Abstract
Background.
A blood urea nitrogen (BUN)/creatinine ratio (BCR) >20 (0.081 in international unit) is used to distinguish pre-renal azotemia (PRA) and acute tubular necrosis (ATN). However, there is little evidence that BCR can distinguish between these two conditions and/or is clinically useful.
Methods.
We conducted a retrospective study using a large hospital database. Patients were divided into three groups: ‘low BCR’ (if BCR when acute kidney injury (AKI) developed was ≤20), ‘high BCR’ (if BCR when AKI developed was >20) and ‘no AKI’ if patients did not satisfy any of the Risk, Injury, Failure, Loss and End-stage kidney disease criteria for AKI during hospitalization.
Results.
Among 20 126 study patients, 3641 (18.1%) had AKI. Among these patients, 1704 (46.8%) had a BCR <20 at AKI diagnosis (‘low BCR’) and 1937 (53.2%) had a BCR >20 (‘high BCR’). The average BCR for the two groups was 15.8 versus 26.1 (P < 0.001). Hospital mortality was significantly less in the ‘low-BCR’ group (18.4 versus 29.9%, P < 0.001). Multivariable logistic regression analysis for hospital mortality (‘no AKI’ as a reference) showed that the odds ratio of ‘high BCR’ (5.73) was higher than that of ‘low BCR’ (3.32).
Conclusions.
Approximately half of the patients with AKI have a BCR >20, the traditional threshold of diagnosing PRA. Unlike PRA patients who have a lower mortality than ATN patients, high BCR patients had higher hospital mortality compared with low BCR patients, which was confirmed with multivariable analysis. These findings do not support BCR as a marker of PRA.
Keywords: acute kidney injury, acute tubular necrosis, blood urea nitrogen, creatinine, pre-renal azotemia
Introduction
Acute kidney injury (AKI) occurs commonly in hospitalized patients and carries a high mortality [1–3]. The causes of AKI are often divided into three groups: pre-renal, intra-renal and post-renal [4–6]. Pre-renal failure, also called pre-renal azotemia (PRA), is described as a reversible increase in serum creatinine and urea concentrations resulting from decreased renal perfusion, which leads to a reduction in the glomerular filtration rate (GFR) [6]. On the other hand, intra-renal diseases affect structures of the nephron such as the glomeruli, tubules, vessels or interstitium, and the most common cause of intra-renal (intrinsic) disease is thought to be acute tubular necrosis (ATN) [5]. These two causes have been reported to account for 66 [1] to 75% [2] of all cases of AKI. Early recognition of the cause of AKI, especially distinguishing PRA and ATN, is widely considered clinically important as fluid resuscitation may improve PRA but can cause tissue edema and worsen ATN. Furthermore, ATN has a much worse prognosis [1, 7].
The blood urea nitrogen (BUN)/creatinine ratio (BCR) is one of the common laboratory tests used to distinguish PRA and ATN, with a typical threshold of 20 (0.081 in international units) being suggested as a useful cut-off point for separating PRA from ATN [8]. However, there is little evidence showing that BCR can distinguish between these two conditions and/or is clinically useful. Therefore, we conducted a study using a large database of patients hospitalized in an academic medical center [3], concentrating on the meaning of BCR in patients with AKI.
Materials and methods
We screened all patients admitted to the Austin Hospital, Melbourne, Australia, between January 2000 and December 2002 using the computerized hospital admissions and discharges database. Patients were excluded if they were younger than 15 years old, if they were on chronic dialysis or had a kidney transplant or if their length of hospital stay was shorter than 24 h. If a patient had more than one admission during the study period, only the last admission was included in the study. The study was approved by the Austin Hospital Human Research Ethics Committee. The need for informed consent was waived as the study required no intervention and no breach of privacy or anonymity as such projects are considered quality improvement activities by the Institutional Ethics Committee.
Serum creatinine and BUN values for all included patients during their hospital stay were obtained from the central laboratory database. AKI was defined according to the Risk, Injury, Failure, Loss and End-stage kidney disease (RIFLE) criteria [9]. We used the GFR criteria only because we could not collect information for urine output. The baseline creatinine was defined in two ways as previously described [3]. In brief, for patients who had more than one admission during the study period, the baseline creatinine was defined as that measured at hospital discharge from the previous admission. For patients with only one admission, the baseline creatinine was estimated using the MDRD equation [10], as recommended by the ADQI workgroup (assuming an average GFR of 75 mL/min in this age group) [9].
