Abstract
Complement alternative pathway dysregulation seems to be the pathophysiological basis of Dense Deposit Disease (DDD). Here, we describe a monoclonal anti-factor H (FH) autoantibody in a woman diagnosed with DDD with a monoclonal gammapathy. Enzyme-linked immunosorbent assays evidenced the presence of anti-FH antibodies in the patient's serum and showed that they were associated with the monoclonal IgG-λ fraction. These autoantibodies recognize the N-terminal region of FH and interfere with its regulatory function. In summary, in the DDD patient described here, the activation of complement alternative pathway was favoured by the presence of anti-FH autoantibodies that recognize the regulatory region of this protein and impede its function and which could ultimately cause the glomerulopathy.
Keywords: anti-factor H autoantibodies, complement, dense deposit disease, monoclonal gammapathy
Background
Dense Deposit Disease (DDD) patients have evidence of complement alternative pathway (AP) dysregulation as reflected by low C3 levels in serum [1–4]. This uncontrolled systemic activation of AP seems to be the pathophysiological basis of the disease. The AP is spontaneously activated at low levels in normal conditions and needs to be strictly controlled by a number of regulators, the most important being complement factor H (FH).
FH is a glycoprotein composed of 20 homologous short consensus repeats (SCRs). The protein includes different interaction sites for C3b and polyanions, with different functional domains at the N- and C-terminal regions of the molecule [3]. FH inhibits the formation and accelerates the decay of the AP C3 convertase and also serves as cofactor for the factor I (FI)-mediated inactivation of C3b.
More than 80% of DDD cases are associated with C3 nephritic factor (C3NeF). Recently, an autoantibody against factor B with similar effect on C3 convertase has been described [5]. Some of the remaining cases are associated with FH deficiencies or mutations that lead to continuous activation of the AP [1, 6]. So far, two cases of DDD associated with FH autoantibodies have been described. One of these was a monoclonal λ light chain dimer which prevented fluid-phase regulation of the C3 convertase by blocking FH cofactor activity [7]. The other case showed monoclonal anti-FH antibodies without determining their impact on protein function [8].
Here, we present the third case of DDD in association with anti-FH autoantibodies, which inhibit FH function and cause a systemic AP activation leading to the glomerular pathology.
Case report
The patient was a 74-year-old woman admitted to the hospital due to general oedema and macrohaematuria. On physical examination, the patient showed mucocutaneous paleness, high arterial blood pressure (199/73 mmHg) and general oedema.
Laboratory findings revealed acute renal failure (serum creatinine 1.8 mg/dL, urea 183 mg/dL), low total protein and albumin levels and anaemia. A monoclonal IgG-λ was detected in the serum. Nephrotic range proteinuria (5.26 g per 24 h) with Bence Jones and haematuria (30–50 red blood cells per field) were observed. Bone marrow aspirate presented 9% plasma cells and cryoglobulins resulted negative.
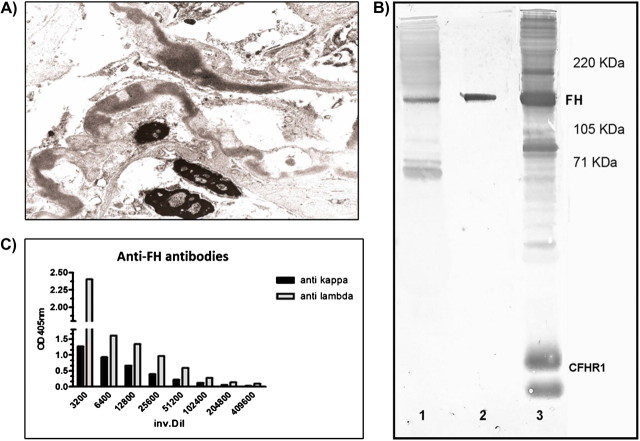
Renal biopsy revealed glomeruli with mesangial expansion and immune deposits distributed in the mesangium and in the peripheral capillary walls in a subendothelial pattern. These deposits were strongly periodic-acid-schiff positive. Immunohistochemistry techniques were negative for detecting kappa and lambda light chains and Congo red staining was also negative. In the ultrastructural study, there were localized fibrillar deposits of an approximate diameter of 27 nm in the mesangium and capillary walls. On the other hand, irregular and discontinuous thickening of glomerular basement membranes because of an electron-dense and homogeneous deposit was observed (Figure 1A).
