SUMMARY
The essential organization of microtubules within neurons has been described, however, less is known about how neuronal actin is arranged and the functional implications of its arrangement. Here we describe in live cells an actin-based structure in the proximal axon that selectively prevents some proteins from entering the axon, while allowing the passage of others. Concentrated patches of actin in proximal axons are present shortly after axonal specification in rat and zebrafish neurons imaged live, and mark positions where anterogradely traveling vesicles carrying dendritic proteins halt and reverse. Patches colocalize with the ARP2/3 complex, and when ARP2/3-mediated nucleation is blocked a dendritic protein mislocalizes to the axon. Patches are highly dynamic, with few persisting longer than 30 minutes. In neurons in culture and in vivo, actin appears to form a contiguous, semipermeable barrier, despite its apparently sparse distribution, preventing axonal localization of constitutively active Myosin Va, but not Myosin VI.
eTOC Blurb
Balasanyan et al. find dynamic patches of actin in proximal axons of live neurons, mature and newly differentiated, in culture and in vivo. Patches contribute to a filter that sequesters some proteins within the somatodendritic domain while allowing others to pass into the axon, leading to polarized localization of proteins.
INTRODUCTION
Evidence from numerous experiments suggests that actin filaments and myosins contribute to the localization of proteins to the somatodendritic compartment (Arnold and Gallo, 2013). Transmembrane proteins on the cell surface and large intracellular molecules are prevented from moving between the somatodendritic and axonal compartments by a mechanism that depends on intact actin filaments (Song et al., 2009; Winckler et al., 1999). Actin filaments are also necessary for the localization of dendritic transmembrane proteins and mRNAs (Balasanyan and Arnold, 2014; Lewis et al., 2009; Song et al., 2009). Interaction with Myosin Va, a plus end-directed motor, is both necessary, and sufficient for somatodendritic localization of transmembrane proteins and mRNAs (Balasanyan and Arnold, 2014; Balasanyan; Lewis et al., 2009). Furthermore, vesicles carrying dendritic proteins halt and reverse in the proximal axon, a process that is blocked by actin depolymerization and by disrupting the function of Myosin Va (Al-Bassam et al., 2012). Conversely, vesicles carrying nonspecifically localized proteins that are induced to interact with Myosin Va halt and reverse. Finally, full length Kif17 localizes to dendrites in part as a result of interaction with the actin cytoskeleton in the proximal axon, which stops its movement into the distal axon (Franker et al., 2016).
Experiments using different modalities have found evidence for actin-based structures in the proximal axon that could mediate differential trafficking of vesicles. Scanning electron microscopy showed images of actin filaments concentrated in patches in the proximal axon (Watanabe et al., 2012). Furthermore, results of functional experiments where myosin motors were inducibly attached to peroxisomes are consistent with actin filaments in the proximal axon having an overall orientation with plus ends facing the cell body (Watanabe et al., 2012). This orientation for actin filaments was corroborated by superresolution microscopy of GFP-Actin (Jensen et al., 2014)(personal communication).
Thus, there is considerable evidence for a model where vesicles carrying dendritic proteins are associated with a plus end-directed myosin motor, which then interacts with patches of actin in the proximal axon that are oriented with plus ends facing the cell body. Nonetheless, other observations have raised questions about this model: (1) Images obtained using platinum replica TEM do not show AIS-specific patches of actin, but instead find actin concentrations corresponding to protrusions throughout the axon and isolated stable actin filaments (Jones et al., 2014). (2) Polarized trafficking occurs prior to assembly of the axon initial segment, and possibly before the appearance of actin patches (Petersen et al., 2014). (3) Although superresolution images taken of live, permeabilized neurons labeled with SiR-Actin find patches in the proximal axon of many neurons, there is a significant minority where patches were not observed (D'Este et al., 2015). (4) Actin may be necessary for correct sorting of proteins into dendritic vesicles. Thus, depolymerizing actin may result in the missorting of dendritic proteins into axonal vesicles, rather than in the elimination of an actin-based barrier to vesicle movement in the proximal axon (Petersen et al., 2014).
Here we address these questions by performing live imaging of neurons in culture and in vivo expressing Utrophin, a protein that specifically labels actin filaments, without disrupting the balance between actin assembly and disassembly (Burkel et al., 2007; Galkin et al., 2002; Ganguly et al., 2015). We find that virtually all neurons examined display labeled actin in the proximal axon. Actin labeling appears as soon as the axon is specified, prior to clustering of Ankyrin G at the axon initial segment. Furthermore, concentrations of actin in the proximal axon mark loci where vesicles carrying dendritic proteins halt and reverse. Actin within the proximal axon forms a barrier to the movement of constitutively active Myosin Va, but not to constitutively active Myosin VI, both in culture and in vivo. In addition, patches are highly dynamic. Patches colocalize with ARP2/3, and disruption of ARP2/3 function leads to an increase in the amount of a dendritic protein, Transferrin Receptor (TfR), in the axon.