Patients were then divided into three groups: ‘low BCR’ if BCR when AKI developed was ≤20, ‘high BCR’ if BCR when AKI developed was >20 and ‘no AKI’ if patients did not satisfy any of the RIFLE criteria for AKI during hospitalization.
Demographic information was collected from the database: age, gender, type of admission, intensive care unit (ICU) admission, use of mechanical ventilation, use of renal replacement therapy (RRT), admission units, length of hospital stay and hospital mortality. The worst class (the highest RIFLE category reached during hospital stay) was chosen from the Risk, Injury or Failure categories. The peak creatinine was defined as the highest creatinine during their hospital admission.
Demographic data are presented as medians (25th–75th quartiles) or percentages. The demographics were compared between ‘low BCR’ and ‘high BCR’ with the Fisher's exact test for nominal values and Mann–Whitney test for numerical variables. Multivariable logistic regression analysis was conducted for hospital mortality. All available variables and renal conditions for patients (‘no AKI’, ‘low BCR’ and ‘high BCR’) were chosen as independent variables in the analysis. ‘General medicine’ was used as the reference for admission units and ‘no AKI’ for renal conditions. To evaluate the meaning of BCR more thoroughly, multivariable analysis was repeated with seven subgroups according to BCR (<10, 10–15, 15–20, 20–25, 25–30, 30–40 and >40). A commercially available statistical package was used (SPSS Statistics 19; IBM Inc., Chicago). A P-value of <0.05 was considered statistically significant.
Results
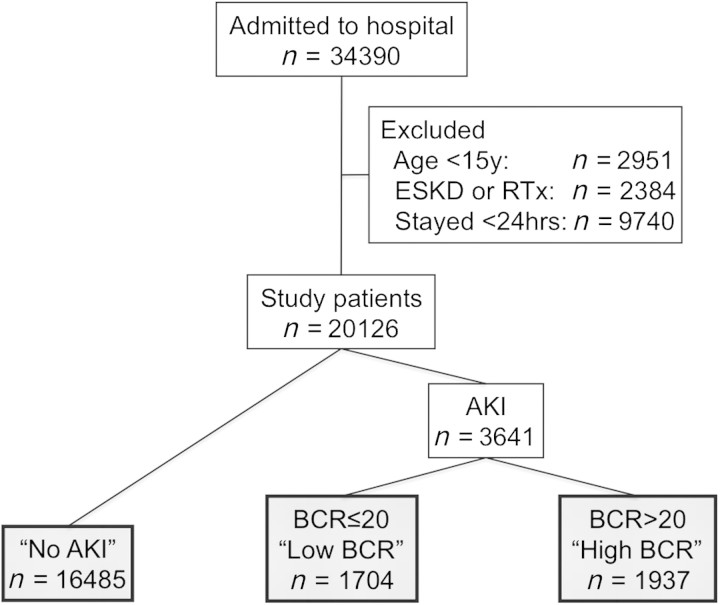
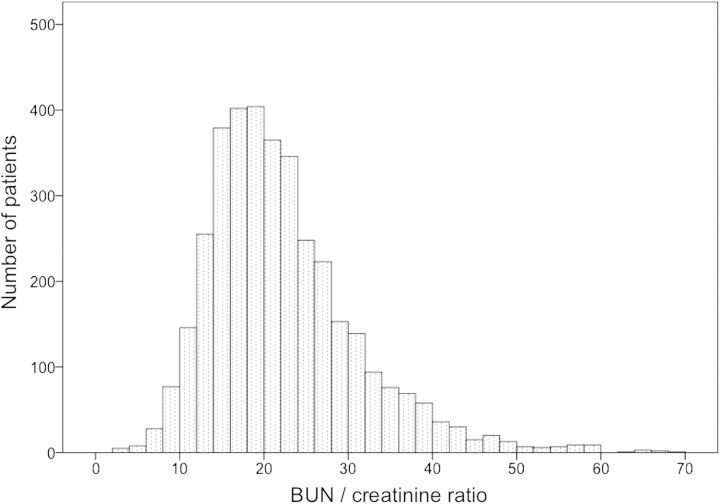
Figure 1 shows the patient selection flow diagram. Among 20 126 study patients, 3641 (18.1%) had AKI. Among these patients, 1704 (46.8%) had a BCR of <20 when AKI was diagnosed (‘low BCR’) and the rest were classified as ‘high BCR’. Figure 2 shows the distribution of the BCR among AKI patients. There was no bimodal distribution in the BCR.