Fig. 1.
(A) High-power microscopic view shows the segmental irregular distribution of the ribbon-like thickenings of the glomerular basement membranes by intramembranous highly electron-dense deposits. (B) Western blot. SDS–PAGE carried out with IgG depleted normal human serum (NHS) in well 1 and 3 (corresponding to lane 1 and 3 in the figure) and with purified FH in well 2. After transfer, lanes 1 and 2 were revealed with IgG purified from the patient and lane 3 with a polyclonal anti-FH antibody that identifies FH and some Complement Factor H-related proteins (CFHR) in NHS. (C) Anti-FH antibody light chain isotype determination by ELISA assay. Anti kappa, anti-human κ light chain antibody; anti lambda, anti-human λ light chain antibody; inv. Dil, serum inversal dilution; CFHR1, complement Factor H-related protein 1.
In summary, the patient was diagnosed with fibrillar glomerulonephritis and ultrastructural findings were compatible with DDD.
At this point, the patient's serum was remitted to Hospital La Paz to extend the immunological study.
Serological study showed normal immunoglobulin and C4 levels but reduced C3 (Supplementary Table 1), which was suggestive of complement AP activation. Immunofixation of serum identified IgG-λ monoclonal gammapathy with residual IgG-κ. C3NeF haemolytic assay was performed and resulted negative. Complement FH measurement by Enzyme-linked immunosorbent assay (ELISA) revealed reduced levels of this protein and anti-FH autoantibodies were detected in the patient's serum and in the patient's purified IgG fraction. Anti-FH activity was confirmed by western blot analysis and another reactivity of the IgG fraction was observed (Figure 1B, lane 1).
The light chain isotype of the autoantibody was determined by ELISA, and the anti-FH activity was associated mainly with monoclonal IgG-λ (Figure 1C).
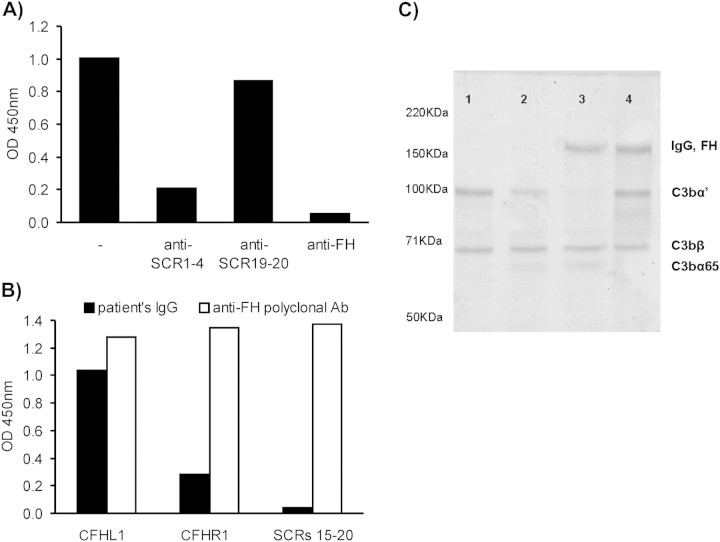
To determine which part of the FH molecule is recognized by the patient's IgG, a competition ELISA with immobilized FH pre-incubated with various anti-FH antibodies was made and it indicated that the autoantibody binds to SCRs 1–4 of FH. Another ELISA assay also showed that the patient's IgG binds to recombinant factor H-like protein 1 (CFHL1), a splicing variant of FH which comprises SCRs 1–7 of the molecule (Figure 2 A and B). This was confirmed by western blot analysis of the patient's IgG (not shown).
Fig. 2.
(A) Competition ELISA, immobilized FH was pre-incubated with different antibodies, and the binding of patient's IgG was determined. (B) Binding of patient's IgG to recombinant proteins. (C) Inhibition of fluid-phase cofactor activity of FH: different mixtures of the required components for the inactivation of C3b by FI were incubated at 37°C and then an 8% SDS–PAGE electrophoresis was carried out under denaturing conditions. Well 1, C3b; Well 2, C3b + FI; Well 3, C3b + FI + FH; Well 4, C3b + FI + FH + patient's IgG.
As the N-terminal region of the FH molecule is where the regulator domain is, the effect of the patient's antibody on fluid-phase cofactor activity of FH was tested. In the presence of FH and patient's IgG, an absence of C3b degradation by FI was observed (Figure 2C).