RESULTS
Actin patches are present in neurons in culture and in vivo
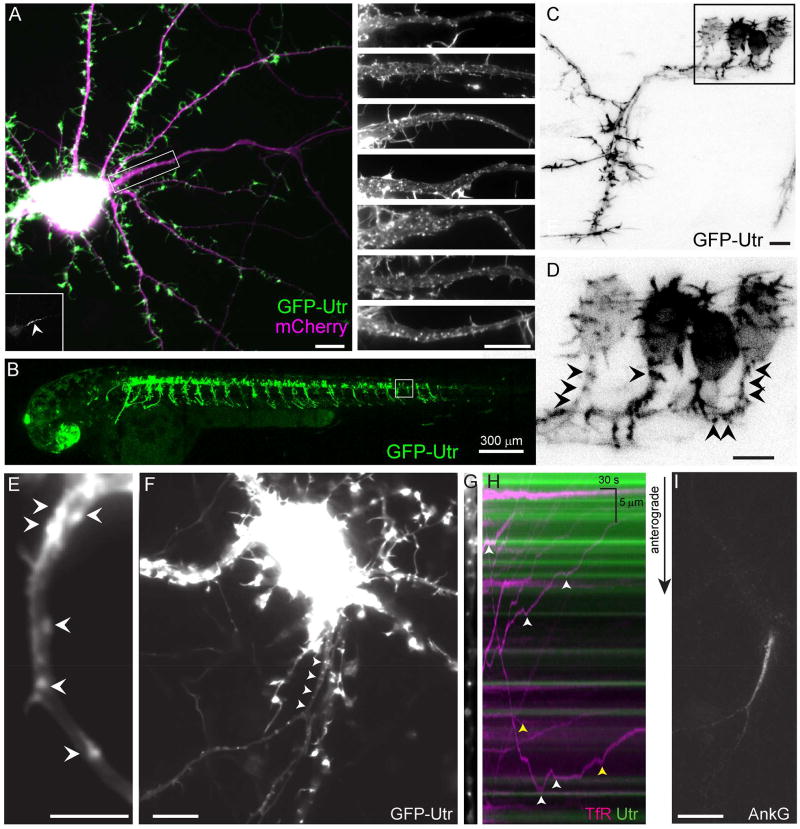
In order to label actin in neurons without fixation or permeabilization we expressed the CH domain of Utrophin (Utr), an actin binding domain that has been shown to label F-actin specifically (Burkel et al., 2007; Ganguly et al., 2015), using a transcriptional regulation system that incorporates negative feedback to ensure low, consistent expression (Figure S1). GFP-Utr labeling colocalized with phalloidin labeling, consistent with GFP-Utr labeling actin with high fidelity (Figure S1). Following expression of GFP-Utr for 3 days in 17 DIV rat cortical neurons in dissociated culture, discrete clusters (patches) were present in 100% of neurons imaged, with an average of 37 +/− 4 patches per neuron (Figure 1A) in the proximal 30 µm of the axon. To determine whether patches are visible in vivo, we expressed GFP-Utr in motor neurons of 1.5–3 days post fertilization (dpf) larval zebrafish. Virtually all motor neurons examined showed patch-like concentrations of GFP-Utr within the proximal axon (Figure 1B–D). The appearance of patches in zebrafish neurons only two days after fertilization suggests that they appear shortly after axonal specification. Similarly, in 3 DIV cortical neurons in culture we observed actin patches in all neurons examined with an average number of 8 +/− 4 (Figures 1E, S2) within the proximal 30 µm of the longest neurite. Thus, actin patches are present, both in culture and in vivo, shortly following axonal specification.
Figure 1.
Actin patches are found in the proximal axons of live neurons and mark positions where vesicles carrying dendritic proteins halt and reverse. (A) 15 DIV cortical neuron in dissociated culture expressing the actin label GFP-Utr (green) and mCherry (purple). The proximal axon is contained within the boxed area. (Inset, lower left) Ankyrin G labeling of the proximal axon (arrowhead). (Right) Upper panel shows magnified image of boxed area. Lower panels show similar areas from other cortical neurons in culture expressing GFP-Utr under similar conditions. N = 20 neurons, 3 cultures. (B) Live 2 dpf zebrafish expressing GFP-Utr in motoneurons. N = 295 cells, 4 fish. (C, D) Higher magnification of area shown in boxed region of (B, C). (D) Arrowheads point to patches of actin in the proximal axon. (E) Proximal axon of 3 DIV cortical neuron expressing GFP-Utr showing actin patches (arrowheads). N = 15 neurons, 4 cultures. (F) 14 DIV cortical neuron in culture expressing TfR-mCherry (arrowheads point to the proximal axon). (G) Straightened image of proximal axon showing GFP-Utr labeling of actin patches. (H) Kymograph of vesicles carrying TfR-mCherry (purple) and actin patches (green). White arrowheads indicate places where TfR containing vesicles halted near actin patches, yellow arrowheads indicate places where halting took place away from patches. (I) Ankyrin G labeling of the axon initial segment of neuron in (F–H). Scale bar 10 µm unless otherwise indicated. See also Figures S1, S2, Movies S1, S2.
Vesicles carrying dendritic proteins halt and reverse at actin patches
To examine the functional significance of actin patches, we observed vesicles carrying exogenous Transferrin Receptor tagged with mCherry (TfR-mCherry) in 13–16 DIV dissociated cortical neurons co-expressing GFP-Utr. We found that 30 of 38 anterogradely moving vesicles halted near sites of concentrated Utr labeling (Figure 1F–I, Movie S1), consistent with actin patches forming barriers to vesicle movement. We observed similar results in 3 DIV neurons, where 41 of 60 anterogradely moving vesicles halted near sites of concentrated actin (Figure S2, Movie S2). Thus, our results are consistent with actin in the proximal axon acting as a barrier to prevent vesicles carrying dendritic proteins from moving into the axon. Furthermore, 6 of 7 neurons examined at 3 DIV did not have appreciable Ankyrin G staining in the proximal axon, despite having actin patches, and vesicles carrying TfR that halted and reversed. This result indicates that formation of actin patches can occur independently of Ankyrin G, a critical organizer of the axon initial segment (Brechet et al., 2008) that is necessary for maintenance of neuronal polarity (Hedstrom et al., 2008; Sobotzik et al., 2009).
