IFN-γ secretion. HLA-A2 mice were immunized with different formulations (a–e), and splenocytes were pulsed ex vivo in the presence of relevant peptide (MAGE) or irrelevant peptide (SIINFEKL) and analyzed for specific IFN-γ secretion. Higher MAGE-A3 epitope-specific IFN-γ secretion was observed for the group that received CpG-MAGE-E2. Data is presented as average ± S.E.M. of 3 independent experiments (n ≥ 3). Statistical significance was determined by two-way ANOVA followed by a Tukey's test (**p < 0.01; ***p < 0.001).
The second MAGE-A3 epitope in this study, MAGE(p2), did not affect the splenocyte IFN-γ secretion when conjugated to E2-CpG compared to unbound peptide and CpG [Fig. S2-C]. The apparent discrepancy between our study compared to a previous investigation52 could be due to the different experimental conditions. In the prior study, much higher doses of peptide MAGE(p2) was used (200 µg total) compared to ours (10 µg total), together with an additional 120 µg of hepatitis B virus core containing a T helper epitope.52
3.5. Higher lysis activity toward MAGE-A3+ cancer cells was observed for the group immunized with CpG-MAGE-E2 nanoparticles
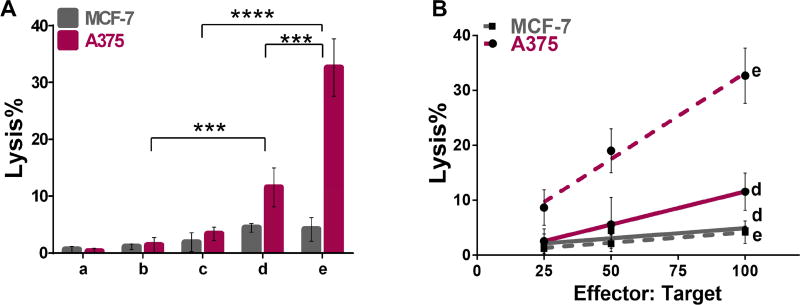
MAGE-specific lytic activity of splenocytes isolated from immunized mice was tested using A375 and MCF-7, which are positive38,53 and negative40 for MAGE-A3, respectively, and results are presented in Figure 5. Splenocytes isolated from mice immunized with CpG-MAGEE2 nanoparticles significantly enhanced the lytic activity toward A375 by 9-fold at a 100:1 effector-to-target ratio [Fig. 5A], compared to free peptide and CpG. As expected, lytic activity observed for the mice immunized with CpG-MAGE-E2 is specific to the cell line expressing MAGE, where no significant lysis was observed for the control cell line MCF-7. As with the results for the NY-ESO-1 antigen on our nanoparticle, our data confirms dose dependency of lysis activity toward target cell line [Fig. 5B]. Our data is also consistent with results previously reporting that the MAGE-specific CD8 T cells generated from immunization of MAGE epitope within bacteriophage are capable of recognizing and lysis of the MAGE-A3+ cell line.54 This data, together with the data generated for NYESO epitope, confirms that the E2 nanoparticle can be used as an effective platform to simultaneously deliver clinically-tested CT antigens and CpG, resulting in an increase in the cellular-mediated immune responses generated to the target epitope.
Figure 5. Results of cell lysis assays using splenocytes from immunization with MAGE-A3 formulations.
A) Summary of average lysis data at 100:1 effector:target ratio. HLA-A2 mice were immunized with different formulations (a–e; see Fig. 4A for schematic), and splenocytes were pulsed ex vivo in the presence of MAGE. Splenocytes isolated from mice immunized with CpG-MAGE-E2 nanoparticles enhanced lysis activity toward A375, relative to free peptide and CpG. B) Cell lysis results at different effector:target ratios. HLA-A2 mice were immunized with different formulations (a–e; see Fig. 4A for schematic), and splenocytes were pulsed ex vivo in the presence of MAGE peptide. Lytic ability toward A375 cell line resulted from CpG-MAGE-E2 nanoparticles immunization is dose dependent. Data is presented as average ± S.E.M. of at least 3 independent experiments. Statistical significance was determined by two-way ANOVA followed by a Tukey's test (***p < 0.001, ****p < 0.0001).
