SUMMARY
Aging brains undergo cognitive decline, associated with decreased neuronal synapse number and function and altered metabolism. Astrocytes regulate neuronal synapse formation and function in development and adulthood, but whether these properties change during aging, contributing to neuronal dysfunction, is unknown. We addressed this by generating aged and adult astrocyte transcriptomes from multiple mouse brain regions. These data provide a comprehensive RNA-seq database of adult and aged astrocyte gene expression, available online as a resource. We identify astrocyte genes altered by aging across brain regions and regionally unique aging changes. Aging astrocytes show minimal alteration of homeostatic and neurotransmission-regulating genes. However, aging astrocytes upregulate genes that eliminate synapses and partially resemble reactive astrocytes. We further identified heterogeneous expression of synapse-regulating genes between astrocytes from different cortical regions. We find that alterations to astrocytes in aging create an environment permissive to synapse elimination and neuronal damage, potentially contributing to aging-associated cognitive decline.
In Brief
The aging brain has reduced synapse number and decreased neuronal activity, functions regulated by neighboring astrocytes. Boisvert et al. investigated if aging astrocytes are contributing to these changes and found that aged astrocytes show increased expression of genes for inflammatory and synapse elimination pathways and decreased cholesterol synthesis enzymes.
INTRODUCTION
In the healthy aging brain, there is a decline in neuronal activity, a loss of synaptic connections, and a decrease in synaptic function (Wang et al., 2011). The magnitudes of these effects vary by brain region (Samson and Barnes, 2013); however, the mechanisms underlying these changes in neuronal and synaptic function are unclear. In the developing and adult brain, non-neuronal glial cells, in particular astrocytes, carry out important functions that regulate synapse number and synaptic communication and support neuronal health and homeostasis (Khakh and Sofroniew, 2015). Whether these astrocytic functions change in the aging brain is not known. Here we explore the hypothesis that decreased neuronal and synaptic function in the aging brain are due to altered properties of astrocytes that negatively affect neurons.
In the forebrain, neuron and astrocyte cell numbers do not significantly change with age, demonstrating that changes in performance are due to altered properties of these cells rather than cell death (Pelvig et al., 2008; Samson and Barnes, 2013). Despite lack of significant cell death, both cortical volume and overall neuronal activity decrease with age, associated with atrophy of neuronal dendrites and an accompanying reduction in dendritic spine number (Pannese, 2011), also evident as a decline in neuronal synapse number. There is a progressive loss of cerebellar Purkinje neurons with age as well as an increase in cerebellar behavioral deficits (Woodruff-Pak et al., 2010). Similarly, extensive stem cell loss is seen in the hypothalamus with age (Zhang et al., 2017), showing that regional differences in aging are present in the brain.
In the developing brain, astrocytes regulate synapse number by secreting synaptogenic signals, such as thrombospondins, hevin, and glypicans, that induce synapse formation (Allen et al., 2012; Christopherson et al., 2005; Kucukdereli et al., 2011) and by eliminating excess synapses by phagocytosis using the receptors Mertk and Megf10 and indirectly by inducing expression of complement cascade components in neurons (Bialas and Stevens, 2013; Chung et al., 2013; Stevens et al., 2007). In the adult brain, astrocytes maintain neuronal and synaptic function, in part through thousands of fine processes that ensheath neuronal synapses (Khakh and Sofroniew, 2015). Functions include recycling neurotransmitters and buffering extracellular potassium to facilitate neuronal transmission (Allen, 2014) and taking up nutrients, including lipids and sugars from the blood, processing them, and delivering them to neurons for energy (van Deijk et al., 2017). Therefore, astrocytes play essential roles in regulating synaptic connectivity and function during development and in the adult.
To find out whether astrocyte function is altered in the aging brain and contributes to decreased neuronal and synaptic function, we took a transcriptomic approach. We isolated mRNA from astrocytes from multiple regions of the adult (4 months) and aging (2 years) mouse brain, allowing us to determine whether astrocyte gene expression changes with age are global or show regional specificity, which is particularly important because heterogeneity exists between astrocytes from different brain areas (Chai et al., 2017). To specifically isolate astrocyte mRNA, we used the ribotag technique to genetically tag ribosomes in astrocytes (Sanz et al., 2009), restricting analysis to astrocytes and enabling purification of astrocyte-associated mRNA throughout the entire cell, including in the fine processes. We used RNA sequencing to identify and quantify astrocyte mRNA, providing unbiased identification of gene expression changes. Prior work has inferred astrocyte changes in aging from whole-brain data (Cribbs et al., 2012; Soreq et al., 2017) or used less physiological isolation methods, such as culturing astrocytes (Bellaver et al., 2017; Jiang and Cadenas, 2014), which alters gene expression, or fluorescence-activated cell sorting (FACS) (Orre et al., 2014), which allows only mRNA from the cell body to be isolated, or used a microarray to identify genes (Orre et al., 2014).
This approach has generated a comprehensive dataset of astrocyte gene expression from multiple regions of the adult and aging brain. We have made this dataset freely available as a resource by generation of a website, allowing anyone to investigate how astrocytes change with age. We used these data to determine key changes to astrocytes in the aging brain and to find out whether these changes could negatively affect synaptic and neuronal function. We further interrogated astrocyte differences between cortical regions as well as between astrocytes from 3 distinct brain regions, identifying differential expression of synapse-regulating genes.
RESULTS
Generation of an Astrocyte-Ribotag Mouse Model for Purification of Astrocyte-Enriched mRNA from the Adult and Aging Brain
To isolate astrocyte-enriched mRNA, we employed a genetic strategy to affinity-tag ribosomal subunits in astrocytes, referred to as astrocyte-ribotag. We crossed floxed-Rpl22-HA mice (a cre-dependent hemagglutinin [HA] tag on ribosomal subunit 22) (Sanz et al., 2009) to a Gfap-cre mouse line (expression of cre recombinase under the Gfap promoter to restrict expression to astrocytes) (Garcia et al., 2004). In cells expressing cre recombinase, an HA-tagged ribosomal subunit is produced, which allows for affinity purification of HA-tagged ribosomes and associated mRNA using an antibody against HA (Figure 1H; Chai et al., 2017; Sanz et al., 2009). Immunostaining against the HA tag demonstrated widespread expression of HA-tagged ribosomes in most brain regions at 4 months, including the cortex, cerebellum, and subcortical areas (Figure 1A). Based on this, we focused our studies on astrocyte aging in the visual cortex (VC), motor cortex (MC), cerebellum (CB) and hypothalamus (HTH) (Figures 1A and 1B).
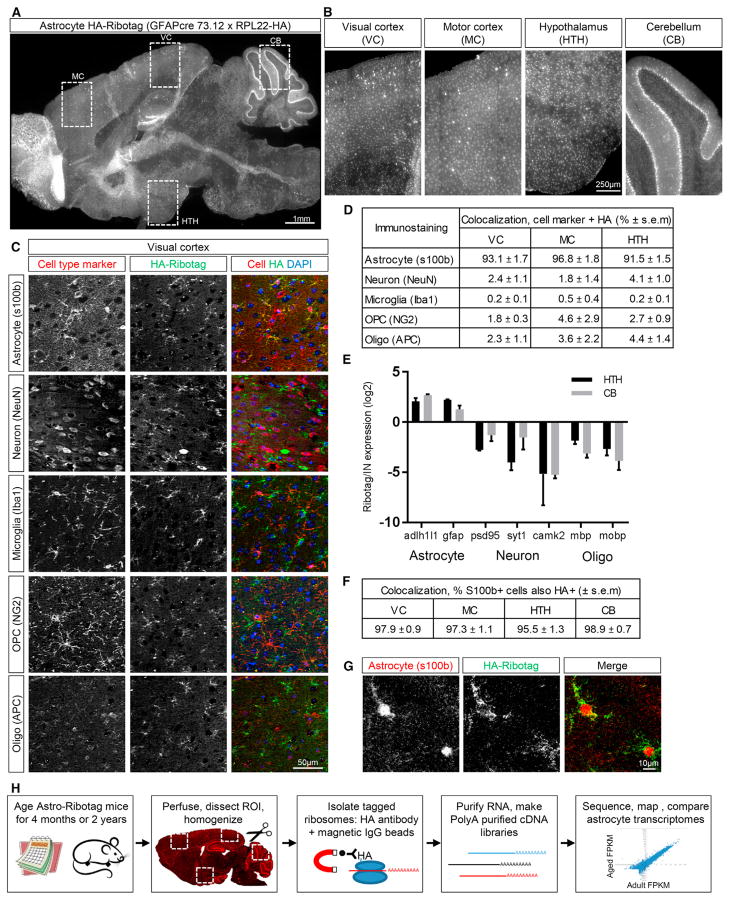
Figure 1. Characterization of the Astrocyte-Ribotag Mouse Model.