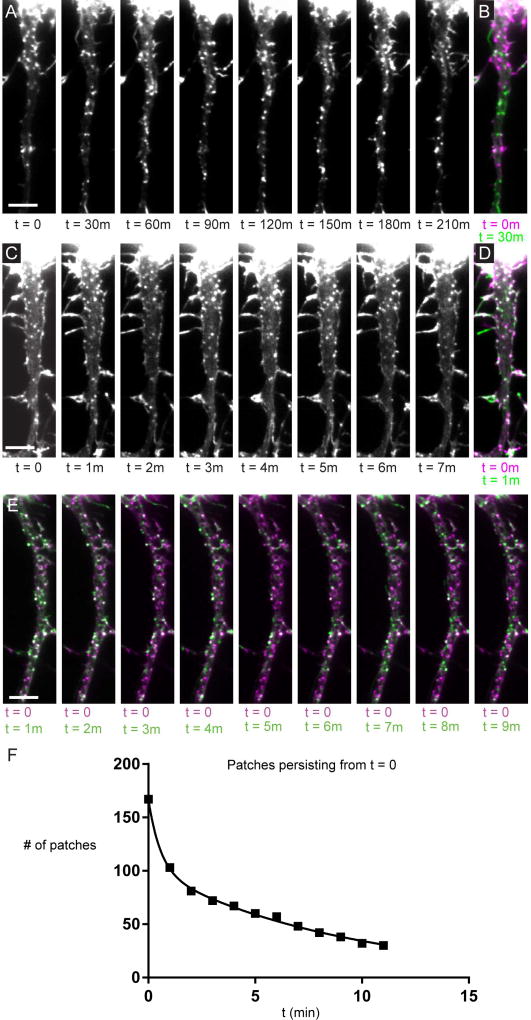
Actin patches are highly dynamic
By imaging Utrophin in live cells we found that most patches are present for less than 30 minutes (Fig. 2A, B), and some are present for less than 1 minute (Fig. 2C–E). Analysis of timelapse movies (Movies S3–S6) indicates that the rate at which patches disappear or move can be fitted with two exponentials, consistent with 39% of patches being short-lived, with an average lifetime of 39 +/− 8 seconds, and 61% being longer-lived, with an average lifetime of 560 +/− 40 seconds (Figure 2F). Furthermore, kymographs of the actin patch movies indicate that patches are stationary and tend to appear and disappear at the same locations (Fig. S3).
Figure 2.
Actin patches are dynamic. (A) A series of timelapse images of the proximal axon of a cortical neuron expressing GFP-Utr taken 30 minutes apart. (B) Merge of the image at t = 0 (purple) and t = 30 minutes (green). White puncta represent patches that have persisted, purple puncta represent patches that have disappeared or moved from that location. Green puncta represent patches that have appeared or moved to that location. (C) Similar images to those in (A) taken 1 minute apart. (D) Merge of image from (C) at t = 0 (purple) and t = 1 minute (green). (E) Merge of timelapse images of GFP-Utr at t = 0 (purple) and t = 1 to 11 minutes (green). (F) Graph of the number of patches that have persisted from t = 0 over time. Solid line corresponds to the fit of 2 exponentials, Tau 1 = 0.7 +/− 0.1 minutes, Tau 2 = 6.5 +/− 0.4 minutes, R2 = 0.9979. Roughly 40% of patches have kinetics corresponding to the shorter Tau, the rest have kinetics corresponding to the longer Tau. Results are a compilation of data from 3 neurons, 3 separate cultures. Scale bar 5 µm. See also Figure S3, Movies S3–S6.
Actin filaments within the proximal axon form a contiguous, semipermeable barrier
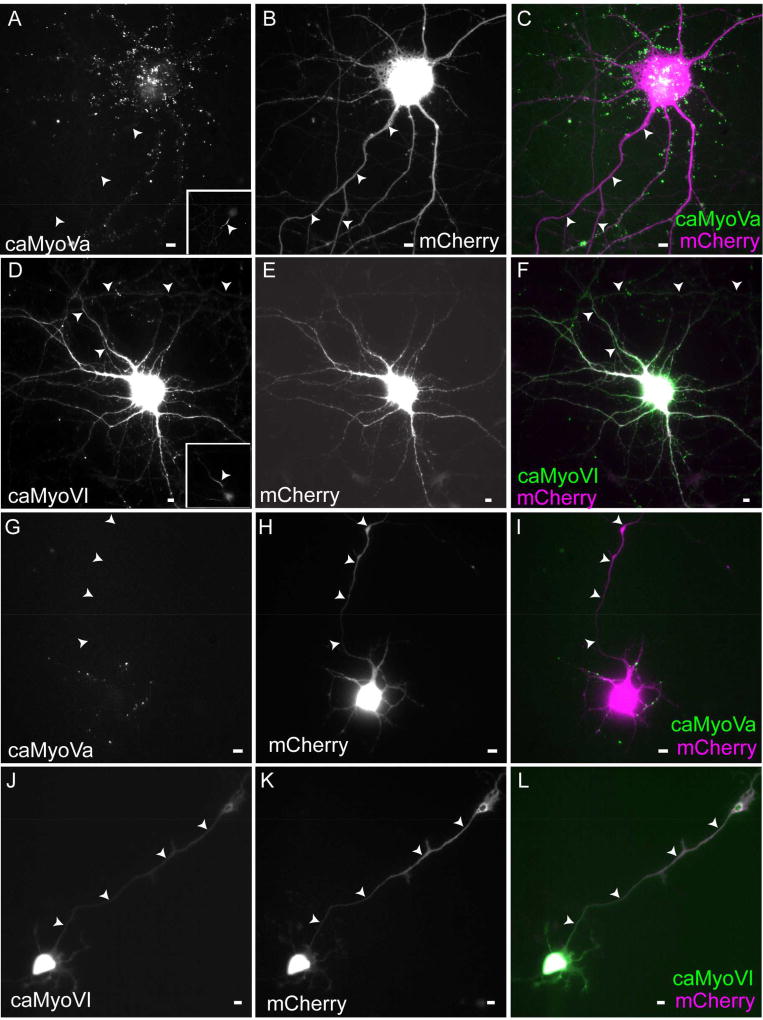
Depolymerizing actin filaments in neurons causes vesicles carrying dendritic proteins to move anterogradely to the distal axon without stopping, consistent with elimination of an actin-based vesicle filter (Al-Bassam et al., 2012). However, this change in trafficking could occur as a result of miss-sorting of dendritic protein into axonal vesicles, rather than of changes in the trafficking of dendritic vesicles (Petersen et al., 2014). To clarify the interpretation of these results, we asked whether actin forms a contiguous barrier to the movement of proteins in the proximal axon using an assay that does not involve vesicular trafficking. Accordingly, we expressed a constitutively active form of Myosin Va (caMyoVa), a plus end-directed motor (Krementsov et al., 2004) in dissociated cortical neurons. After 24 hours of expression caMyoVa localized very specifically to the soma and dendrites (ADR = 0.1 +/− 0.02, Figure 3A–C). An ADR less than one indicates somatodendritic localization whereas an ADR equal to one indicates nonspecific localization. Many neurons in this analysis exhibited clustered caMyoVA in the somatodendritic compartment. However, when diffuse labeling of caMyoVa was examined we found that caMyoVa was also localized to the somatodendritic compartment (ADR = 0.2 +/− 0.03, Fig. S4). Disruption of actin filaments using cytochalasin D causes caMyoVa to distribute to axons, as well as to the somatodendritic compartment (ADR = 0.9 +/− 0.04; Figure S4) in contrast to its somatodendritic localization in the presence of DMSO (ADR = 0.2 +/− 0.02; Figure S4), confirming that the polarized distribution of caMyoVa in wild-type cells is a result of interaction with actin filaments.