3.6. Co-immunization with nanoparticles bearing both NYESO and MAGE epitopes yielded an additive IFN-γ effect and increased the lysis activity
Prior studies have demonstrated that targeting a tumor through multiple antigen sources can decrease the possibility of tumor escape. For example, Banchereau et al. demonstrated clinical benefit for patients who received vaccines composed of four different melanoma antigens if at least two out of four antigen peptides were immunogenic in patients, rather than one peptide alone.55–56 Similar observations have been made for renal cell cancer.57 Therefore, we investigated the effects of simultaneous immunization with CpG-NYESO-E2 and CpG-MAGE-E2 nanoparticles.
We observed that immunization with the CpG-NYESO-E2 and CpG-MAGE-E2 nanoparticles together (25 µg each, 50 µg total per dose) significantly increased the NYESO and MAGE epitope-specific IFN-γ secretion, compared to immunization with simultaneous vaccination of unbound CpG, NYESO, and MAGE peptides [Fig. 6B]. As expected, we observed negligible IFN-γ for the cells pulsed with an irrelevant SIINFEKL peptide [Fig. 6B], confirming the specificity of immune response generated from immunization.
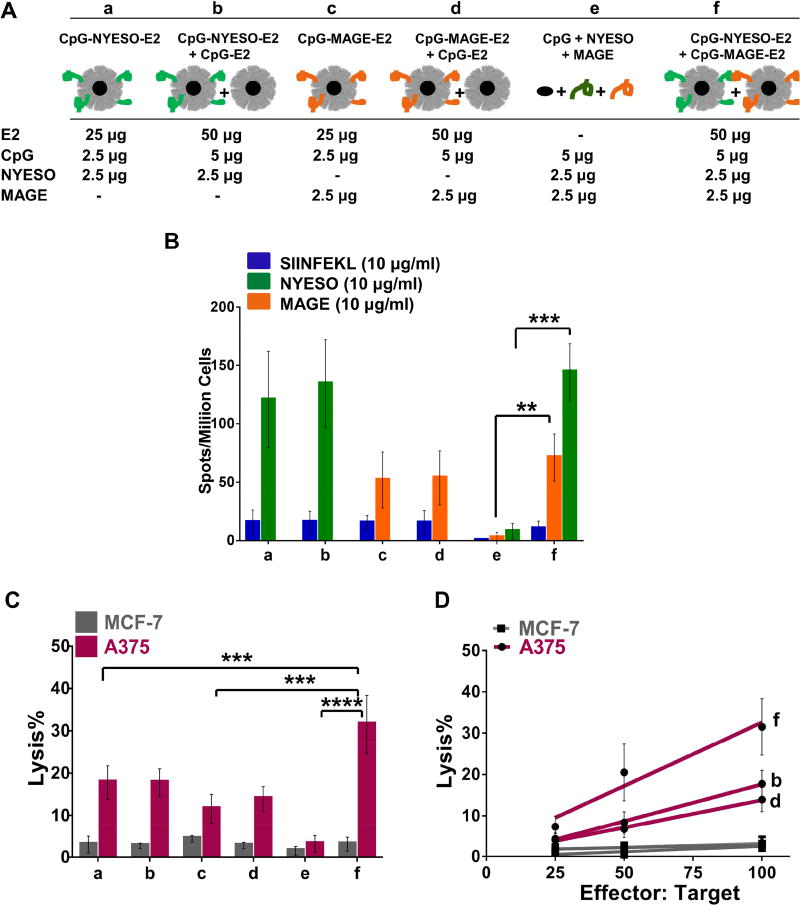
Figure 6. Cell-mediated immune responses using splenocytes from co-immunization with NYESO-1 and MAGE-A3 formulations.