(A) Sagittal brain section from 4-month-old astrocyte-ribotag mouse immunostained for HA to visualize ribosomes; image is a mosaic of tile images acquired at 10x.
(B) Zoomed-in images from (A), regions of HA-tagged ribosome purification (VC, MC, HTH, CB); images are cropped from the mosaic tile image.
(C) Immunostaining for HA and cell-specific markers in VC to determine cell-type expression of HA-tagged ribosomes. Images cropped from a larger mosaic of tiles acquired at 20x.
(D) Quantification of cell-specific staining shows majority of HA-tagged ribosomes are in astrocytes. n = 3 × 4 month mice/brain region.
(E) qRT-PCR from HTH and CB astrocyte-ribotag-isolated mRNA shows enrichment for astrocyte mRNAs over other cell types, compared to total (input) mRNA. n = 3 × 2 year mice/brain region; graph mean ± SEM.
(F) Quantification of S100b positive cells that are also HA positive; n = 3 × 4 month mice/brain region.
(G) Immunostaining for HA and S100b shows ribosomes present throughout astrocyte processes in VC.
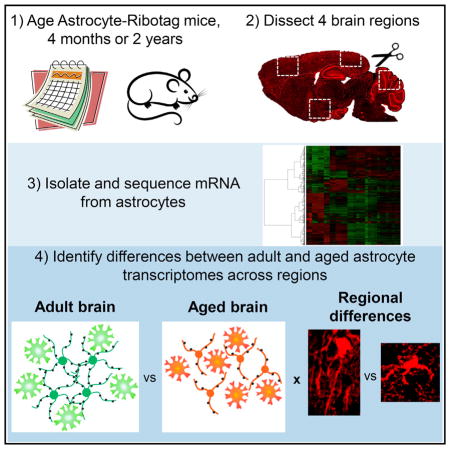
(H) Schematic of the experimental paradigm—purification of astrocyte-enriched mRNA from multiple brain regions of adult (4-month-old) and aging (2-year-old) mice, followed by RNA-seq and analysis.
See also Figures S1 and S2.
To determine whether astrocytes are the major cell type expressing HA-tagged ribosomes, we immunostained brain sections for HA along with antibodies against cell-specific markers (astrocytes, S100b; neurons, neuronal nuclear antigen [NeuN]; oligodendrocyte precursor cells, NG2; oligodendrocytes, APC; microglia, Iba1; Figure 1C, VC; Figures S1 and S2A, other regions). We found a 92%–97% overlap between HA and the astrocyte marker S100b in the VC, MC, and HTH (Figure 1D), with the rest of the HA-positive cells being neurons (2%–4%) or of the oligodendrocyte lineage (2%–4%). In the HTH, a subset of ependymal cells lining the third ventricle expressed HA (α tanycytes, which derive from the GFAP lineage and share astrocyte properties (Goodman and Hajihosseini, 2015; Figures S2B and S2C). The high density of immunostaining in the CB made quantification challenging, so we used qRT-PCR to determine cell type enrichment. In both the CB and HTH (as a comparison), mRNA for astrocyte markers is strongly enriched in the pull-down compared with total mRNA from all cells (input), and expression of neuronal and oligodendrocyte lineage genes is decreased (Figure 1E). The majority of astrocytes in each brain region also express HA-tagged ribosomes (96%–99% of S100b cells are HA-positive; Figure 1F). We further confirmed that HA-tagged ribosomes are present throughout the astrocyte, including distal processes where local translation occurs (Figure 1G; Sakers et al., 2017).
Purification and Sequencing of Astrocyte-Enriched mRNA from Multiple Brain Regions of Adult and Aging Mice
To find out whether astrocyte gene expression is altered in the aging brain, we isolated astrocyte-enriched mRNA from multiple brain regions, as validated in Figure 1—the VC, MC, HTH, and CB. We looked at 2 time points: 4 months (adult), when developmental changes to astrocyte gene expression have ceased, and 2 years (aging), before age-dependent pathology develops. We used RNA sequencing (RNA-seq) to identify and quantify the mRNA. For each time point and brain region, we sequenced 3 astrocyte-ribotag samples from separate mice and an input sample (mRNA from all cells) to provide a reference for determining astrocyte enrichment (Figure 1H). To quantify astrocyte enrichment, we developed a list of cell-specific markers from published RNA-seq and microarray data of purified cortical cell types from the developing brain (see Table S1 for a list; Cahoy et al., 2008; Zhang et al., 2014). Comparison of astrocyte-ribotag mRNA levels with the input shows a 6-fold enrichment for astrocyte genes in all brain regions at both ages, along with a depletion of neuronal and microglial genes and decreased levels of endothelial and oligodendrocyte lineage genes (Figure 2A). We further compared the cerebellar data with cerebellar cell-type-specific microarray data (Doyle et al., 2008), finding a 3- to 5-fold enrichment for astrocyte and Bergmann glia genes and a depletion of other cell types (Figure S1C).
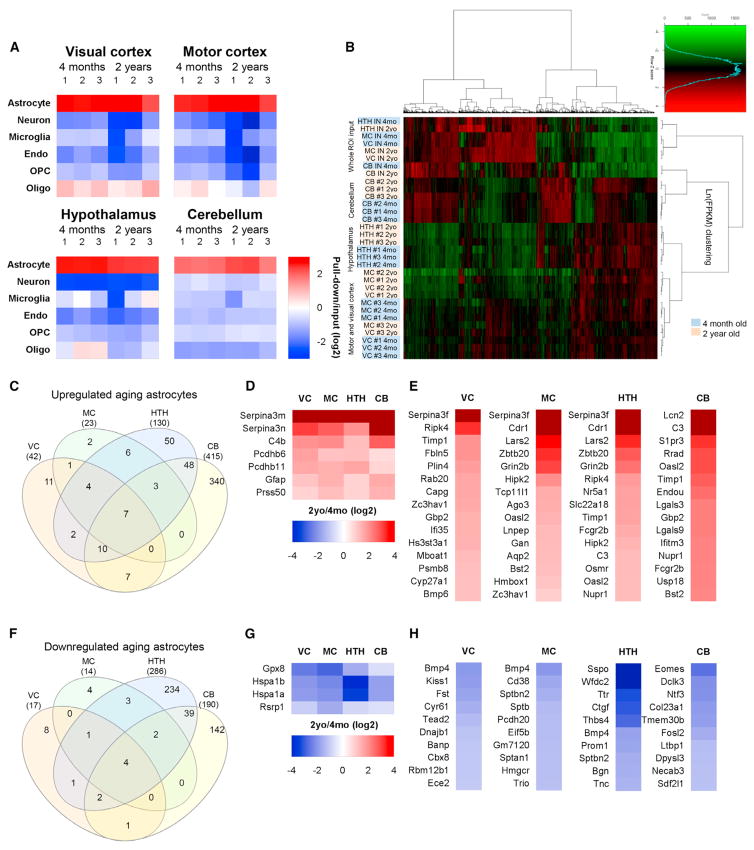
Figure 2. Purification and RNA-Seq of Astrocyte-Enriched mRNA Identifies Differentially Expressed Genes between Adult and Aging Astrocytes.
(A) Analysis of RNA-seq data for cell-specific mRNA demonstrates enrichment for astrocytes over other cell types in the pull-down (astrocyte mRNA) compared with input (total mRNA) (see Table S1 for the gene list).
(B) Clustering analysis of the top 4,401 expressed genes shows that astrocyte-ribotag pull-down samples cluster away from input mRNA; samples cluster by region of isolation and, within region, by age. n = 3 mice for each brain region and age (astrocyte samples). See Table S2 for FPKM for all genes.
(C) Venn diagram showing overlap of genes upregulated in astrocytes from 4 different brain regions between 4 months and 2 years.
(D) Heatmap of genes that are significantly upregulated in astrocytes by aging in all regions.
(E) Heatmaps of top 15 genes upregulated in astrocytes in each brain region by aging (excluding those in D; see Tables S3 and S4 for a full list).
(F) Venn diagram of genes downregulated in astrocytes from 4 different brain regions between 4 months and 2 years.
(G) Heatmap of genes that are significantly downregulated in astrocytes by aging in all regions.
(H) Heatmaps of the top 10 genes downregulated in astrocytes in each brain region by aging (excluding those in G; see Tables S3 and S4 for a full list). All heatmaps presented as fold change 2 years/4 months (log2). See also Figure S1 and Tables S1, S2, S3, and S4.
Having validated that astrocyte-ribotag samples are enriched for astrocyte mRNA, we performed a clustering analysis on all samples using the top expressed genes. This showed that biological replicates from the same brain region group together and that input samples (all cells) segregate away from astrocytes. Astrocytes from each cortical region are more similar to each other than astrocytes from non-cortical regions, and, within a brain region, the majority of samples cluster by age at isolation (Figure 2B). This shows that astrocytes from different brain regions have distinct gene expression profiles and that these profiles change with age. This now provides a dataset we can use to find out how astrocyte gene expression changes in the aging brain (Table S2), available online as a resource at http://igc1.salk.edu:3838/astrocyte_aging_transcriptome/.