Figure 3.
Constitutively active Myosin Va, but not Myosin VI, is excluded from the axon in cultured neurons. (A–C) caMyoVa expressed in a 16 DIV cortical neuron for 24 hours is localized exclusively in the somatodendritic compartment. Inset shows Ankyrin G staining (n = 9 neurons, 2 cultures). (D–F) caMyoVI expressed in a 16 DIV cortical neuron for 24 hours is localized to both the axonal and somatodendritic compartments (n = 12 neurons, 4 cultures). (G–I) Somatodendritic localization of caMyoVa expressed in a 3 DIV cortical neuron (n = 10 neurons, 3 cultures). (J–L) Somatodendritic and axonal localization of caMyoVI expressed in a 3 DIV cortical neuron (n = 9 neurons, 3 cultures). Arrowheads point to axon. Scale bar 10 µm. See also Figure S4.
If the barrier were formed by actin filaments predominantly oriented with plus ends facing the cell body, one would expect that a myosin motor that moves to the minus end of actin filaments would be able to enter axons. Following 24 hours expression in cortical neurons, constitutively active myosin VI (caMyoVI) localized to both the axon and to the soma and dendrites (ADR = 0.9 +/− 0.03; Figure 3D–F), with an ADR value significantly different from that of caMyoVa (P < 0.001, Mann Whitney). These results are consistent with the proximal axon containing a contiguous barrier comprised of actin filaments oriented predominantly with their plus ends facing the cell body.
Selective dendritic transport occurs shortly after axonal specification (Petersen et al., 2014; Silverman et al., 2001), and thus if the actin-based filter were to play a role in dendritic transport, one would expect it to be present at a similarly early time in development (Petersen et al., 2014). To test whether the actin filter acts as a contiguous barrier to movement of proteins into the axon, we expressed caMyoVa in 3 DIV cultured neurons. We found that caMyoVa localized almost exclusively within the somatodendritic compartment, with virtually no expression in the axon (Figure 3G–I; ADR = 0.15 +/− 0.02). In contrast, caMyoVI localized to both axonal and somatodendritic compartments following expression in 3 DIV cultured neurons (ADR = 1.14 +/− 0.04; Figure 3J–L, P < 0.001, Mann Whitney).
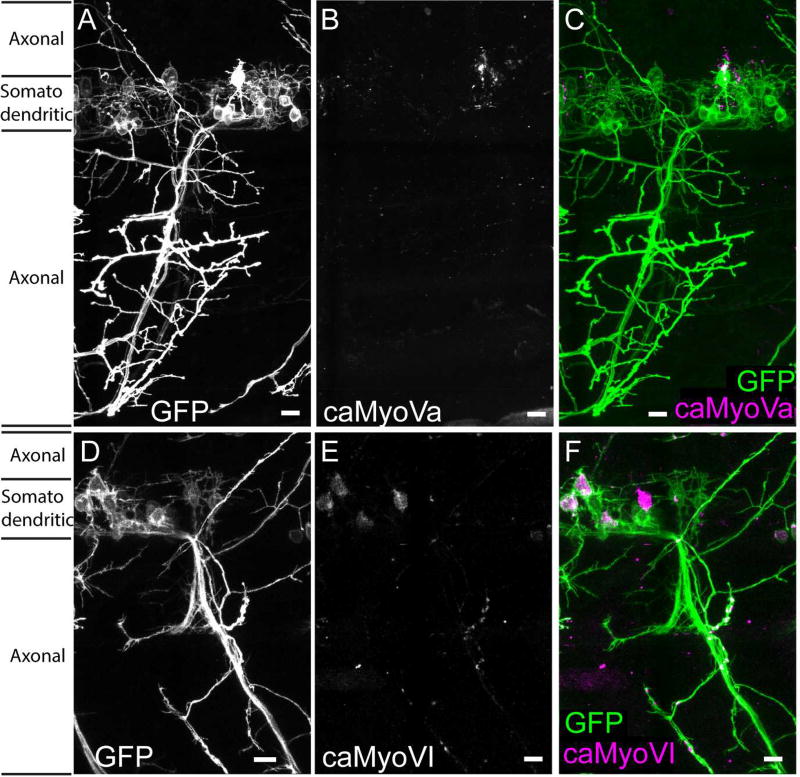
To determine whether an actin barrier is present in vivo we expressed constitutively active myosins in zebrafish motoneurons and examined their localizations at 2 dpf. caMyoVa-mCherry localized specifically to the somatodendritic compartment (ADR = 0.11 +/− 0.01; Figure 4A–C), whereas caMyoVI-mCherry localized in both the axonal and somatodendritic compartments (ADR = 0.61 +/− 0.04; Figure 4D–F; P < 0.001, Mann Whitney). Together these results are consistent with actin in the proximal axon forming a functional filter that permits the entrance of a minus end-directed myosin motor, but blocks entrance of a plus end-directed motor shortly after neuronal specification.
Figure 4.
Constitutively active Myosin Va, but not Myosin VI, is excluded from the axon in vivo. Spinal motoneuron in 2 dpf zebrafish expressing (A) GFP, (B) caMyoVa-mCherry, (C) caMyoVa-mCherry (purple) and GFP (green) (n = 9 neurons, 3 fish) (D) GFP, (E) caMyoVI-mCherry, (F) caMyoVI-mCherry (purple) and GFP (green) (n = 9 neurons, 3 fish). Scale bar 10 µm.