A) Vaccine components per dose of different formulation groups (a–f). B) Summary of averaged ELISpot data, which evaluated antigen-specific IFN-γ secretion. HLA-A2 mice were immunized with different formulations (a–f). Splenocytes of immunized mice were pulsed ex vivo in the presence of relevant peptide (NYESO or MAGE) or irrelevant peptide (SIINFEKL) and analyzed for specific IFN-γ secretion. Data is presented as average ± S.E.M. of at least 3 independent experiments. Statistical significance was determined by two-way ANOVA followed by a Bonferroni's test (**p < 0.01; ***p < 0.001). C) Summary of average lysis activity at 100:1 effector: target ratio. Mice received immunizations with different formulations (a–f), and splenocytes were incubated with target cells (A375 or MCF-7). Co-immunization with CpG-NYESO-E2 and CpG-MAGE-E2 nanoparticles significantly enhanced the lytic activity toward HLA-A2+ and MAGE-A3+ cell line (A375), relative to immunization with one antigen. D) Cell lysis results at different effector:target ratios. Data show dose-dependent cell lysis for both individual and combined antigens when bound to E2 and CpG, relative to unconjugated peptides and CpG. Data is presented as average ± S.E.M. of at least 3 independent experiments. Statistical significance was determined by two-way ANOVA followed by a Tukey's test (**p < 0.01; ***p < 0.001).
We also obtained the same levels of IFN-γ secretion to the individual NYESO and MAGE epitopes when CpG-NYESO-E2 and CpG-MAGE-E2 nanoparticles were co-administered, relative to each nanoparticle formulation separately [Fig. 6B]; this shows that the specific IFN-γ responses to individual epitopes were preserved after co-immunization. The result is an additive effect to IFN-γ secretion, with the total IFN-γ frequencies of group f (sum of NYESO-and MAGE-specific IFN-γ) being approximately equal to the sum of individual NYESO-specific IFN-γ (group b) and MAGE-specific IFN-γ (group d). Furthermore, this additive effect to IFN-γ secretion from the simultaneous immunization is entirely antigen-specific and is not due to any adjuvant effect due the CpG-E2 nanoparticle itself, since the addition of CpG-E2 alone did not promote further specific IFN-γ secretion (compare formulations a to b, and c to d; Fig. 6B).
Consistent with the ELISpot data, splenocytes isolated from mice simultaneously immunized with CpG-NYESO-E2 and CpG-MAGE-E2 nanoparticles (25 µg each, 50 µg total per dose) significantly enhanced the lytic activity toward A375 [Fig. 6C], in a dose dependent manner [Fig. 6D], relative to unbound CpG, NYESO, and MAGE epitopes. As expected, the lytic activity was specific to A375, with no lysis observed for the control cell line MCF-7 [Fig. 6C]. Furthermore, an elevated and additive lysis activity was observed for the mice that were coimmunized with CpG-NYESO-E2 and CpG-MAGE-E2 formulations compared to each formulation separately [Fig. 6C].
However, co-immunization with higher doses of CpG-NYESO-E2 and CpG-MAGE-E2 nanoparticles (50 µg each, 100 µg total per dose) did not amplify the specific-IFN-γ secretion [Fig. S3] or lysis activity [Fig. S4], relative to each formulation separately; in fact, both IFN-γ and lysis effects were lower than the effects of each individual antigen-nanoparticle alone. This observation could be due to T cell exhaustion47 or peptide competition on the MHC of the antigen presenting cells (e.g., DCs) or on the T cell receptors.58,59 This data, together with our data for the 100 µg CpG-NYESO-E2 results [Fig. S1], shows that for our current immunization schedule, the optimal dose for maximal immunogenic response is 50 µg total nanoparticle (either 50 µg for one antigen, or 25 µg for two antigens).
Overall, our results show that co-delivery of a multi-epitope nanoparticle vaccine can elicit higher cell-mediated immune responses than single-epitope formulations. Although dosing multiple (unconjugated) peptides in solution has shown to increase immune responses in the clinic,39–41 and others have investigated other classes of antigen combinations,60,61 our study is the first investigation, to our knowledge, that has examined the efficacy of administering multiple cancer-testis epitopes peptides within the context of nanoparticles. Our data show that (1) coimmunization of our E2 nanoparticle vaccines can preserve the individual cell-mediated effects against cancer-testis antigens and (2) that these IFN-γ and lytic effects are additive.