Fig. 1.
Patient selection flow diagram for current study. RTx, renal transplantation.
Fig. 2.
Distribution for BUN/creatinine ratio.
Table 1 shows the demographics of patients. Compared with ‘low BCR’ patients, patients in the ‘high BCR’ group were older, had more emergency operations, but required fewer ICU admissions or mechanical ventilation (P < 0.0001 for all comparisons). They were also more likely to be female and had lower baseline creatinine values. At the time of AKI, their serum creatinine level was slightly lower and their BUN higher, with an average BCR difference of >10 (15.8 versus 26.1, P < 0.001).
Table 1.
Demographics of patientsa
| No AKI | Low BCR | High BCR | Low versus High | |
| Number of patients | 16 485 | 1704 | 1937 | |
| Age, years | 65 (49–76) | 75 (65–82) | 79 (71–85) | <0.001 |
| Male gender, % | 56.1 | 55.3 | 45.1 | <0.001 |
| Re-admission, % | 29.3 | 25.8 | 32.8 | <0.001 |
| Emergency admission, % | 55.7 | 65.3 | 75.4 | <0.001 |
| ICU admission, % | 12.3 | 28.4 | 22.8 | <0.001 |
| Mechanical ventilation, % | 7.3 | 20.4 | 14.5 | <0.001 |
| Baseline Cr, mg/dL | 1.01 (0.81–1.08) | 1.00 (0.79–1.04) | 0.82 (0.78–1.03) | <0.001 |
| Baseline Cr, (μmol/L) | 89 (71–95) | 88 (70–92) | 72 (69–91) | |
| Operation, % | 36.7 | 33.6 | 23.1 | <0.001 |
| Admission units, % | <0.001 | |||
| General medicine | 19.6 | 30.7 | 45.6 | |
| Cardiology | 12.9 | 7.8 | 6.6 | |
| Gastroenterology | 3.1 | 5.4 | 4.2 | |
| Hematology | 1.6 | 2.6 | 2.6 | |
| Neurology | 4.9 | 1.8 | 2.1 | |
| Oncology | 7.6 | 5.6 | 7.8 | |
| Renal medicine | 0.6 | 5.7 | 3.0 | |
| Respiratory medicine | 3.6 | 3.2 | 3.8 | |
| Stroke unit | 2.9 | 3.0 | 2.5 | |
| Other medical units | 3.7 | 1.4 | 1.9 | |
| Cardiac surgery | 4.5 | 8.7 | 4.6 | |
| General surgery | 12.7 | 8.3 | 5.6 | |
| Neurosurgery | 5.4 | 0.9 | 1.3 | |
| Orthopedics | 4.6 | 2.2 | 2.6 | |
| Thoracic surgery | 4.0 | 1.8 | 1.2 | |
| Urology | 3.0 | 5.0 | 0.9 | |
| Vascular surgery | 2.7 | 4.3 | 2.9 | |
| Other surgical units | 2.6 | 1.3 | 0.8 | |
| Admission to AKI, days | N/A | 2 (1–4) | 2 (1–4) | 0.18 |
| Cr at AKI occurrence, mg/dL | N/A | 1.74 (1.43–2.33) | 1.64 (1.32–2.05) | <0.001 |
| Cr at AKI occurrence, (μmol/L) | N/A | 153 (126–205) | 144 (116–180) | |
| BUN at AKI occurrence, mg/dL | N/A | 28.0 (22.1–36.1) | 44.5 (34.7–62.2) | <0.001 |
| Urea, mmol/L | N/A | 10.0 (7.9–12,9) | 15.9 (12.4–22.2) | |
| BUN/Cr ratio | N/A | 15.8 (13.3–17.8) | 26.1 (22.7–31.7) | <0.001 |
| Urea mmol/L/Cr μmol/L | N/A | 0.064 (0.054–0.072) | 0.106 (0.092–0.129) |
Admission to AKI, duration between hospital admission and AKI occurrence; Cr, creatinine; N/A, not available.