ARP2/3 plays a critical role in the maintenance of actin patches
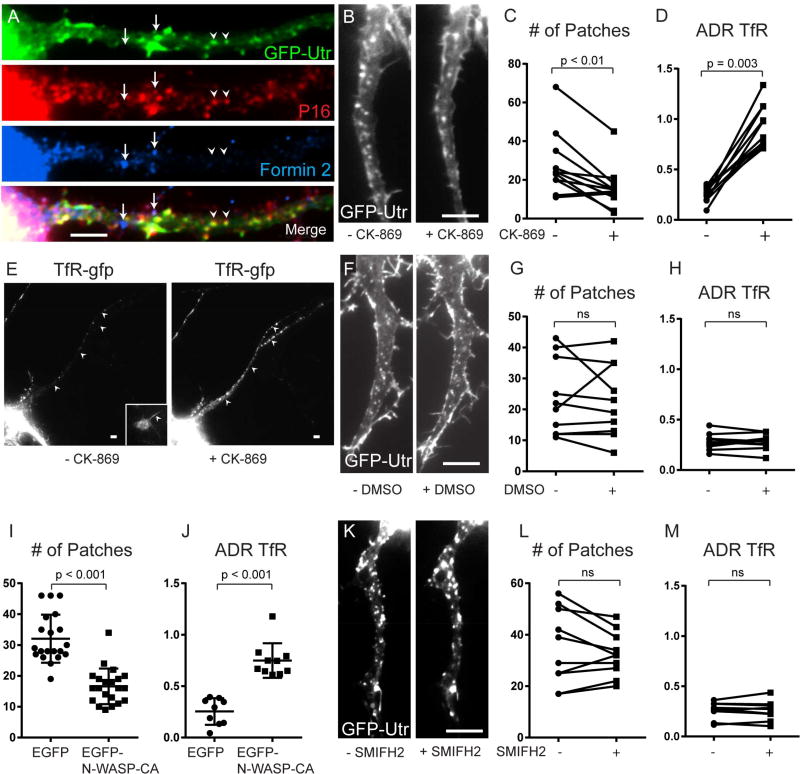
The actin nucleator ARP2/3 plays a major role in shaping the actin networks in dendritic spines, which have web-like patterns that are similar to those seen in actin patches (Korobova and Svitkina, 2010; Watanabe et al., 2012). To test whether ARP2/3 associates with actin patches in the proximal axon, we immunostained dissociated neurons expressing GFP-Utr with an anti-P16-ARC antibody and found colocalization between patches and ARP2/3 (Figure 5A). In contrast, Formin 2, a member of a family of proteins involved in elongation of individual actin filaments, was not colocalized with either actin patches or with ARP2/3 (Figure 5A).
Figure 5.
Actin patches are dependent on ARP2/3, but not on Formin 2. (A) 13 DIV cortical neuron expressing GFP-Utr and co-immunostained for P16 (red), Formin 2 (blue) and GFP (green) shows colocalization between actin patches and the ARP2/3 complex, but not Formin 2. (B) Axon expressing GFP-Utr before and after exposure to CK-869 for 30 minutes. (C) Numbers of actin patches in the proximal axon before and after exposure to CK-869 for 30 minutes (n = 11 neurons, 4 cultures). (D) ADR (axon to dendrite ratio) showing the relative amount of TfR-GFP in the proximal axon before and after exposure to CK-869 (n = 11 neurons, 4 cultures). (E) Cortical neuron expressing TfR-GFP before and after exposure to CK-869. Inset shows Ankyrin G staining. Arrowheads point to axon. (F) Proximal axon in cortical neuron expressing GFP-Utr before and after exposure to DMSO. (G) Number of actin patches in proximal axon before and after exposure to DMSO (n = 10 neurons, 5 cultures). (H) ADR of TfR before and after exposure to DMSO (n = 10 neurons, 5 cultures). (I) Number of actin patches in proximal axon of neurons expressing EGFP (n = 20 neurons, 3 cultures) and neurons expressing EGFP-NWASP-CA (n = 21 neurons, 3 cultures). Neurons co-expressed TagRFP-Utr. (J) ADR of TfR in cortical neurons co-expressing either EGFP (n = 9 neurons 2 cultures) or EGFP-N-WASP-CA (10 neurons, 3 cultures). (K) Proximal axon in neuron expressing GFP-Utr before and after exposure to SMIFH2. (L) Number of actin patches in proximal axon before and after exposure SMIFH2 (n = 10 neurons, 2 cultures). (M) ADR of TfR before and after exposure to SMIFH2 (n = 10 neurons, 2 cultures). Scale bar 5 µm. See also Figure S5.
To test whether ARP2/3 is necessary for maintenance of patches, we examined the same cortical neurons expressing TfR mCherry and GFP-Utr before and after exposure to CK-869, an ARP2/3 inhibitor, for 30 minutes. Following CK-869 exposure the number of actin patches labeled by Utr in the proximal axon was substantially reduced (28 +/− 5 patches initially, to 16 +/− 4 for the same cells after exposure to CK-869; P < 0.01, Wilcoxon; Figure 5B, C). A decrease in the number of patches was also found when comparing neurons expressing EGFP-N-WASP-CA, a blocker of ARP2/3 function (Strasser et al., 2004), to control neurons expressing EGFP (17 +/− 1 vs. 32 +/− 4, P < 0.001, Wilcoxon; Figure 5I). To investigate a possible functional significance of the reduction in actin patches, we examined the distribution of exogenously expressed TfR-mCherry in the same cortical neurons before and after exposure to CK-869 for 30 minutes. We found that the ADR of TfR in neurons exposed to CK869 was 3-fold higher vs. control neurons (ADR = 0.9 +/− 0.2 vs. 0.3 +/− 0.07; P = 0.003, Wilcoxon; Figure 5D, E). In addition, the ADR of TFR in cells expressing EGFP-N-WASP-CA was significantly higher than in cells expressing EGFP alone (ADR = 0.8 +/− 0.05 vs. 0.3 +/− 0.04, P < 0.001, Mann Whitney, Figures 5J, S5). These increases in ADR are consistent with leakage of TfR into the axon, which indicates a breach in the barrier between the somatodendritic and axonal compartments. In contrast, exposure to DMSO alone caused no change in the number of patches (24 +/− 4 patches before DMSO to 23 +/− 4 patches after exposure to DMSO; P > 0.4, Wilcoxon; Figure 5F, G). In addition, these cells did not show a change in the ADR of exogenous TfR in control cells vs. those exposed to DMSO (ADR = 0.3 +/− 0.08 vs. 0.3 +/− 0.08, P > 0.3, Wilcoxon; Figure 5H). These results are consistent with ARP2/3 being necessary for actin patches and for the patches being necessary for localization of TfR to the somatodendritic compartment.