Vaccination of cytotoxic T-cell epitopes in combination with helper epitopes have also been investigated. While some studies show this to be a successful strategy,62 other evidence has demonstrated that the CD8 T cell response can actually be reduced after co-immunization with T helper epitopes.62,63 Thus, using T helper epitopes within our nanoparticle vaccines could be another important approach to examine towards increasing efficacy.
4. Conclusion
In this study, we have investigated the effects of incorporating clinically-relevant human cancer-testis antigens into a nanoparticle platform that had been previously shown to increase cancer vaccine efficacy. We examined whether higher cell-mediated immune responses can be achieved by simultaneously packaging of CT antigens and CpG adjuvant to the E2 nanoparticle. In a HLA-A2 transgenic mouse model, we found that immunization with nanoparticle formulations containing CpG and CT antigens resulted in a significantly higher specific IFN-γ frequencies compared to unbound antigen and CpG. Further, we observed an elevated lysis activity towards a target cancer cell line (A375). Additionally, simultaneous delivery of CpG-NYESO-E2 and CpG-MAGE-E2 nanoparticles preserved the effects of the individual antigennanoparticles, and resulted in an additive IFN-γ secretion and lysis activity relative to each separate nanoparticle formulation. Altogether, this work shows the advantages of using the E2 nanoparticle as an effective vaccine platform to deliver cancer-testis antigens for higher cell-mediated activation.
Supplementary Material
Acknowledgments
We thank Dr. Jin Wook Choi, Pamela B. Besada-Lombana, and Dr. Nancy Da Silva for access to and assistance with HPLC. DLS measurements and mass spectrometry analysis were carried out at the UCI Laser Spectroscopy Facility and the UCI Mass Spectrometry Facility, respectively. We are grateful to Dr. Sergey Ryazantsev at the California NanoSystems Institute at UCLA for assistance with obtaining TEM images. We also thank Dr. Christine E. McLaren at UCI's Chao Family Comprehensive Cancer Center's Biostatistics Shared Resource Facility for consultation and advice with statistical analyses. This work was supported by the National Institute of Health (R21EB017995) and the National Cancer Institute of the National Institutes of Health (P30CA062203). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Even-Desrumeaux K, Baty D, Chames P. State of the art in tumor antigen and biomarker discovery. Cancers. 2011;3:2554–2596. doi: 10.3390/cancers3022554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer immunotherapy with peptide-based vaccines : what have we achieved ? where are we going ? J. Natl. Cancer Inst. 1991;94:805–818. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 4.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H. Therapeutic vaccines for cancer : an overview of clinical trials. Nat. Rev. Clin. Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 6.Gao F, Sun M, Ma W, Wu X, Liu L, Kuang, Xu C. A Singlet Oxygen Generating Agent by Chirality-dependent Plasmonic Shell-Satellite Nanoassembly. Adv. Mater. 2017;29:1–8. doi: 10.1002/adma.201606864. [DOI] [PubMed] [Google Scholar]
- 7.Ma W, Fu P, Sun M, Xu L, Kuang H, Xu C. Dual Quantification of MicroRNAs and Telomerase in Living Cells. J. Am. Chem. Soc. 2017;139:11752–11759. doi: 10.1021/jacs.7b03617. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 9.Soema PC, Willems G, Jiskoot W, Amorij J, Kersten GF. European Journal of Pharmaceutics and Biopharmaceutics Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015;94:427–435. doi: 10.1016/j.ejpb.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H. Gold Nanoparticles as a vaccine platform : influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 11.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 12.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly (propylene sulfide) nanoparticles. J. of Controlled Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature Biotechnology. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C, Convertine A, Stayton PS. pH-responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molino NM, Neek M, Anne J, Nelson EL, Wang S. Biomaterials Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials. 2016;86:83–91. doi: 10.1016/j.biomaterials.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molino NM, Anderson AKL, Nelson EL, Wang SW. Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano. 2013;7:9743–9752. doi: 10.1021/nn403085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molino NM, Neek M, Tucker JA, Nelson EL, Wang SW. Display of DNA on Nanoparticles for Targeting Antigen Presenting Cells. ACS Biomater. Sci. Eng. 2017;3:496–501. doi: 10.1021/acsbiomaterials.7b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domingo GJ, Chauhan HJ, Lessard IAD, Fuller C, Perham RN. Self-assembly and catalytic activity of the pyruvate dehydrogenase multienzyme complex from Bacillus stearothermophilus. Eur. J. Biochem. 1999;266:1136–1146. doi: 10.1046/j.1432-1327.1999.00966.x. [DOI] [PubMed] [Google Scholar]
- 19.Dalmau M, Lim S, Chen HC, Ruiz C, Wang SW. Thermostability and molecular encapsulation within an engineered caged protein scaffold. Biotechnol. Bioeng. 2008;101:654–664. doi: 10.1002/bit.21988. [DOI] [PubMed] [Google Scholar]
- 20.Dalmau M, Lim S, Wang S. Design of a pH-Dependent Molecular Switch in a Caged Protein Platform. Nano Lett. 2009;9:160–166. doi: 10.1021/nl8027069. [DOI] [PubMed] [Google Scholar]
- 21.Ren D, Dalmau M, Randall A, Shindel MM, Baldi P, Wang SW. Biomimetic design of protein nanomaterials for hydrophobic molecular transport. Adv. Funct. Mater. 2012;22:3170–3180. doi: 10.1002/adfm.201200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren D, Kratz F, Wang SW. Engineered drug-protein nanoparticle complexes for folate receptor targeting. Biochem. Eng. J. 2014;89:33–41. doi: 10.1016/j.bej.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren D, Kratz F, Wang SW. Protein nanocapsules containing doxorubicin as a pH-responsive delivery system. Small. 2011;7:1051–1060. doi: 10.1002/smll.201002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnadas DK, Bai F, Lucas K. Cancer testis antigen and immunotherapy. ImmunoTargets Ther. 2013;2:1–19. doi: 10.2147/ITT.S35570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fratta E, Coral S, Covre A, Parisi G, Colizzi F, Danielli R, Nicolay H, Sigalotti L, Maio M. The biology of cancer testis antigens : Putative function, regulation and therapeutic potential. Mol. Oncol. 2011;5:164–182. doi: 10.1016/j.molonc.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caballero OL, Chen Y. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholaou T, Ebert L, Davi ID, Robson N, Klein O, Maraskovsky E, Chen W, Cebon J. Directions in the immune targeting of cancer: Lessons learned from the cancer-testis Ag NY-ESO-1. Immunol and Cell Biology. 2006;84:303–317. doi: 10.1111/j.1440-1711.2006.01446.x. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H, Song Y, Hoon DSB, Appella E, Celis E. Tumor-reactive T helper lymphocytes recognize a promiscuous MAGE-A3 epitope presented by various major histocompatibility complex class II alleles. Cancer Res. 2001;61:4773–4778. [PubMed] [Google Scholar]
- 29.Olson BM, McNeel DG. Antigen loss and tumor-mediated immunosuppression facilitate tumor recurrence. Expert Rev. Vaccines. 2012;11:1315–7. doi: 10.1586/erv.12.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of Human Solid Tumors from T–Cell Recognition: Molecular Mechanisms and Functional Significance. Adv. Immunol. 1999;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 31.Petrulio CA, Kaufman HL. Development of the PANVAC-VF vaccine for pancreatic cancer. Expert Rev Vaccines. 2006;5:9–19. doi: 10.1586/14760584.5.1.9. [DOI] [PubMed] [Google Scholar]
- 32.Chen JL, Dunbar PR, Gilead iU, Jäger E, Gnjatic S, Nagata Y, Stockert E, Panicali DL, Chen YT, Knuth A, Old LJ, Cerundolo V. Identification of NY-ESO-1 Peptide Analogues Capable of Improved Stimulation of Tumor-Reactive CTL. J. Immunol. 2000;165:948–955. doi: 10.4049/jimmunol.165.2.948. [DOI] [PubMed] [Google Scholar]