Identification of Gene Expression Changes Between Adult and Aging Astrocytes from Multiple Brain Regions
We first determined the number of genes that are significantly upregulated or downregulated (p < 0.05 adjusted for multiple comparisons) in astrocytes by aging in each individual brain region. We found the least changes in cortical astrocytes (< 100 genes) and the most in the HTH and cerebellum (> 500) (Table S3). We then filtered for genes that are significantly altered (adjusted p < 0.05), expressed at an appreciable level (fragments per kilobase of transcript per million mapped reads [FPKM] > 1) and are astrocyte-expressed (pull-down/input > 0.75) (Experimental Procedures). This identified 42 upregulated genes in the VC, 23 in the MC, 130 in the HTH, and 415 in the CB (Figure 2C; Table S4; top 15 upregulated genes/region, Figure 2E). To identify core aging-upregulated genes, we looked for overlap in gene expression changes between all brain regions and identified 7 genes (Figure 2D), including markers of reactive as-trocytes, Gfap and Serpina3n (Zamanian et al., 2012), and a component of the complement cascade involved in synapse elimination, C4b (Sekar et al., 2016). β-Protocadherins are upregulated in all astrocytes by aging, and although little is known about their function, the related γ-protocadherins have important roles in developmental synapse formation (Peek et al., 2017). To determine whether any of the upregulated genes are region-specific, we generated lists of genes that are uniquely upregulated in astrocytes in one brain region or showed overlap with 1 or 2 other regions (Table S4). This showed a specific increase in Bmp6 in VC astrocytes with age, along with the negative synaptic regulator Sparc (see below). The CB has unique pro-inflammatory changes with age, with caspase-1 and -12, the chemokine Cxcl5, and the key inflammasome receptors Tlr2 and 4 all going up.
We applied the same filtering criteria to identify genes that are downregulated in aging astrocytes. This identified 17 genes in the VC, 14 in the MC, 286 in the HTH, and 190 in the CB (Figure 2F; Table S4; top 10 decreased genes/region, Figure 2H). There are 4 core genes significantly downregulated in astrocytes in all brain regions, 2 of which are heat shock proteins (Figure 2G), protective molecular chaperones that prevent protein damage (Bobkova et al., 2015). We next generated lists of genes that are uniquely downregulated in astrocytes in one brain region or showed overlap with 1 or 2 other regions (Table S4). Bmp4 is downregulated with aging in the VC, MC, and HTH but not the CB, appearing to oppose the increase in Bmp6 observed with age in the VC. In the HTH, the extracellular matrix factor Tnc, associated with the astrocyte injury response, goes down, as do multiple amino acid transporters. These data identify core changes that are common to all astrocytes in the aging brain as well as unique region-dependent aging responses.
Validation of Aging Astrocyte Gene Expression Changes by In Situ Hybridization
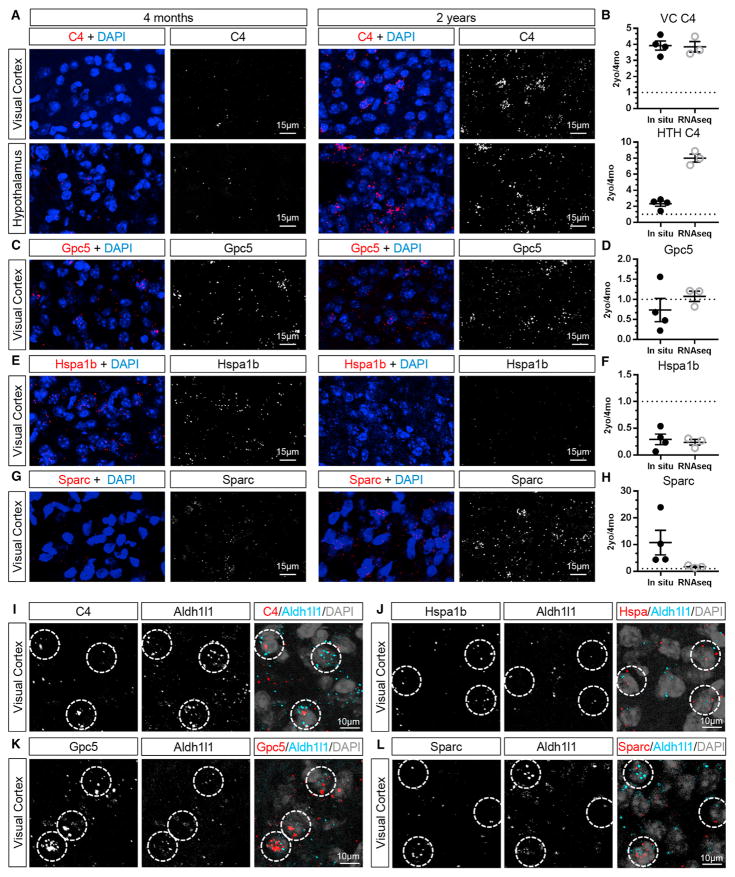
We next validated the ability of RNA-seq to reliably detect mRNA changes in aging astrocytes using RNAscope in situ hybridization in 4-month- and 2-year-old VCs of wild-type mice. We examined upregulated genes (C4 and Sparc), a downregulated gene (Hspa1b), and a gene that did not change with age (Gpc5) in the RNA-seq analysis. In the aging brain, we detected high levels of autofluorescence from lipofuscin granules, an accumulation of lipids characteristic of aging brain tissue (Gray and Woulfe, 2005). The lipofuscin colocalizes with neuronal and microglial markers in the cortex and HTH, with little found in astrocytes (Figures S3C and S3D), whereas extensive lipofuscin is seen in Bergmann glia in the CB (Figure S3D). To allow image analysis, the lipofuscin signal was removed by masking (example images in Figures S3A and S3B; Experimental Procedures). We quantified the total signal intensity of the probe and detected a similar fold change in mRNA as in the RNA-seq—an increase in C4 and Sparc with age, no change in Gpc5, and a decrease in Hspa1b (Figures 3A–3H; Figures S4A–S4D). C4 mRNA was also increased in the aging HTH, matching the RNA-seq (Figures 3A and 3B; Figure S4E). As an alternative approach, we quantified the total volume of the mRNA signal, which yielded the same fold change in mRNA level (Figure S4F). We next validated astrocyte expression of target genes by probing for the gene of interest along with the astrocyte marker Aldh1l1, showing overlap between Aldh1l1 and all of the genes of interest (C4, Sparc, Gpc5, and Hspa1b) (Figures 3I–3L). As an additional validation, we normalized the target probe signal to the Aldh1l1 signal as an internal control and again found the same trends in altered gene expression with age (Figure S4G). Taken together, this shows that astrocyte-ribotag RNA-seq reliably detects upregulated, downregulated, and unchanging gene expression in aging astrocytes.
Figure 3. Validation of Aging-Induced Changes in Astrocyte Gene Expression by In Situ Hybridization.
(A, C, E, and G) In situ hybridization to detect mRNA for candidate genes in 4-month-old (left) and 2-year-old (right) mouse brains, with lipofuscin subtracted for clarity (as in Figure S3). Shown are in situ hybridization for a gene upregulated in astrocytes by aging in the VC and HTH, C4 (A); unchanged in the VC, Gpc5 (C); downregulated in the VC, Hspa1b (E); and upregulated in the VC, Sparc (G). For whole-region images encompassing the area analyzed, see Figure S4.
(B, D, F, and H) Quantification of fold change in mRNA expression detected by in situ hybridization between 2 years and 4 months compared with fold change detected by RNA-seq, showing similar fold change (B: C4; D: Gpc5; F: Hspa1b; H: Sparc). Data are presented as a scatterplot of individual points with mean ± SEM. In situ, n = 4 4-month-old and 4 2-year-old mice; RNA-seq, n = 3 4-month-old and 3 2-year-old mice.
(I–L) Double in situ hybridization with the astrocyte marker Aldh1l1 and C4 (I), Gpc5 (K), and Sparc (L) in 2-year-old VC and Hspa1b (J) in 4-month-old VC. Circles mark astrocytes expressing the target probe and Aldh1l1.
See also Figures S3 and S4.