In contrast to results with ARP2/3, blocking Formin 2 using the inhibitor SMIFH2 did not change the number of patches following 30 minutes of exposure (35 +/− 5 patches vs. 33 +/− 3 patches, P > 0.8, Wilcoxon, Figure 5K, L). The ADR of TfR also was not significantly different in control cells vs. those exposed to SMIFH2 (ADR = 0.3 +/− 0.03 vs. 0.3 +/− 0.03, P > 0.8; Figure 5M), indicating that Formin 2 function is not necessary for dendritic localization of TfR. We also asked whether actin patches are dependent on microtubules by exposing neurons expressing GFP-Utr to 15 µM Nocodazole. We found that exposure to 15 µM Nocodazole for 30 minutes, which was sufficient to dramatically affect microtubule integrity (Figure S5), did not affect the number of actin patches (26 +/− 3 with Nocodazole vs. 25 +/− 2 without Nocodazole; P > 0.6, Wilcoxon, Figure S5), or the ADR of TfR (ADRTfR = 0.3 +/− 0.02; ADRTfR, nocodazole = 0.3 +/− 0.03; P > 0.8, Mann Whitney, Figure S5). Thus, the number of actin patches is not changed by microtubule depolymerization.
DISCUSSION
We examined the distribution of actin in living neurons in culture and in vivo using an actin binding domain of Utrophin as a vital label. Close to 100% of labeled neurons showed patches of actin in the proximal axon. Possible discrepancies with results of previous experiments could be caused by fixation and/or permeabilization, which have been shown to cause depolymerization of actin filaments (Small et al., 1999). Patches were found in both mature neurons and those with newly formed axons, both in culture and in vivo, indicating that patch formation occurs early in axonal differentiation, even prior to expression of Ankyrin G. Actin is present in discrete patches that mark spots in the proximal axon where vesicles carrying a dendritic protein and moving anterogradely tend to halt and reverse, suggesting that they form part of a barrier that prevents such vesicles from moving to the distal axon in both young and mature neurons.
Note that although previous experiments are consistent with Utrophin not disrupting the equilibrium between actin assembly and disassembly (Bement et al., 2007), and although we found in neurons that Utrophin and Phalloidin staining overlap (Figure S1), it is difficult to establish unequivocally that Utrophin had no effect on actin in these experiments.
To ask whether actin forms a contiguous barrier between the axonal and somatodendritic compartments, we examined the distribution of exogenously expressed, constitutively active Myosin Va. The absence of caMyoVa in the axon in both young and mature neurons in culture, and in vivo, would suggest that actin forms an impervious barrier to the movement of some proteins. Furthermore, the presence of Myosin VI in the axon would suggest that actin acts as a filter. Importantly, this experiment did not rely on vesicular trafficking, and thus its interpretation is not complicated by possible manipulation of protein sorting, as has been suggested for previous experiments (Petersen et al., 2014). It is important to note that the inability of constitutively active Myosin Va to enter the axon does not imply that full-length Myosin Va does not enter the axon. As in the case of dynein, which enters the axon despite microtubules that are oriented in a direction that prevents its entry, Myosin Va could be transported into the axon directly by a kinesin motor (Twelvetrees et al., 2016). Alternatively, it could be transported by slow axonal transport or on a vesicle in an inactive, closed position.
One explanation for the discrepancy between actin’s apparent distribution and the efficiency with which it mediates the somatodendritic localization of caMyoVa could be that the filter is comprised of components other than patches that are difficult to visualize with standard epifluorescence microscopy combined with Utrophin labeling. This idea is consistent with the observation that some halting and reversing events take place in areas that appear to be devoid of actin patches (Figures 1, S2). Interestingly, experiments using STED microscopy or TEM of platinum replicas have noted the presence of relatively long actin filaments within the proximal axon that one study referred to as actin bundles (D'Este et al., 2015; Jones et al., 2014), and which could be part of the filter. The question of whether bundles or other, as of yet undescribed, structures form part of the filter will require its visualization at high resolution in a preparation where the integrity of actin filaments is preserved. Nonetheless, all of our results are consistent with actin filaments in the proximal axon being oriented such that overall their barbed ends face towards the cell body. This intrinsic polarity causes anterogradely moving vesicles associated with plus end-directed myosins to halt and reverse and retrogradely moving vesicles to slow down, and sometimes pause.
In addition to documenting the presence and functional significance of actin patches, our experiments suggested clues about their structural components. We found that a component of ARP2/3, a complex that stimulates actin polymerization and is found in dendritic spines (Wegner et al., 2008), is also present in patches. Furthermore, its presence is necessary for maintenance of patches and for the localization of dendritic proteins. This finding is consistent with the presence of branched arrangements of actin seen in electron micrographs of both spines (Korobova and Svitkina, 2010) and patches (Watanabe et al., 2012). The actin networks in spines and in patches have functions that are similar: directing proteins to postsynaptic sites in the case of spines (Schulz et al., 2004), and to the somatodendritic compartment in the case of patches (Al-Bassam et al., 2012).
Colocalization with ARP2/3 is also a feature of actin patches that are found in the distal axon (Spillane et al., 2011). These patches are similar to the patches in the proximal axon in that they are made up of branched filaments, however the distal axon patches are associated with filopodia, whereas the actin patches in the proximal axon are not (Loudon et al., 2006). In addition, both types of actin patches are highly dynamic. The mean lifespan of distal actin patches is roughly 40 seconds, almost identical to that of the 40% of proximal actin patches that turn over quickly. The 60% that turn over slowly have an average lifetime closer to 9 minutes. In contrast to ARP2/3, Formin 2 was found not to be colocalized with the proximal actin patches and blocking the function of Formin 2 with an inhibitor did not affect the localization of a dendritic protein. In this manner proximal actin patches are different from dynamic actin structures in the shaft of the axon, known as actin trails, which are associated with Formins, but not with ARP2/3 (Ganguly et al., 2015).