Table 2 shows renal and hospital outcomes. Patients in the ‘high BCR’ group had lower RIFLE classes and required RRT less frequently. Sixteen hundred patients recovered renal function during their hospital stay. Both groups had the same median duration of AKI of 2 days (P = 0.25). However, hospital mortality was significantly higher than that in the ‘low-BCR’ group (29.9 versus 18.4%; P < 0.001).
Table 2.
Renal and hospital outcomesa
| No AKI | Low BCR | High BCR | Low versus High | |
| RRT requirement, % | N/A | 5.7 | 2.7 | <0.001 |
| RIFLE-max, % | <0.001 | |||
| Risk | N/A | 47.3 | 53.2 | |
| Injury | N/A | 27.2 | 30.5 | |
| Failure | N/A | 25.5 | 16.3 | |
| AKI duration (days) | N/A | 2 (1–4) | 2 (1–4) | 0.25 |
| Hospital stay, days | 5 (3–9) | 8 (4–16) | 9 (5–18) | 0.001 |
| Hospital mortality, % | 4.4 | 18.4 | 29.9 | <0.001 |
RIFLE-max, the worst RIFLE class during hospitalization; N/A, not available.
Table 3 shows the results of the multivariable logistic regression analysis for hospital mortality. After adjusting for confounding variables, the odds ratio (OR) for mortality among ‘high-BCR’ patients (5.732) was higher than for ‘low-BCR’ patients (3.321).
Table 3.
Multivariable logistic regression analysis for hospital mortalitya
| Variables | ORs (95% CI) | P-values |
| Age, years | 1.036 (1.031–1.041) | <0.001 |
| Male gender | 1.204 (1.065–1.361) | 0.003 |
| Re-admission | 1.727 (1.521–1.961) | <0.001 |
| Emergency admission | 1.509 (1.298–1.753) | <0.001 |
| ICU admission | 2.957 (2.325–3.762) | <0.001 |
| Mechanical ventilation | 5.421 (4.142–7.095) | <0.001 |
| Baseline Cr, mg/dL | 1.571 (1.384–1.784) | <0.001 |
| Operation | 0.759 (0.625–0.921) | 0.005 |
| Admission units | ||
| General medicine | 1.000 (reference) | |
| Cardiology | 0.402 (0.298–0.542) | <0.001 |
| Gastroenterology | 1.393 (0.999–1.941) | 0.050 |
| Hematology | 2.760 (1.988–3.833) | <0.001 |
| Oncology | 4.386 (3.609–5.331) | <0.001 |
| Renal medicine | 0.316 (0.181–0.552) | <0.001 |
| Stroke unit | 2.137 (1.624–2.812) | <0.001 |
| Cardiac surgery | 0.088 (0.059–0.131) | <0.001 |
| General surgery | 0.531 (0.401–0.705) | <0.001 |
| Thoracic surgery | 0.544 (0.329–0.901) | 0.018 |
| Urology | 0.145 (0.058–0.360) | <0.001 |
| Vascular surgery | 0.407 (0.268–0.617) | <0.001 |
| Other surgical units | 0.193 (0.076–0.491) | 0.001 |
| Renal condition | ||
| No AKI | 1.000 (reference) | |
| Low BCR | 3.321 (2.816–3.916) | <0.001 |
| High BCR | 5.732 (4.973–6.606) | <0.001 |
Cr, creatinine.