Aging Astrocytes Show Altered Expression of Synapse-Regulating Genes
Our hypothesis is that changes in astrocyte properties, reflected as changes in gene expression, are contributing to impaired synaptic and neuronal function in the aging brain. We therefore asked whether genes expressed by astrocytes that regulate synapses during development are altered in aging and contribute to synapse loss (Figure 4A). We examined known synapse-inducing genes, thrombospondins (Thbs) and Sparcl1 (Hevin) (Christopherson et al., 2005; Kucukdereli et al., 2011), as well as astrocytic regulators of synaptic strength, Glypican 4 and 6 (Allen et al., 2012). Glypican and hevin expression are not altered in any brain region between 4 months and 2 years, and Thbs1, 2, and 4 are not altered in the aging cortex. There is a significant decrease in Thbs4 in the aging HTH (Figure 4B), whereas, in the CB, there is a significant decrease in Thbs1 and an increase in Thbs2 with age (Figure 4B). Sparc inhibits synapse function by blocking synapse formation and decreasing synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Allen, 2014). There is a significant 2-fold increase in Sparc in the aging VC, confirmed by in situ hybridization (Figure 3G), with the same trend in the MC, suggesting that, in the aging cortex, increased production of Sparc may inhibit synaptic function and block new synapse formation (Figure 4B).
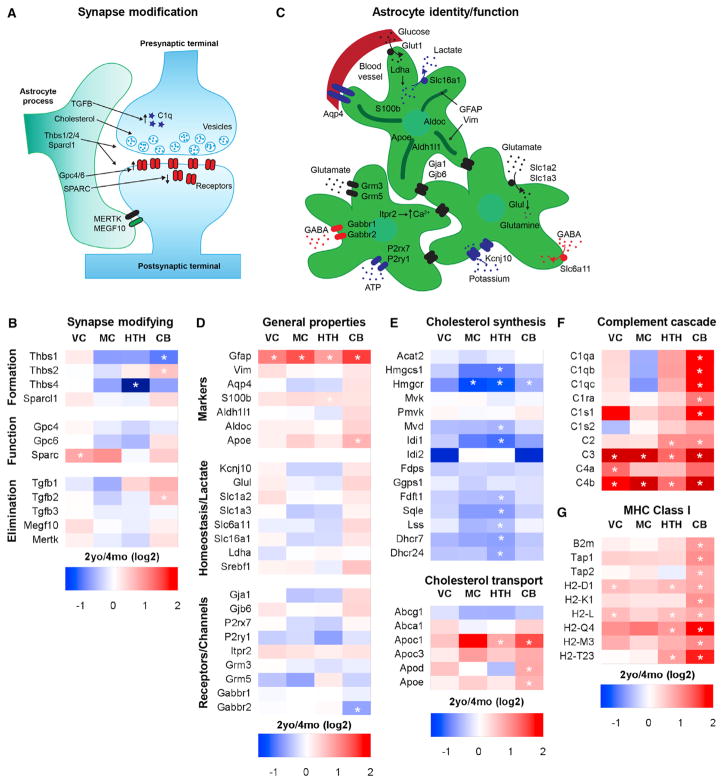
Figure 4. Astrocyte Marker and Synapse-Modifying Gene Expression in the Aging Brain.
(A and B) Schematic of astrocyte-expressed genes that regulate neuronal synapse formation, function, and elimination (A), with the corresponding heatmap (B) demonstrating region-specific increased expression of genes that inhibit synaptic function and eliminate synapses.
(C and D) Schematic of genes important for astrocyte identity and function: astrocyte markers and metabolism (top astrocyte), homeostatic functions (right astrocyte), and neurotransmitter receptors (left astrocyte) (C), with the corresponding heatmap (D) showing minimal changes in aging.
(E) Enzymes in the cholesterol synthesis pathway are downregulated in aging astrocytes, whereas cholesterol-transporting proteins are upregulated.
(F and G) Immune/antigenic response pathways implicated in synapse elimination, the complement cascade (F), and MHC (G), are upregulated in aging astrocytes. All heatmaps are presented as 2-year/4-month (log2) fold change for each brain region. *, significantly altered between 2-year and 4-month astrocytes.
See also Table S5.
Astrocytes regulate synapse elimination during development by secreting transforming growth factor β (TGF-β), which induces components of the complement cascade in neurons (C1q), and by direct phagocytosis of synapses using Mertk and Megf10 (Bialas and Stevens, 2013; Chung et al., 2013; Stevens et al., 2007). There is no change in TGF-β1–3 expression in aging astrocytes, except for a significant 2-fold increase in TGF-β2 in cerebellar astrocytes (Figure 4B). Interestingly, C3, a part of the classical complement cascade required for synapse elimination (Stevens et al., 2007), is strongly upregulated in aging astrocytes in all brain regions examined, as are other members of the complement cascade (see below) (Figure 4F). Expression of Megf10 and Mertk is not altered, suggesting that there is no increase in direct phagocytosis of synapses by aging astrocytes (Figure 4B). Taken together, these data support an active role for astrocytes in eliminating synapses in the aging brain by upregulating expression of genes known to be involved in synapse elimination, with limited alterations in the expression of genes that induce synapse formation.
Astrocyte Homeostasis Genes Are Largely Unchanged with Aging
We next asked whether genes considered markers of astrocytes are altered by aging, including the cytoskeletal markers Gfap and vimentin, the cytosolic markers S100b, Aldoc, and Aldh1l1, the lipoprotein Apoe, and the water channel Aqp4 (Figure 4C). Of these, only Gfap is significantly altered in astrocytes in all brain regions by aging, with a 2- to 3-fold increase in expression (Figure 4D). Gfap is a known marker of reactive astrocytes, and this is explored more in Figure 5 (Zamanian et al., 2012). In the adult brain, astrocytes regulate synaptic transmission and neuronal homeostasis through a number of pathways (Figure 4C; Allen, 2014). Astrocyte expression of Kir4.1 (Kcjn10), the major channel involved in potassium uptake, is unchanged by aging. The glutamate uptake transporters Glt1 and glutamate aspartate transporter (GLAST) (Slc1a2 and Slc1a3), the glutamate-metabolizing enzyme glutamine synthetase (Glul), and the γ-aminobutyric acid (GABA) transporter GAT3 (Slc6a11) are not significantly altered by aging (Figure 4D). Astrocytes express numerous channels and neurotransmitter receptors and can respond to activation of receptors with increases in intracellular calcium and release of neuromodulators (Figure 4C; Khakh and Sofroniew, 2015). Examination of genes related to these functions, including connexins, metabotropic glutamate and GABA receptors, calcium channels, and ATP receptors, revealed no significant change in expression with aging, except for a decrease in CB Gabbr2 (Figure 4D). Components of the lactate shuttle (e.g., Ldha and Mct1), which supply neurons with lactate for metabolism, are also unaltered in aging astrocytes, as is the transcription factor regulating lipid synthesis, sterol regulatory element-binding proteins (Srebf1) (Figure 4D). Therefore, the expression of genes related to major functions of astrocytes in relation to synaptic transmission and neuronal homeostasis is not altered in the aging brain, demonstrating that there is not a general loss of astrocyte support of neurons with age.
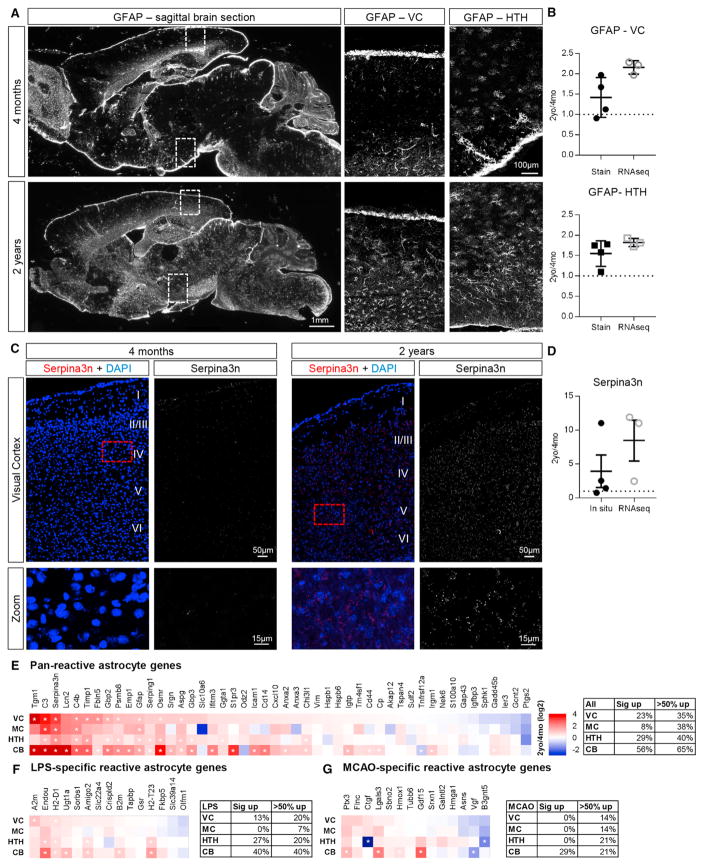
Figure 5. Aging Astrocytes Upregulate Expression of Genes that Overlap with Reactive Astrocytes.