Although actin plays an important role in targeting of dendritic proteins, it seems likely that dynein also contributes to this process. In particular, experiments examining the trafficking of full-length Kif-17 identified distinct roles for the two motor proteins (Franker et al., 2016). Actin appears to be involved in halting Kif-17-mediated movement of peroxisomes following entry into the proximal axon, in a manner similar to halting that was observed for vesicles carrying dendritic proteins (Al-Bassam et al., 2012). However, subsequent movements of the peroxisomes were mediated by dynein, which is consistent with our previous observations that actin patches are comprised of relatively short fibers and myosin-mediated movements in the proximal axon tend to be 1 µm or shorter (Watanabe et al., 2012). Together, our results and those related to Kif-17 trafficking would suggest the following model (Figure S6): 1. Vesicles that are to be prevented from moving to the distal axon are designated as such through association, directly or indirectly, with plus end-directed Myosin motors. 2. Interaction of Myosin with the actin filter in the proximal axon stops the movement of vesicles, likely by disengaging kinesin motors from microtubules. 3. Halting of the kinesin-mediated vesicle movement allows dynein to engage microtubules and move vesicles back to the somatodendritic compartment. In this manner interactions of myosin and actin modulate the actions of kinesin and dynein, causing them to selectively transport specific vesicles to the axon or dendrites.
EXPERIMENTAL PROCEDURES
Patch quantification
To quantify patches in rat cortical neurons, live 12–17 day old dissociated cortical neurons expressing regulated GFP-Utr for 48 hrs were imaged. Puncta of relatively uniform diameter of less than 3 µm in the proximal 30 µm of the axon were considered to be patches. In order to observe actin in vivo 4 zebrafish (3 dpf) were imaged live using confocal microscopy. Roughly 300 motoneurons expressing GFP-Utr were examined using the same criteria that were used to identify patches and bundles in dissociated cells.
Live imaging of neurons in culture
For live imaging experiments, coverslips with transfected or nucleofected neurons on them were mounted on an open Warner Chamber using vacuum grease, and pre-warmed imaging medium was added on top of neurons. Warner chambers were then fixed on a microscope stage with environmental control to ensure viability of neurons, and left for 10–15 min. prior to the start of imaging to ensure minimal drift. Live images were obtained using either a 60×/1.2 NA water objective or a 100×/1.4NA oil objective on an Olympus IX71 wide-field microscope.
Axon to dendrite ratio (ADR) calculation
To calculate the ADR the mean amount of fluorescence per pixel in all of the dendrites that were present in a particular image, as well as in the axon was measured (with background subtracted from both). ADR was calculated as the ratio of mean fluorescence in the axon to that in the dendrite. This value was used for quantifying the localization of TfR (Figure 5). To normalize, the value calculated above was divided by the ratio of the mean fluorescence per pixel in the axon to the mean fluorescence in the dendrites of a nonspecifically localized protein such as GFP. This value was used for quantifying the localization of caMyoVa and caMyoVI (Figures 3 and 4). In cells where the ADR of diffuse caMyoVa was calculated regions of diffuse staining in the dendrites were used (Fig. S4). All analysis was done by blinded observers.
Statistical Methods
No statistical methods were used to predetermine sample size. Nonparametric tests were used throughout, as the data are not normally distributed. Data are represented as mean ± SEM. Statistical comparisons are between groups with similar variances.
Preparation of zebrafish embryos and dissociated cultures of rat neurons
Experimental protocols were conducted according to the US National Institutes of Health guidelines for animal research and were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Detailed methods can be found in Supplemental Experimental Procedures.
Supplementary Material
Movie S1 Vesicles carrying TfR, a dendritic protein, halt and reverse at actin patches, related to Fig. 1. 14 DIV cortical neuron in culture expressing TfR-mCherry (purple) and GFP-Utr (green). Vesicles carrying TfR-mCherry halt and reverse at locations marked by actin patches (arrows). Images collected at 1.5 s intervals. Scale bar 10 µm.
Movie S2 Vesicles carrying TfR, a dendritic protein, halt and reverse at actin patches, related to Fig. 1. 3 DIV cortical neuron in culture expressing TfR-mCherry (purple) and GFP-Utr (green). Vesicles carrying TfR-mCherry halt and reverse at locations marked by actin patches (arrows). Images collected at 1.5 s intervals. Scale bar 10 µm.
Movie S3 Proximal axon of cortical neuron expressing GFP-Utr, related to Fig. 2. Images were taken at intervals of 30 minutes. Scale bar 5 µm.
Movie S4 Proximal axon of cortical neuron expressing GFP-Utr, related to Fig. 2. Images were taken at intervals of 1 minute. Scale bar 5 µm.
Movie S5 Proximal axon of cortical neuron expressing GFP-Utr, related to Fig. 2. Images were taken at intervals of 1 minute. Scale bar 5 µm.
Movie S6 Proximal axon of cortical neuron expressing GFP-Utr, related to Fig. Images were taken at intervals of 1 minute. 2. Scale bar 5 µm.
Highlights.
Dynamic actin patches are present in the proximal axon of live neurons
Patches are present shortly after axonal specification in culture and in vivo
Constitutively active plus end-directed myosins localize to the soma and dendrites
ARP2/3 is present in patches and is necessary for localization of dendritic proteins
Acknowledgments
We thank M. Roth and J. Oliver for technical support, M. Griset for contributing to the graphical abstract and E. Liman for helpful suggestions on the manuscript. This work was supported by NIH (grants NS041963 and NS081687 to D.B.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Experiments in mammalian neurons were designed by V.B., K.W., T.L.L and D.B.A, and performed by V.B., K.W. and T.L.L. Experiments in zebrafish were designed by V.B., W.P.D. and D.B.A and were performed by V.B., W.P.D., K.W. and assisted by L.A.T. The paper was written by D.B.A. with assistance from V.B. and K.W.