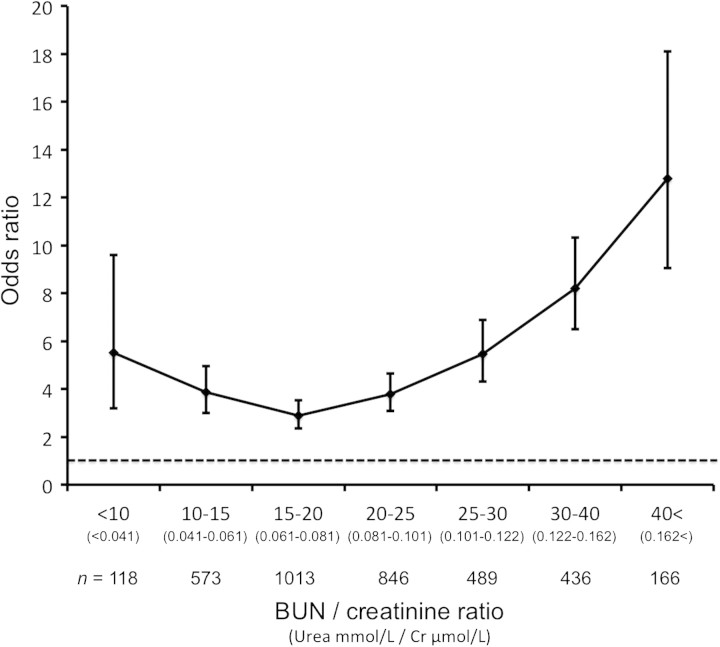
Figure 3 shows the ORs for hospital mortality among patients with different BCRs. The OR curve was J-shaped, and patients with a BCR of 15–20 had the lowest OR for hospital mortality. Patients with BCR of >40 had a very high OR (12.8) for hospital mortality. Patient characteristics and outcomes among patients with different BCRs are shown in Supplementary Table 1.
Fig. 3.
ORs for hospital mortality among different BUN/creatinine ratios.
Since approximately three quarters of the patients did not have a known baseline creatinine and it was calculated with the MDRD equation, sensitivity analysis was conducted separately by studying only patients with more than one admission (measured baseline creatinine available). Demographics of patients, renal and hospital outcome and multivariable logistic regression analysis for hospital mortality are shown in Supplementary Tables 2, 3 and 4. These results were almost identical to those for all patients.
Discussion
Key findings
We performed a retrospective study of a large database of patients admitted to hospital to determine whether a BCR >20 was useful in separating patients with functional AKI secondary to pre-renal factors (so-called PRA) from patients with structural AKI (so called ATN). We found that this diagnostic test failed on several grounds. Firstly, we did not find a bimodal distribution of BCR. This observation indicates that BCR is continuous and cannot be divided into values diagnostic of two separate conditions (PRA versus ATN). Secondly, ATN carries a worse prognosis than PRA [1, 7], yet, we found that patients with a high BCR (traditionally considered a marker of PRA) actually had a higher morality rate than those with low BCR (traditionally considered a marker of ATN). This indicates that BCR cannot diagnose the type of AKI and that PRA and ATN cannot be distinguished by this test. Additionally, we confirmed that, even after adjustment for several major confounding variables, the association between a high BCR and mortality remained greater than for a low BCR value. Finally, when we analyzed the association between BCR and outcome, we found that it had a J-shaped curve with the lowest mortality in the 15–20 range.
Relationship with previous studies
BCR has been used to distinguish PRA and ATN for decades, although more recently the usefulness of such terms has been called into question and may not be appropriate or correct [3, 11]. The theory behind the value of BCR as a means of distinguishing PRA and ATN is that elevated levels of antidiuretic hormone in PRA increase reabsorption of both water and urea, which increases serum BUN concentration more than serum creatinine [12]. However, actual clinical evidence to support the theory is scarce and dated [13, 14]. In fact, >30 years ago, Morgan et al. [15] suggested that BCR was not useful to distinguish PRA and intrinsic renal failure. More recently, Tariq et al. [16] studied 191 hospitalized patients for cholera, with an average duration of symptoms prior to hospitalization of 3.8 days. Although the majority of the patients (92%) presented with dehydration, the mean BCR was as low as 11.6 at hospital admission. Two recent studies have challenged the value of distinguishing PRA and ATN [17, 18]. Traditional methods used to distinguish PRA and ATN, e.g. urinalysis and Fe-Na, are similarly being challenged [19, 20]. Although the current study does not directly challenge traditional practice, our findings are consistent with more recent thought that most, if not all, biochemical- or urinalysis-based studies do not have sufficient scientific robustness in classifying patients into clinically syndrome, which logically fit into the PRA/ATN paradigm [21].