- 33.Khong HT, Yang JC, Topalian SL, Sherry RM, Mavroukakis SA, Whit DE, Rosenberg SA. Immunization of HLA-A*0201 and/or HLA-DPbeta1*04 patients with metastatic melanoma using epitopes from the NY-ESO-1 antigen. J. Immunother. 2004;27:472–7. doi: 10.1097/00002371-200411000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jäger E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jäger D, Arand M, Wada H, Noguchi Y, Stockert E, Old LJ, Knuth A. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J. Exp. Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song S, Wang F, He X, He Y, Li D, Sun H. Evaluation of antitumor immunity efficacy of epitope-based vaccine with B16 cell line coexpressing HLA-A2/H-2kb and CTL multiepitope in HLA transgenic mice. Vaccine. 2007;25:4853–4860. doi: 10.1016/j.vaccine.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Voskens CJ, Sewell D, Hertzano R, DeSanto J, Rollins S, Lee M, Taylor R, Wolf J, Suntharalingam M, Gastman B, Papadimitriou JC, Lu C, Tan M, Morales R, Cullen K, Celis E, Mann D, Strome SE. Induction of mage-A3 and HPV-16 immunity by Trojan vaccines in patients with head and neck carcinoma. Head Neck. 2012;34:1734–1746. doi: 10.1002/hed.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunda V, Frederick DT, Bernasconi MJ, Wargo JA, Parangi S. A potential role for immunotherapy in thyroid cancer by enhancing NY-ESO-1 cancer antigen expression. Thyroid. 2014;24:1241–1250. doi: 10.1089/thy.2013.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapoport AP, Aqui NA, Stadtmauer EA, et al. Combination immunotherapy after asct for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells. Clin. Cancer Res. 2014;20:1355–1365. doi: 10.1158/1078-0432.CCR-13-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klar AS, Gopinadh J, Kleber S, Wadle A. Treatment with 5-Aza-2'-Deoxycytidine Induces Expression of NY-ESO-1 and Facilitates Cytotoxic T Lymphocyte-Mediated Tumor Cell Killing. PLoS One. 2015;1:1–15. doi: 10.1371/journal.pone.0139221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kayser S, Watermann I, Rentzsch C, Weinschenk T, Wallwiener D, Gückel B. Tumor-associated antigen profiling in breast and ovarian cancer : mRNA, protein or T cell recognition ? J. Cancer Res. Clin. Oncol. 2003;129:397–409. doi: 10.1007/s00432-003-0445-7. [DOI] [PubMed] [Google Scholar]
- 41.Kochenderfer JN, Chien CD, Simpson JL, Gress RE. Maximizing CD8+ T cell responses elicited by peptide vaccines containing CpG oligodeoxynucleotides. Clin. Immunol. 2007;124:119–130. doi: 10.1016/j.clim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davila E, Kennedy R, Celis E. Generation of Antitumor Immunity by Cytotoxic T Lymphocyte Epitope Peptide. Cancer Res. 2003;63:3281–3288. [PubMed] [Google Scholar]
- 43.Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J, Kluger H, Tejwani S, Green J, Ramakrishna V, Crocker A, Vitale L, Yellin M, Davis T, Keler T. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. 2014;6:1–11. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maraskovsky E, Sjölander S, Drane DP, Schnurr M, Thuy, Le TT, Mateo L, Luft T, Masterman KN, Tai T-Y, Chen Q, Green S, Sjölander A, Pearse MJ, Lemonnier FA, Chen W, Cebon J, Suhrbier A. NY-ESO-1 protein formulated in ISCOMATRIX adjuvant is a potent anticancer vaccine inducing both humoral and CD8+ T-cell-mediated immunity and protection against NY-ESO-1+ tumors. Clin Cancer Res. 2004;10:2879–2890. doi: 10.1158/1078-0432.ccr-03-0245. [DOI] [PubMed] [Google Scholar]
- 45.Palmowski MJ, Lopes L, Ikeda Y, Salio M, Cerundolo V, Collins MK. Intravenous Injection of a Lentiviral Vector Encoding NY-ESO-1 Induces an Effective CTL Response. J. Immunol. 2004;172:1582–1587. doi: 10.4049/jimmunol.172.3.1582. [DOI] [PubMed] [Google Scholar]