References
- Al-Bassam S, Xu M, Wandless TJ, Arnold DB. Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. Cell Rep. 2012;2:89–100. doi: 10.1016/j.celrep.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DB, Gallo G. Structure meets function: actin filaments and myosin motors in the axon. J Neurochem. 2013 doi: 10.1111/jnc.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasanyan V, Arnold DB. Actin and myosin-dependent localization of mRNA to dendrites. PLoS One. 2014;9:e92349. doi: 10.1371/journal.pone.0092349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasanyan V, A DB. Myosin-dependent localization of mRNA to dendrites. doi: 10.1371/journal.pone.0092349. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement WM, Yu HY, Burkel BM, Vaughan EM, Clark AG. Rehabilitation and the single cell. Curr Opin Cell Biol. 2007;19:95–100. doi: 10.1016/j.ceb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel BM, von Dassow G, Bement WM. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil Cytoskeleton. 2007;64:822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Este E, Kamin D, Gottfert F, El-Hady A, Hell SW. STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep. 2015;10:1246–1251. doi: 10.1016/j.celrep.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Franker MA, Esteves da Silva M, Tas RP, Tortosa E, Cao Y, Frias CP, Janssen AF, Wulf PS, Kapitein LC, Hoogenraad CC. Three-Step Model for Polarized Sorting of KIF17 into Dendrites. Curr Biol. 2016;26:1705–1712. doi: 10.1016/j.cub.2016.04.057. [DOI] [PubMed] [Google Scholar]
- Galkin VE, Orlova A, VanLoock MS, Rybakova IN, Ervasti JM, Egelman EH. The utrophin actin-binding domain binds F-actin in two different modes: implications for the spectrin superfamily of proteins. J Cell Biol. 2002;157:243–251. doi: 10.1083/jcb.200111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Tang Y, Wang L, Ladt K, Loi J, Dargent B, Leterrier C, Roy S. A dynamic formin-dependent deep F-actin network in axons. J Cell Biol. 2015;210:401–417. doi: 10.1083/jcb.201506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183(4):635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CS, Watanabe S, Rasmussen HB, Schmitt N, Olesen SP, Frost NA, Blanpied TA, Misonou H. Specific sorting and post-Golgi trafficking of dendritic potassium channels in living neurons. J Biol Chem. 2014;289:10566–10581. doi: 10.1074/jbc.M113.534495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Korobova F, Svitkina T. Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. J Cell Biol. 2014;205:67–81. doi: 10.1083/jcb.201401045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krementsov DN, Krementsova EB, Trybus KM. Myosin V: regulation by calcium, calmodulin, and the tail domain. J Cell Biol. 2004;164:877–886. doi: 10.1083/jcb.200310065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Jr, Mao T, Svoboda K, Arnold DB. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat Neurosci. 2009;12(5):568–576. doi: 10.1038/nn.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF, Jr, Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 2006;66:847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JD, Kaech S, Banker G. Selective microtubule-based transport of dendritic membrane proteins arises in concert with axon specification. J Neurosci. 2014;34:4135–4147. doi: 10.1523/JNEUROSCI.3779-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TW, Nakagawa T, Licznerski P, Pawlak V, Kolleker A, Rozov A, Kim J, Dittgen T, Kohr G, Sheng M, et al. Actin/alpha-actinin-dependent transport of AMPA receptors in dendritic spines: role of the PDZ-LIM protein RIL. J Neurosci. 2004;24:8584–8594. doi: 10.1523/JNEUROSCI.2100-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MA, Kaech S, Jareb M, Burack MA, Vogt L, Sonderegger P, Banker G. Sorting and directed transport of membrane proteins during development of hippocampal neurons in culture. Proc Natl Acad Sci U S A. 2001;98:7051–7057. doi: 10.1073/pnas.111146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J, Rottner K, Hahne P, Anderson KI. Visualising the actin cytoskeleton. Microsc Res Tech. 1999;47:3–17. doi: 10.1002/(SICI)1097-0029(19991001)47:1<3::AID-JEMT2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Sobotzik JM, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, Schultz C. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc Natl Acad Sci U S A. 2009;106:17564–17569. doi: 10.1073/pnas.0909267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136(6):1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol. 2011;71:747–758. doi: 10.1002/dneu.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM. Arp2/3 is a negative regulator of growth cone translocation. Neuron. 2004;43:81–94. doi: 10.1016/j.neuron.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Twelvetrees AE, Pernigo S, Sanger A, Guedes-Dias P, Schiavo G, Steiner RA, Dodding MP, Holzbaur EL. The Dynamic Localization of Cytoplasmic Dynein in Neurons Is Driven by Kinesin-1. Neuron. 2016;90:1000–1015. doi: 10.1016/j.neuron.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Al-Bassam S, Miyazaki Y, Wandless TJ, Webster P, Arnold DB. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep. 2012;2:1546–1553. doi: 10.1016/j.celrep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem. 2008;283:15912–15920. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1 Vesicles carrying TfR, a dendritic protein, halt and reverse at actin patches, related to Fig. 1. 14 DIV cortical neuron in culture expressing TfR-mCherry (purple) and GFP-Utr (green). Vesicles carrying TfR-mCherry halt and reverse at locations marked by actin patches (arrows). Images collected at 1.5 s intervals. Scale bar 10 µm.
Movie S2 Vesicles carrying TfR, a dendritic protein, halt and reverse at actin patches, related to Fig. 1. 3 DIV cortical neuron in culture expressing TfR-mCherry (purple) and GFP-Utr (green). Vesicles carrying TfR-mCherry halt and reverse at locations marked by actin patches (arrows). Images collected at 1.5 s intervals. Scale bar 10 µm.
Movie S3 Proximal axon of cortical neuron expressing GFP-Utr, related to Fig. 2. Images were taken at intervals of 30 minutes. Scale bar 5 µm.
Movie S4 Proximal axon of cortical neuron expressing GFP-Utr, related to Fig. 2. Images were taken at intervals of 1 minute. Scale bar 5 µm.
Movie S5 Proximal axon of cortical neuron expressing GFP-Utr, related to Fig. 2. Images were taken at intervals of 1 minute. Scale bar 5 µm.
Movie S6 Proximal axon of cortical neuron expressing GFP-Utr, related to Fig. Images were taken at intervals of 1 minute. 2. Scale bar 5 µm.