Naturally, a high BCR can come from high BUN or a low creatinine or both. In this study, however, BUN had much larger influence on BCR (28.0 versus 44.5, 59% increase) than creatinine (1.74 versus 1.64, 6% decrease). BUN has been known to be a risk factor for mortality in variety of conditions: e.g. acute and chronic heart failure [22], coronary artery bypass graft [23], acute pancreatitis [24], pneumonia [25] and bone marrow transplant [26]. BUN is also included in general severity scores for critically ill patients [27]. Recently, Beier et al. [28] studied 26 288 adult patients with a serum creatinine of 0.80–1.30 mg/dL admitted to 20 ICUs in two teaching hospitals. They found that an elevated BUN in patients with normal creatinine was independently associated with mortality (ORs for BUN >40 mg/dL and 20–40 were 2.93 and 1.49, respectively, compared with BUN 10–20 mg/dL as a reference). Feinfeld et al. [29] studied 19 patients who acutely developed markedly increased BUN levels (>100 mg/dL) with only modest elevation of creatinine (<5 mg/dL) for possible causes of disproportionate azotemia. They found likely ‘PRA’ as a cause of azotemia only in 9 among the 19 patients. Interestingly, they also found that fractional sodium excretion was <1% only in 4 among 11 patients in whom it was measured. These observations strongly suggest that BUN is modulated by a number of mechanisms (e.g. parenteral nutrition, protein catabolism, steroid administration, gastrointestinal bleeding, etc.) and is a surrogate marker of illness severity independent of renal function. These observations provide further pathophysiological explanation of why a BCR is unlikely to help distinguish PRA from ATN.
Apart from mortality, we found that there were several differences between high BCR and low BCR that suggests that patient characteristic and the nature and severity of disease are more important determinants of the BCR than renal factors.
Strengths and limitations
This study contains several limitations. Firstly, this is a single-center study that limits its generalizability. However, it was conducted in a large academic center, which shares the typical characteristics of other similar centers in resource-rich countries. In addition, to our knowledge, this is the first study to examine the meaning of BCR in patients with AKI using a large database (including >20 000 patients). As such, it should provide useful information to help clinicians understand the nature of BCR as a diagnostic and prognostic test. Secondly, the threshold of high and low BCR used in this study (BCR of 20) was arbitrary. However, this threshold is typically used in reviews and textbooks [8]. Furthermore, we also conducted a multivariable analysis for morality dividing BCR into several subgroups and confirmed that higher BCR was related to higher mortality, with an exception of a very low BCR (<10). Thirdly, creatinine may be a less sensitive marker of AKI in sicker individuals as acute and chronic illness can reduce muscle creatinine generation rate, slowing the rate of rise in creatinine after a fall in GFR. Thus, urea may have had time to rise higher when first meeting RIFLE R in a sicker population.
Implications for clinicians
Although BCR has been used to distinguish PRA and ATN, our study suggests that it might be more useful as a prognostic indicator of mortality. The use of BCR to diagnostically separate PRA from ATN cannot be justified.
Future studies
Our study was the first to look at the clinical meaning of BCR. Our findings (relationship between high BCR and mortality) therefore need to be confirmed or refuted in other studies and in different health care systems. Future studies could focus on whether BCR, combined with other potential diagnostic tests used to separate PRA from ATN (urinary sodium, fractional excretion of sodium or fractional excretion of urea), can still offer diagnostic value in patients with AKI.
Conclusions
In conclusion, we found that approximately a half of hospital patients with AKI have a BCR >20, the traditional threshold for the diagnosing PRA. However, we found that the BCR did not have a bimodal or near bimodal distribution and that the relationship between BCR and mortality was J-shaped. Additionally, and contrary to expectations, patients with suspected functional AKI had a higher hospital mortality compared with patients with a lower BCR, a finding confirmed with multivariable analysis. These findings suggest that BCR cannot be used to distinguish functional low-mortality AKI (PRA) from structural high-mortality AKI (ATN).
Supplementary data
Supplementary data is available online at http://ckj.oxfordjournals.org.
Acknowledgments
We would like to thank Mr Harvey Sutcliffe for his assistance in obtaining the central laboratory data and Mr Peter Davey for his assistance in obtaining the admissions and discharges information.
Funding. This study was supported by the Austin Hospital Anaesthesia and Intensive Care Trust Fund.
Conflict of interest statement. None declared.