- 46.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4 + CD25 + regulatory T cells. Nature Immunology. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 47.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;2:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 49.Pollack SM, Jones RL, Farrar EA, Lai IP, Lee SM, Cao J, Pillarisetty VG, Hoch BL, Gullett A, Bleakley M, Conrad EU, Eary JF, Shibuya KC, Warren EH, Carstens JN, Heimfeld S, Riddell SR, Yee C. Tetramer guided cell sorter assisted production of clinical grade autologous NY-ESO-1 specific CD8+ T cells. J.Immunother. Cancer. 2014;2:1–10. doi: 10.1186/s40425-014-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wargo JA, Robbins PF, Li Y, Zhao Y, El-Gamil M, Caragacianu D, Zheng Z, Hong JA, Downey S, Schrump DS, Rosenberg SA, Morgan RA. Recognition of NY-ESO-1+ tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol Immunother. 2009;58:383–394. doi: 10.1007/s00262-008-0562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Domingo GJ, Caivano A, Sartorius R, Barba P, Bäckströma M, Piatier-Tonneau D, Guardiola J, Berardinis P, Perham RN. Induction of specific T-helper and cytolytic responses to epitopes displayed on a virus-like protein scaffold derived from the pyruvate dehydrogenase multienzyme complex. Vaccine. 2003;21:1502–1509. doi: 10.1016/s0264-410x(02)00664-3. [DOI] [PubMed] [Google Scholar]
- 52.Chinnasamy N, Wargo J, Yu Z, Rao M, Frankel T, Riley J, Hong J, Parkhurst M, Feldman S, Schrump D, Restifo N, Robbins P, Rosenberg S, Morgan R. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J. Immunol. 2011;186:685–96. doi: 10.4049/jimmunol.1001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa F, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE-A3– directed T cells. Sci. Transl. Med. 2013;5:1–11. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sartorius R, Pisu P, D’Apice L, Pizzella L, Romano C, Cortese G, Giorgini A, Santoni A, Velotti F, De Berardinis P. The use of filamentous bacteriophage fd to deliver MAGE-A10 or MAGE-A3 HLA-A2-restricted peptides and to induce strong antitumor CTL responses. J. Immunol. 2008;180:3719–3728. doi: 10.4049/jimmunol.180.6.3719. [DOI] [PubMed] [Google Scholar]
- 55.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 56.Fay JW, Palucka AK, Johnston DA, Burkeholder S. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34+ progenitor-derived dendritic cells. Cancer Immunol. 2006;10:1209–1218. doi: 10.1007/s00262-005-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich P, Mendrzy R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 58.Adodni L, Nagy ZA. Peptide competition for antigen presentation. Immunology Today. 1990;11:21–24. doi: 10.1016/0167-5699(90)90006-u. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg SA, Sherry RM, Morton KE, Yang JC, Topalian SL, Royal RE, Kammula US, Restifo NP, Hughes MS, Schwarz SL, Ngo LT, Mavroukakis SA, White DE. Altered CD8(+) T-cell responses when immunizing with multiepitope peptide vaccines. J Immunother. 2006;29:224–231. doi: 10.1097/01.cji.0000190399.98802.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCormick AA, Corbo TA, Wykoff-Clary S, Palmer KE, Pogue GP. Chemical conjugate TMV - Peptide bivalent fusion vaccines improve cellular immunity and tumor protection. Bioconjug. Chem. 2006;17:1330–1338. doi: 10.1021/bc060124m. [DOI] [PubMed] [Google Scholar]
- 61.Tan S, Sasada T, Bershteyn A, Yang K, Ioji T, Zhang Z. Combinational delivery of lipid-enveloped polymeric nanoparticles carrying different peptides for anti-tumor immunotherapy. Nanomedicine. 2014;9:635–647. doi: 10.2217/nnm.13.67. [DOI] [PubMed] [Google Scholar]
- 62.Slingluff CL. The Present and Future of Peptide Vaccines for Cancer: Single or Multiple, Long or Short, Alone or in Combination? Cancer J. 2012;17:343–350. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noguchi M, Moriya F, Koga N, Matsueda S. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low dose cyclophosphamide in patients with metastatic castration resistant prostate cancer. Cancer Immunol. Immunother. 2016;65:151–160. doi: 10.1007/s00262-015-1781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.