(A) Immunohistochemistry for Gfap in 4-month (top) and 2-year (bottom) mouse brain demonstrates increased Gfap protein in multiple regions (VC and HTH) of the aging brain; overview images are mosaic of tile images acquired at 10x (left) and zoomed-in images are cropped from mosaic of tiles acquired at 20x (right).
(B) Quantification of Gfap immunostaining in the VC (top) and HTH (bottom) compared with RNA-seq.
(C) In situ hybridization for Serpina3n in 4-month (left) and 2-year (right) VC demonstrates increased Seprina3n in the aging VC. Images are mosaic of tiles acquired at 20x.
(D) Quantification of Serpina3n mRNA in the VC, comparing in situ hybridization with RNA-seq.
(B and D) Data are presented as scatterplots of individual points with mean ± SEM. Immunostaining and in situ, n = 4 4-month-old and 4 2-year-old mice; RNA-seq, n = 3 4-month-old and 3 2-year-old mice.
(E–G) Comparison of fold change in expression of reactive astrocyte genes between adult and aging astrocytes shows that overlap varies by brain region. (E) Pan-reactive astrocyte genes.
(F) LPS-specific reactive astrocyte genes.
(G) MCAO-specific reactive astrocyte genes.
Heatmaps are presented as 2-year/4-month (log2) fold change for each brain region. *, significantly altered between 2-year and 4-month astrocytes.
Pathway Analysis Identified that Aging Astrocytes Increase Expression of Immune Pathways and Decrease Cholesterol Synthesis
As an unbiased approach to identify alterations in aging astrocytes, we carried out a pathway analysis of the top changed genes in each brain region (Table S5). Fitting with the pattern of individual gene expression changes, the majority of pathways altered in aging were in the CB and HTH rather than in the cortex. A pathway downregulated in aging astrocytes in multiple brain regions is the cholesterol synthesis pathway (Figure 4E), with the major rate-limiting enzyme, Hmgcr, significantly decreased in HTH, MC, and CB astrocytes and the majority of the genes involved in cholesterol synthesis (Martin et al., 2010) decreasing in the HTH. Conversely, expression of genes responsible for cholesterol transport is increased in the CB and HTH (Figure 4E). Astrocytes are a major source of lipid synthesis in the brain and provide neurons with cholesterol, essential for presynaptic vesicle formation (Figure 4A), suggesting that a lack of cholesterol production in aging astrocytes may inhibit the release of neurotransmitter and decrease presynaptic function (Mauch et al., 2001; van Deijk et al., 2017).
Pathways upregulated in aging astrocytes include the complement system, cytokines, and major histocompatibility complex (MHC) (Table S5). MHC class I is upregulated in the CB, HTH, and VC (Figure 4G), and because this pathway presents antigen, its upregulation may be a sign of cellular stress (Cribbs et al., 2012), although, in the developing brain, MHC class I plays a role in promoting synapse elimination (Huh et al., 2000). Multiple components of the classical complement cascade are significantly upregulated in aging astrocytes across brain regions, including C3 and C4, which are upregulated in all astrocytes (Figure 4F). In development, synapse elimination is induced by complement, with C1q tagging synapses for elimination (Stevens et al., 2007) and recruiting C3, leading to phagocytosis of synapses by microglia (Schafer et al., 2012). These pathway changes show that aging astrocytes provide an environment that allows synapse elimination via upregulation of complement and MHC and decrease synaptic support by downregulation of cholesterol synthesis.
Aging Astrocyte Gene Changes Overlap with Reactive Astrocyte Gene Changes
The analysis of individual genes and pathways upregulated in aging astrocytes revealed changes associated with reactive astrocytes, including upregulation of Gfap, Serpina3n, the complement cascade, and cytokines (Sofroniew, 2015; Zamanian et al., 2012). These changes persist on the level of protein, with immunohistochemistry against Gfap showing an ~1.5 fold increase between 4-month- and 2-year-old VC and HTH, a similar upregulation to that detected by RNA-seq (Figures 5A and 5B). We additionally asked whether Serpina3n is upregulated in aging astrocytes using in situ hybridization and found a 4-fold upregulation of Serpina3n in the VC, similar to that detected by RNA-seq (Figures 5C and 5D). Increases in Gfap and Serpina3n are distributed throughout the brain, showing a general increase in astrocyte reactivity across the brain with age rather than a localized response to injury or damage.
Reactive astrocytes are characterized by altered expression of hundreds of genes, not just an upregulation of Gfap, and show varying gene expression changes depending on the type and severity of the insult (Sofroniew, 2015; Zamanian et al., 2012). We therefore compared changes in aging astrocytes with those induced by different injury paradigms: peripheral lipopoly-saccharide (LPS) to induce neuroinflammation (purportedly damaging reactivity) and middle cerebral artery occlusion (MCAO) to induce a stroke (purportedly protective reactivity) (datasets from Zamanian et al., 2012). We first looked at pan-reactive astrocyte genes (upregulated in response to both LPS and MCAO) and found that between 8% and 56% of genes are significantly upregulated in aging astrocytes, with the MC showing the least changes and the CB the most (Figure 5E). We then investigated changes unique to each paradigm and again identified overlap between the genes increased in aging and the modality-specific reactive astrocyte genes, with most changes in the CB and the least in cortical astrocytes (Figures 5F and 5G). Our data show that aging astrocytes have gene expression changes that partially overlap with reactive astrocyte changes, reflecting a mild to moderate reactive state.
Adult Cortical Astrocytes Show Region-Dependent Gene Expression
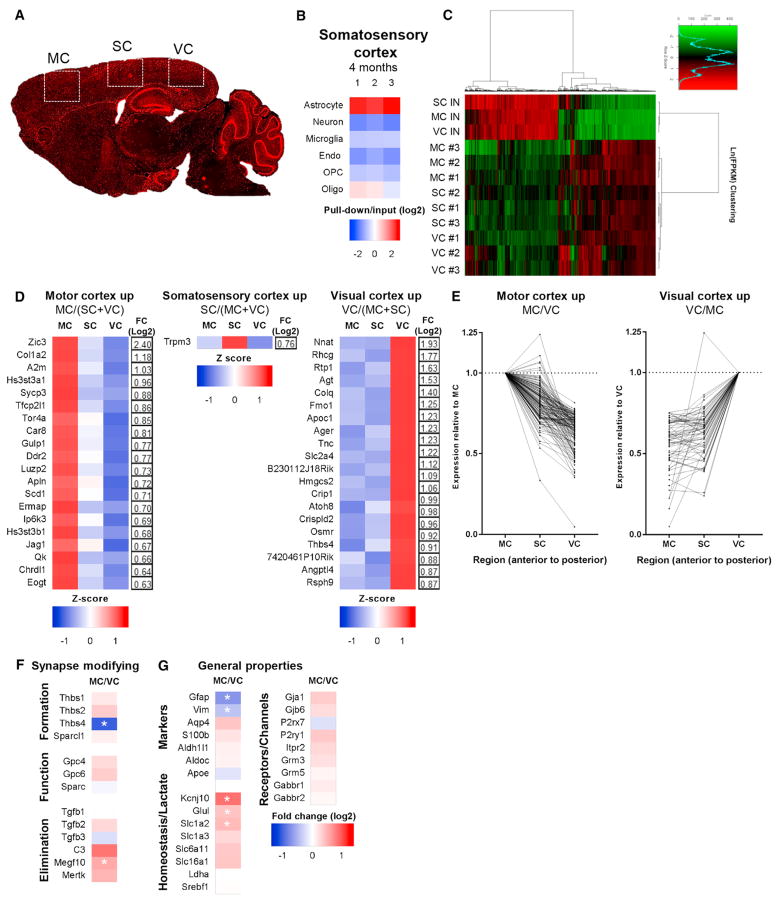
In addition to identifying alterations in gene expression in aging astrocytes, our dataset allows us to determine how astrocytes differ between regions of the adult brain. We concentrated on differences within cortical regions (Figure 6) and between the cortex (CTX) and other brain regions (Figure 7). We added a 4-month somatosensory cortex (SC) condition to enable comparison of 3 distinct cortical areas (Figure 6A); SC samples exhibit 6-fold astrocyte enrichment over input and depletion of other cell types (Figure 6B; Table S1). Clustering analysis using the top expressed genes demonstrated that each cortical region clusters separately from other cortical regions, indicating region-specific astrocyte gene expression (Figure 6C). We determined the number of genes that significantly change between astrocytes in different cortical regions (Table S6), and, to identify astrocyte-specific genes, we filtered for genes that are significantly altered (adjusted p < 0.05), expressed at an appreciable level (FPKM > 1), and astrocyte-specific (pull-down/input > 3) (Experimental Procedures; Table S7). MC and VC astrocytes have the most differences in gene expression compared to all other cortical astrocytes, with 102 and 112 genes upregulated, respectively (Figure 6D; Table S7). SC astrocytes have the least region-specific genes, with an intermediate gene expression profile reflective of their anatomical position between the VC and the MC (Figure 6C). We further generated a list of genes with differential expression comparing the VC to the MC (Table S7) and found that SC astrocyte gene expression is between that of the MC and VC for 93% of the 227 significantly changed astrocyte-enriched genes between the MC and VC (Figure 6E), demonstrating an anterior-to-posterior gene expression gradient in cortical astrocytes for these genes.