Another serum protein recognized by the patient's IgG was plasminogen, identified by mass spectrometry from a Coomassie stained-gel band, after 8% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) (Proteomics Unit, Universidad Complutense, Madrid, Spain). Anti-plasminogen activity was then tested in the purified IgG and in patient's serum by ELISA and western blot (Supplementary Figure 1).
Because of the probable association of monoclonal gammapathy with two glomerular diseases, the patient started treatment with cyclophosphamide and dexamethasone.
Discussion
This is the third report of a patient with DDD and FH autoantibodies, the second demonstrating blocking of FH function by autoantibodies.
The patient reported here had a monoclonal IgG-λ that was associated with the anti-FH activity. When the patient started her treatment, monoclonal IgG titres diminished and so did FH autoantibodies. Renal function improved and C3 and FH serum levels achieved normal values (Supplementary Table 1).
The patient's antibodies recognized the regulatory region of FH and they blocked FH cofactor activity for FI. As antibodies impeded FH function, complement AP was constitutively activated. The effect of these autoantibodies on FH function seems to be analogous to a mutation in SCR4 described previously in which cell-binding activity was normal but complement control was defective [9].
The patient's antibodies prevented fluid-phase FH functions, and this inhibitory effect promotes systemic complement activation, diminishes C3 serum levels, and C3 and terminal components of complement cascade deposition on the kidney. FH autoantibodies are relatively frequent in atypical haemolytic uraemic syndrome, where they are directed to SCRs 19–20, impairing FH binding to surface-bound C3b and cell membranes and limiting its capacity to protect cells from complement lysis. This causes a localized damage on the surface of renal endothelial cells by inadequate protection from complement activation, not the systemic activation seen in DDD.
The patient's autoantibodies also recognize plasminogen, whose presentation could be related with anti-FH autoantibodies. Numerous pathogens have surface proteins that bind both host proteins to avoid elimination by complement during the innate immune response [10]. Thus, complement regulators and other proteins attached to the pathogen's surface would be recognized as antigens and the host would develop antibodies against these self-proteins.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Acknowledgments
This work was supported by a grant from Ministerio de Ciencia e Innovación (PS09/00122) and Centro de Investigación Biomédica en Red de Enfermedades Raras (INTRA/10/738.1) and supported in part by the Deutsche Forschungsgemeinschaft (JO 844/1-1).
We thank Dr Xavier Fulladosa for his helpful advice.
Conflict of interest statement: None declared.
References
- 1.Smith RJ, Alexander J, Barlow PN, et al. New approaches to the treatment of dense deposit disease. J Am Soc Nephrol. 2007;18:2447–2456. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker PD. Dense deposit disease: new insights. Curr Opin Nephrol Hypertens. 2007;16:204–212. doi: 10.1097/MNH.0b013e3280bdc0f4. [DOI] [PubMed] [Google Scholar]
- 3.Pickering MC, Cook HT. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin Exp Immunol. 2008;151:210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zipfel PF, Heinen S, Józsi M, et al. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol. 2006;43:97–106. doi: 10.1016/j.molimm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Strobel S, Zimmering M, Papp K, et al. Anti-factor B autoantibody in dense deposit disease. Mol Immunol. 2010;47:1476–1483. doi: 10.1016/j.molimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Licht C, Fremeaux-Bacchi V. Hereditary and acquired complement dysregulation in membranoproliferative glomerulonephritis. Thromb Haemost. 2009;101:271–278. [PubMed] [Google Scholar]
- 7.Jokiranta TS, Solomon A, Pangburn MK, et al. Nephritogenic lambda light chain dimer: a unique human miniautoantibody against complement factor H. J Immunol. 1999;163:4590–4596. [PubMed] [Google Scholar]
- 8.Sethi S, Sukov WR, Zhang Y, et al. Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am J Kidney Dis. 2010;56:977–982. doi: 10.1053/j.ajkd.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Licht C, Heinen S, Józsi M, et al. Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II) Kidney Int. 2006;70:42–50. doi: 10.1038/sj.ki.5000269. [DOI] [PubMed] [Google Scholar]
- 10.Seling A, Siegel C, Fingerle V, et al. Functional characterization of Borrelia Spielmanii outer surface proteins that interact with distinct members of the human factor H protein family and with plasminogen. Infect Immun. 2010;78:39–48. doi: 10.1128/IAI.00691-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.