Figure 6. Adult Cortical Astrocytes Show Region-Dependent Gene Expression.
(A) Schematic of cortical regions examined at 4 months. MC and VC, same as before; somatosensory cortex (SC), new.
(B) Analysis of RNA-seq data for cell-specific mRNA demonstrates enrichment for SC astrocytes over other cell types in the pull-down (astrocyte mRNA) compared with input (total mRNA) (see Table S1 for the gene list).
(C) Clustering analysis of the top 3,487 expressed genes shows that astrocyte-ribotag pull-down samples cluster by cortical region. n = 3 4-month-old mice per region.
(D) Heatmaps and fold change of the top 20 genes significantly upregulated in astrocytes in individual cortical regions compared with all other cortical regions (MC, SC, and VC), presented as row Z score.
(E) Genes significantly differentially expressed between the MC versus VC show a gradient of gene expression, normalized to the MC (left) or VC (right). One gene (Cdr1) exceeds the axis range and is excluded for clarity (see Table S7 for the full list).
(F and G) Heatmap of synapse-modifying (F) and general astrocyte property (G) gene expression changes between the MC and VC, expressed as log2 (fold change). *, significantly altered between regions.
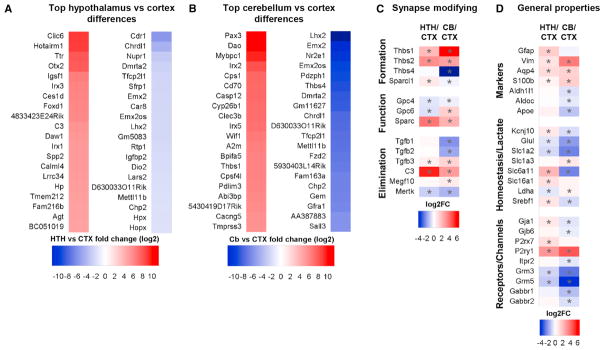
Figure 7. Regional Differences between Adult Astrocytes.
(A and B) Heatmaps of the top 20 regional astrocyte-enriched genes between adult regions, presented as log2 fold change of HTH (A) or CB (B) FPKM/combined CTX FPKM (see Table S7 for the full list).
(C and D) Comparison of expression of genes that are important for astrocyte regulation of synapses (C) and for astrocyte functions (D), between the HTH and CTX, and CB and CTX, presented as log2 fold change of HTH or CB/CTX. *, significantly altered between regions.
We then asked whether cortical astrocytes from distinct regions have differential expression of synapse-modifying genes (Figures 4A and 4C). Because of the expression gradient, we compared expression between MC and VC astrocytes (Table S7; Figures 6F and 6G). VC astrocytes show significantly increased expression of the cytoskeletal protein Gfap, the synapse inducer Thbs4, and a synapse-eliminating complement cascade component, C4b. MC astrocytes express significantly higher levels of the glutamate uptake transporter Slc1a2 (Glt1), the potassium channel Kcnj10 (Kir4.1), and the monoamine oxidase Maoa, all of which could affect ongoing synaptic transmission. Additionally, MC astrocytes have increased Gpc5, which may affect synaptic function, as well as phagocytic receptor Megf10, involved in synapse elimination. Therefore, even within the cortex, astrocytes show differential expression of synapse-regulating genes.
Identification of Regionally Enriched Genes in Adult Astrocytes
We next focused on astrocyte-enriched gene expression differences between astrocytes from distinct brain regions. Comparing the CTX and HTH, there are 748 astrocyte-enriched genes significantly upregulated in the HTH and 158 significantly upregulated in the CTX (Table S7; Figure 7A). HTH astrocytes express higher levels of synapse-modifying genes (Figures 4A and 7C), with Sparc expression 15-fold greater, Thbs1 and 2 4-fold increased, and hevin (Sparcl1) significantly increased. Gpc4 and 6, however, show significantly lower expression in HTH astrocytes than in CTX astrocytes. HTH astrocytes have increased mRNA for complement factors involved in synapse elimination, C3 (44-fold) and C4b (4.8-fold), and a significant decrease in the phagocytic receptor Mertk. In the HTH, the astrocyte water channel Aqp4 is doubled, the astrocyte marker Gfap is increased 3-fold, and the purinoreceptors P2rx7 and P2ry4 are significantly increased (Figures 4C and 7D). The primary astrocyte GABA/glycine transporters Gat3 (Slc6a11), Slc6a9, and Gat1 (Slc6a1) are enriched in the HTH, whereas the glutamate transporter Glt1 (Slc1a2) is enriched in the CTX (Figure 7D). Pathway analysis of differentially expressed genes (Table S8) identified “metabolism of lipids and lipoproteins” as enriched in the HTH, whereas CTX astrocytes are enriched for “cholesterol biosynthesis” and “steroid metabolism,” suggesting differential lipid processing and synaptic support between the two brain regions.
We carried out the same comparison between CTX and CB astrocytes, identifying 326 astrocyte-enriched genes increased in the CB, with 656 genes upregulated in the CTX (Table S7; Figure 7B). Synapse-modifying genes also differ between the regions (Figure 7C), with Thbs4 expressed over 500-fold more in the CTX and Thbs1 and 2, Gpc6, and Sparc higher in the CB. CTX astrocytes express more Wnt genes (e.g., Lrp4 and the frizzled family members Sfrp1, Sfrp5, Fzd2, and Fzd10), which have been implicated in remodeling adult synapses and may confer neuroprotective benefits in aging (Oliva et al., 2013). The neurotransmitter transporters Glt1 (Slc1a2) and Gat3 (Slc6a11) as well as metabotropic glutamate receptors (Grm3 and 5) and GABA receptors (Gabbar1 and 2) are enriched in CTX astrocytes, whereas the glutamate transporter GLAST (Slc1a3) and the ionotropic glutamate receptors GluA1 and 4 (Gria1,4) are higher in CB astrocytes (Figure 7D). We finally asked whether these regional differences between adult astrocytes are maintained in the aging brain and confirmed that the majority are (Tables S6 and S7), showing that regional astrocyte identity is largely maintained in aging.
DISCUSSION
We performed RNA-seq on astrocytes from multiple regions of the adult and aging mouse brain to investigate whether alterations in astrocyte properties lead to the loss of neuronal synapses in aging. We found little change in the expression of synapse supportive factors, except for a decrease in cholesterol synthesis, showing that loss of astrocyte support does not lead to senescence of synapses. Instead, we found that astrocytes up-regulate the expression of inflammatory factors that can damage synapses and cause their elimination, demonstrating that astrocytes become actively damaging to synapses in the aging brain. There was variability in the number of genes that changed with age in astrocytes from different brain regions, with cortical astrocytes showing the least changes and cerebellar astrocytes the most. This demonstrates the regional dependency of astrocyte aging, with more alterations in areas that experience cell death (Woodruff-Pak et al., 2010; Zhang et al., 2017). Altogether, we provide a comprehensive and validated analysis of gene expression changes in astrocytes between the healthy adult and aging brain, which we have made available as a resource online. We have focused on changes to astrocytes that may affect synaptic function, the hypothesis we set out to test.
Our adult astrocyte gene expression datasets are in broad agreement with other microarray and RNA-seq profiles of astrocytes from the developing and adult brain (Cahoy et al., 2008; Chai et al., 2017; Doyle et al., 2008; Morel et al., 2017; Zhang et al., 2014). We now add to these resources by extending the analysis to examine aging astrocyte gene expression from multiple brain regions using astrocyte-ribotag and RNA-seq. Ribo-tag restricts analysis to ribosome-associated mRNAs, which are more likely to be actively being made into proteins, providing a representation of the translational state of the cells, a similar approach to that used by others (Chai et al., 2017; Doyle et al., 2008; Morel et al., 2017). Studies using FACS (Cahoy et al., 2008) or immunopanning (Zhang et al., 2016) to isolate astrocyte mRNA offer the ability to look at the whole transcriptome, including non-coding RNAs, but do not highlight mRNAs that are being functionally utilized and are restricted to RNAs present in the cell body because of the isolation method. A difference between ribotag and bacterial artificial chromosome (BAC) trap as a way of tagging ribosomes is that ribotag only requires transient cre recombinase expression to irreversibly express an HA tag on the endogenous ribosomal Rpl22 subunit (Sanz et al., 2009; Doyle et al., 2008), so after recombination, the production of tagged ribosomes is determined by the normal program of that cell, regardless of cre expression. We have validated that the majority of astrocytes in all brain regions examined (~98%, Figure 1) express tagged ribosomes in the adult brain, and, because adult astrogenesis and astrocyte loss are rare, the population of astrocyte-ribotag cells should remain constant between the adult and aged time points and encompass the majority of cells. This is particularly important to note because we used Gfap-cre to induce recombination, and Gfap increases in aging astrocytes. In the astrocyte-ribotag model, ~95% of tagged ribosomes are expressed by astrocytes, with the rest in non-astrocyte cells, including a subset of neurons and oligodendrocytes (Figures 1 and 2; Table S1). To focus on gene changes occurring in astrocytes, we restricted our analysis to genes that showed an enrichment in the astrocyte pull-down sample compared with the whole-brain input, which should prevent gene expression changes caused by ribosome tagging in a small subset of other cells from being detected. Finally, as with all large datasets, we encourage users to independently validate genes of interest.
The majority of genes implicated in synaptogenesis in the developing brain are unchanged between adult and aging astrocytes. It is surprising that so many of these genes, including those for hevin, thrombospondin, and glypican family members, remain expressed throughout life, long after developmental synapse formation has ceased. This points to additional roles for these factors in the adult brain, perhaps in relation to learning and memory. The decreased thrombospondin expression in the HTH with aging may contribute to alterations in synapse number and dendritic spine morphology observed with age, as thrombospondins regulate these features (Christopherson et al., 2005). A similar stability of gene expression between the adult and aging brain was found for synaptic transmission regulators, including potassium channels and neurotransmitter uptake transporters. This lack of age-dependent changes points against dysregulated astrocyte homeostasis being involved in altered synaptic transmission in aging.
We found that substantially more genes increase with age than decrease, a trend that mirrors whole-brain data (Cribbs et al., 2012). Of the consistent decreases, the most robust is in the cholesterol synthesis pathway. The brain synthesizes the majority of the cholesterol it needs in situ because circulating cholesterol is unable to readily cross the blood-brain barrier. The aging human and rodent brain have decreased cholesterol levels, but the reasons for this decrease are not known (Martin et al., 2010; Thelen et al., 2006). We identified decreased expression of cholesterol synthesis genes, with a significant decrease in the rate-limiting enzyme Hmgcr in aging astrocytes across brain regions, providing a potential mechanism for decreased cholesterol levels in the aging brain. Astrocyte-derived cholesterol is essential for neuronal dendritic elaboration and presynaptic function during development (Mauch et al., 2001), and, given that dendrites atrophy and presynaptic release decreases in the aging brain, decreased astrocyte cholesterol synthesis with age may contribute to these deficits.
A major change we detected in aging astrocytes was an upregulation of genes that have an important role in eliminating excess synapses during development, including the complement pathway and MHC class I (Huh et al., 2000; Stevens et al., 2007). Recently, these same pathways have been implicated in synapse elimination in neurological disorders; for example, C4 is upregulated in schizophrenia, and complement is involved in eliminating synapses in Alzheimer’s disease (Hong et al., 2016; Sekar et al., 2015, 2016). These pathways are among the top genes we find upregulated in astrocytes in the aging brain across regions, strongly suggesting that aging astrocytes provide an environment that is permissive for synapse elimination. Interestingly, the mechanism of synapse elimination involves complement tagging of synapses for phagocytosis by microglia, showing a potential for cross-talk between different glial cell types in aging, which may be bidirectional (Liddelow et al., 2017; Schafer et al., 2012). Microglia also show alterations in gene expression in the aging brain, and, as with astrocytes, these changes vary by brain region, with cerebellar microglia showing the most changes with age, the same result we found for astrocytes (Grabert et al., 2016). The cerebellum is the only region of the brain where age-dependent neuronal death is observed and the only region with astrocyte accumulation of lipofuscin (Figure S3), so it is interesting that this is where the most gene expression changes in glial cells occur. In the future, it will be important to investigate whether glial gene expression changes are causative, preceding Purkinje neuron death, or occur later, as a response to the change in environment caused by neuronal loss.
We find that astrocytes in the aging brain exhibit gene expression changes consistent with reactive astrocytes, albeit with changes of a lesser magnitude, showing a mild to moderate level of reactivity (Sofroniew, 2015). Previous studies have shown increased Gfap levels in the aging brain (Bellaver et al., 2017); however, Gfap is just one of hundreds of genes that can be up-regulated in reactive astrocytes, so our data allowed us to investigate how reactive aging astrocytes are. This analysis identified differential gene expression changes between normal aging and pathological astrocytes, providing a resource to distinguish between astrocytes in healthy aging and disease (Berchtold et al., 2014; Sekar et al., 2015). Interestingly the top 2 genes upregulated by aging in all regions were the reactive astrocyte genes Serpina3m and n, variations in which have been associated with cognitive function in human aging and Alzheimer’s disease vulnerability (Cluett et al., 2010). The cause of the moderate reactivity of astrocytes in the aging brain is unclear because the gene expression changes overlap with those induced by both peripheral inflammation, which induces inflammatory reactive astrocytes, and stroke, which induces purportedly protective reactive astrocytes. Given that many of the upregulated genes reflect inflammatory changes, however, it is interesting that treatment with low doses of the anti-inflammatory drug ibuprofen is protective against aging-induced cognitive deficits in rodents, raising the possibility that a contributing factor in this protection is a reduction in astrocyte reactivity (Rogers et al., 2017).
In the adult brain, we identified significant differences in gene expression between astrocytes in different cortical regions, showing that, even within the cortex, astrocytes may be specialized to regulate the functions of each cortical area. Interestingly, the majority of differentially expressed genes show a gradient of expression across the cortex. These include synapse regulators such as Thbs4, which induces synapse formation, and C4b and Megf10, which eliminate synapses, extracellular matrix factors, and enzymes that process neurotransmitters. When we compared astrocytes from distinct brain regions, many more differences in gene expression were found. Hypothalamic astrocytes express higher levels of genes that regulate synapses compared with cortical astrocytes, indicative of a continued role for astrocytes in adult synaptogenesis in the HTH (Theodosis et al., 2008). Interestingly, hypothalamic astrocytes are enriched in genes involved in lipid metabolism, whereas cortical astrocytes are enriched in genes for lipid synthesis, suggesting different lipid processing between the two areas. Further, adult cerebellar astrocytes are enriched for inflammatory caspases, which may contribute to the greater aging response observed in these cells.
Overall, we find that astrocytes in the aging brain alter their gene expression to produce an environment that will allow synapse elimination and decrease presynaptic function. It has been proposed that aging changes predispose the brain to diseases of aging such as Alzheimer’s disease and Parkinson’s disease by providing conditions that allow them to develop and progress. Our data support this hypothesis and show that changes in astrocytes could be major contributors. This suggests that targeting astrocytes is a viable approach to protect synapses in the aging brain and in aging-related diseases.
EXPERIMENTAL PROCEDURES
All mouse work was approved by the Salk Institute Institutional Animal Care and Use Committee.
Ribotag Pull-Down
Male mice heterozygous for flox-Rpl22-HA (The Jackson Laboratory, 011029) and hemizygous for Gfap-cre (The Jackson Laboratory, 012886) (astrocyte-ribotag) were used to isolated astrocyte mRNA based on a modified ribotag protocol (Sanz et al., 2009). Astrocyte-ribotag mice were aged for either 4 months or 2 years, and the VC, MC, SC, HTH, and CB were micro-dissected on ice in chilled physiological buffer using an adult brain reference atlas (www.brain-map.org) and neuroanatomical landmarks to identify regions (n = 3 mice, 4 months; 3 mice, 2 years).
RNA-Seq Library Generation and Sequencing
RNA quantity and quality were measured with a Tape Station (Agilent) and Qubit fluorimeter (Thermo Fisher Scientific); see the Supplemental Experimental Procedures for RIN integrity number (RIN) scores. 100–400 ng of RNA was used to make libraries; to avoid batch effects, library preparation and sequencing were done at the same time for all samples. mRNA was extracted with oligo-dT beads, capturing poly(A) tails, and cDNA libraries were made with the Illumina TruSeq Stranded mRNA Library Preparation Kit (RS-122-2101) by the Salk Institute Next Generation Sequencing (NGS) Core. Samples were sequenced on an Illumina HiSeq 2500 with single-end 50-bp reads at 25–60 million reads per sample.
RNA-Seq Mapping, Analysis, and Statistics
Raw sequencing data were demultiplexed and converted into FASTQ files using consensus assessment of sequence and variation (CASAVA) (v1.8.2) and quality-tested with FASTQC v0.11.2. Analysis was done in R v3.1.1 with Bio-conductor package v2.13. Alignment to the mm10 genome was performed using the spliced transcripts alignment to a reference (STAR) aligner, version 2.4.0k (Dobin et al., 2013). Mapping was carried out using default parameters (up to 10 mismatches per read and up to 9 multi-mapping locations per read), and a high ratio of uniquely mapped reads (> 75%) was confirmed, with exonic alignment inspected to ensure that reads were mapped predominantly to annotated exons. Raw gene expression was quantified across all genes (RNA-seq) using the top-expressed isoform as a proxy for gene expression, and differential gene expression was carried out using the EdgeR package, version 3.6.8, with default normalization, using replicates to compute within-group dispersion and, when cortical samples were pooled, batch correction to account for the differences between mice (Robinson et al., 2010). The standard EdgeR normalization and differential expression pipeline was used to ensure that mean-variance relationships were appropriately modeled prior to differential expression testing. Significance for differential expression was defined as adjusted p < 0.05, calculated with EdgeR using Benjamini-Hochberg’s procedure for multiple comparisons adjustment.
Characterization of Astrocyte-Ribotag
To determine the astrocyte specificity of the astrocyte-ribotag line, we performed 2 analyses: immunostaining against the ribotag epitope (HA) and cell type-specific markers (astrocytes, microglia, oligodendrocytes, neurons, and oligodendrocyte precursor cells) and qRT-PCR for cell type-specific mRNA from the ribotag pull-down compared with the input.
In Situ Hybridization
The RNAscope 2.5 HD Multiplex Fluorescent Manual Assay kit (ACDbio, 320850) was used with modifications to detect mRNA changes between 4-month- and 2-year-old mice. 4 mice (biological replicates) were analyzed per time point, with 3 brain sections/animal/probe (technical replicates) imaged on a confocal microscope, and the amount of signal was quantified in Imaris (Bitplane). See the Supplemental Experimental Procedures for further details.
Supplementary Material
Highlights.
Provide astrocyte transcriptome from multiple regions of aged and adult mouse brain
Astrocytes have a region-dependent aging response, are moderately reactive in aging
Aged astrocytes increase synapse elimination genes, decrease cholesterol synthesis
Most astrocyte-enriched genes are differentially expressed between regions
Acknowledgments
We thank Alex Kopelevich and Cari Dowling for technical support, Luisa Amaral for website design and construction, Mat LeBlanc and Ye Zheng for advice, the NIA for aged WT mice, and members of the Allen lab and MNL for discussions. This project was funded by an Ellison Medical Foundation New Scholar in Aging Award (to N.J.A.). M.M.B. received support from the Chapman Foundation. Work in the lab of N.J.A. is supported by NIH-NINDS R01 NS089791 the Hearst Foundation, the Pew Foundation, the Dana Foundation, and the Whitehall Foundation. Work in the Razavi Newman Integrative Genomics and Bioinformatics Core Facility of the Salk Institute is funded by NIH-NCI CCSG P30 014195 and the Helmsley Trust. This work was supported by the NGS Core Facility of the Salk Institute with funding from NIH-NCI CCSG P30 014195, the Chapman Foundation, and the Helmsley Charitable Trust.
Footnotes
DATA AND SOFTWARE AVAILABILITY
The accession number for the sequencing data reported in this paper is GEO: GSE99791. Analyzed reads (FPKM) are available from the GEO, http://igc1.salk.edu:3838/astrocyte_aging_transcriptome/, and Table S2.
Supplemental Information includes Supplemental Experimental Procedures, four figures, and eight tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2017.12.039.
AUTHOR CONTRIBUTIONS
M.M.B. and N.J.A. designed the experiments and wrote the manuscript with input from other authors. N.J.A. conceived the project. M.M.B. performed all experiments except as noted. N.J.A., M.M.B., G.A.E., and M.N.S. analyzed data.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Allen NJ. Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol. 2014;30:439–463. doi: 10.1146/annurev-cellbio-100913-013053. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaver B, Souza DG, Souza DO, Quincozes-Santos A. Hippocampal Astrocyte Cultures from Adult and Aged Rats Reproduce Changes in Glial Functionality Observed in the Aging Brain. Mol Neurobiol. 2017;54:2969–2985. doi: 10.1007/s12035-016-9880-8. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Sabbagh MN, Beach TG, Kim RC, Cribbs DH, Cotman CW. Brain gene expression patterns differentiate mild cognitive impairment from normal aged and Alzheimer’s disease. Neurobiol Aging. 2014;35:1961–1972. doi: 10.1016/j.neurobiolaging.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas AR, Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bobkova NV, Evgen’ev M, Garbuz DG, Kulikov AM, Morozov A, Samokhin A, Velmeshev D, Medvinskaya N, Nesterova I, Pollock A, Nudler E. Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proc Natl Acad Sci USA. 2015;112:16006–16011. doi: 10.1073/pnas.1516131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopher-son KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron. 2017;95:531–549. e9. doi: 10.1016/j.neuron.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluett C, Brayne C, Clarke R, Evans G, Matthews F, Rubinsztein DC, Huppert F, Llewellyn DJ, Rice N, Henley W, et al. Polymorphisms in LMNA and near a SERPINA gene cluster are associated with cognitive function in older people. Neurobiol Aging. 2010;31:1563–1568. doi: 10.1016/j.neurobiolaging.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ADR, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Goodman T, Hajihosseini MK. Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front Neurosci. 2015;9:387. doi: 10.3389/fnins.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DA, Woulfe J. Lipofuscin and aging: a matter of toxic waste. Sci SAGE KE. 2005;2005:re1. doi: 10.1126/sageke.2005.5.re1. [DOI] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Cadenas E. Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell. 2014;13:1059–1067. doi: 10.1111/acel.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci USA. 2011;108:E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Dotti CG, Ledesma MD. Brain cholesterol in normal and pathological aging. Biochim Biophys Acta. 2010;1801:934–944. doi: 10.1016/j.bbalip.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nägler K, Schumacher S, Göritz C, Müller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Morel L, Chiang MSR, Higashimori H, Shoneye T, Iyer LK, Yelick J, Tai A, Yang Y. Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J Neurosci. 2017;37:8706–8717. doi: 10.1523/JNEUROSCI.3956-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva CA, Vargas JY, Inestrosa NC. Wnts in adult brain: from synaptic plasticity to cognitive deficiencies. Front Cell Neurosci. 2013;7:224. doi: 10.3389/fncel.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, Klooster J, Bossers K, Hol EM. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging. 2014;35:1–14. doi: 10.1016/j.neurobiolaging.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct Funct. 2011;216:85–89. doi: 10.1007/s00429-011-0308-y. [DOI] [PubMed] [Google Scholar]
- Peek SL, Mah KM, Weiner JA. Regulation of neural circuit formation by protocadherins. Cell Mol Life Sci. 2017;74:4133–4157. doi: 10.1007/s00018-017-2572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29:1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bio-conductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Liu CC, Zhao N, Wang J, Putzke T, Yang L, Shinohara M, Fryer JD, Kanekiyo T, Bu G. Subacute ibuprofen treatment rescues the synaptic and cognitive deficits in advanced-aged mice. Neurobiol Aging. 2017;53:112–121. doi: 10.1016/j.neurobiolaging.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakers K, Lake AM, Khazanchi R, Ouwenga R, Vasek MJ, Dani A, Dougherty JD. Astrocytes locally translate transcripts in their peripheral processes. Proc Natl Acad Sci USA. 2017;114:E3830–E3838. doi: 10.1073/pnas.1617782114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RD, Barnes CA. Impact of aging brain circuits on cognition. Eur J Neurosci. 2013;37:1903–1915. doi: 10.1111/ejn.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci USA. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Ya-masaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar S, McDonald J, Cuyugan L, Aldrich J, Kurdoglu A, Adkins J, Serrano G, Beach TG, Craig DW, Valla J, et al. Alzheimer’s disease is associated with altered expression of genes involved in immune response and mitochondrial processes in astrocytes. Neurobiol Aging. 2015;36:583–591. doi: 10.1016/j.neurobiolaging.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, et al. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2015;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq L, Rose J, Soreq E, Hardy J, Trabzuni D, Cookson MR, Smith C, Ryten M, Patani R, Ule J UK Brain Expression Consortium; North American Brain Expression Consortium. Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Rep. 2017;18:557–570. doi: 10.1016/j.celrep.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Thelen KM, Falkai P, Bayer TA, Lütjohann D. Cholesterol syn- thesis rate in human hippocampus declines with aging. Neurosci Lett. 2006;403:15–19. doi: 10.1016/j.neulet.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SHR. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- van Deijk AF, Camargo N, Timmerman J, Heistek T, Brouwers JF, Mogavero F, Mansvelder HD, Smit AB, Verheijen MHG. Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia. 2017;65:670–682. doi: 10.1002/glia.23120. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AFT. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KPT, Comalli DM, Kennard JA, Agelan A, Thompson RF. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci USA. 2010;107:1624–1629. doi: 10.1073/pnas.0914207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52–57. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.