References
- 1.Liaño F, Pascual J Madrid Acute Renal Failure Study Group. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 2.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Bellomo R, Goldsmith D, et al. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 4.Klahr S, Miller SB. Acute oliguria. N Engl J Med. 1998;338:671–675. doi: 10.1056/NEJM199803053381007. [DOI] [PubMed] [Google Scholar]
- 5.Singri N, Ahya SN, Levin ML. Acute renal failure. JAMA. 2003;289:747–751. doi: 10.1001/jama.289.6.747. [DOI] [PubMed] [Google Scholar]
- 6.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 7.Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med. 2002;137:744–752. doi: 10.7326/0003-4819-137-9-200211050-00010. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal M, Swartz R. Acute renal failure. Am Fam Physician. 2000;61:2077–2088. [PubMed] [Google Scholar]
- 9.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 11.Macedo E, Mehta RL. Prerenal failure: from old concepts to new paradigms. Curr Opin Crit Care. 2009;15:467–473. doi: 10.1097/MCC.0b013e328332f6e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blantz RC. Pathophysiology of pre-renal azotemia. Kidney Int. 1998;53:512–523. doi: 10.1046/j.1523-1755.2003_t01-1-00784.x. [DOI] [PubMed] [Google Scholar]
- 13.Marshall S. Urea-creatinine ratio in obstructive uropathy and renal hypertension. JAMA. 1964;190:719–720. doi: 10.1001/jama.1964.03070210025004. [DOI] [PubMed] [Google Scholar]
- 14.Dossetor JB. Creatininemia versus uremia. The relative significance of blood urea nitrogen and serum creatinine concentrations in azotemia. Ann Intern Med. 1966;65:1287–1299. doi: 10.7326/0003-4819-65-6-1287. [DOI] [PubMed] [Google Scholar]
- 15.Morgan DB, Carver ME, Payne RB. Plasma creatinine and urea: creatinine ratio in patients with raised plasma urea. Br Med J. 1977;2:929–932. doi: 10.1136/bmj.2.6092.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tariq M, Memon M, Jafferani A, et al. Massive fluid requirements and an unusual BUN/creatinine ratio for pre-renal failure in patients with cholera. PLoS One. 2009;4:e7552. doi: 10.1371/journal.pone.0007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian J, Barrantes F, Amoateng-Adjepong Y, et al. Rapid reversal of acute kidney injury and hospital outcomes: a retrospective cohort study. Am J Kidney Dis. 2009;53:974–981. doi: 10.1053/j.ajkd.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Uchino S, Bellomo R, Bagshaw SM, et al. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–1839. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 19.Bagshaw SM, Langenberg C, Bellomo R. Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48:695–705. doi: 10.1053/j.ajkd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Bagshaw SM, Langenberg C, Wan L, et al. A systematic review of urinary findings in experimental septic acute renal failure. Crit Care Med. 2007;35:1592–1598. doi: 10.1097/01.CCM.0000266684.17500.2F. [DOI] [PubMed] [Google Scholar]
- 21.Bellomo R, Bagshaw S, Langenberg C, et al. Pre-renal azotemia: a flawed paradigm in critically ill septic patients? Contrib Nephrol. 2007;156:1–9. doi: 10.1159/000102008. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC, Adams KF, Jr, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 23.Hartz AJ, Kuhn EM, Kayser KL, et al. BUN as a risk factor for mortality after coronary artery bypass grafting. Ann Thorac Surg. 1995;60:398–404. doi: 10.1016/0003-4975(95)00358-r. [DOI] [PubMed] [Google Scholar]
- 24.Wu BU, Bakker OJ, Papachristou GI, et al. Blood urea nitrogen in the early assessment of acute pancreatitis: an international validation study. Arch Intern Med. 2011;171:669–676. doi: 10.1001/archinternmed.2011.126. [DOI] [PubMed] [Google Scholar]
- 25.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 26.Bacigalupo A, Oneto R, Bruno B, et al. Early predictors of transplant-related mortality (TRM) after allogeneic bone marrow transplants (BMT): blood urea nitrogen (BUN) and bilirubin. Bone Marrow Transplant. 1999;24:653–659. doi: 10.1038/sj.bmt.1701953. [DOI] [PubMed] [Google Scholar]
- 27.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 28.Beier K, Eppanapally S, Bazick HS, et al. Elevation of blood urea nitrogen is predictive of long-term mortality in critically ill patients independent of “normal” creatinine. Crit Care Med. 2011;39:305–313. doi: 10.1097/CCM.0b013e3181ffe22a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinfeld DA, Bargouthi H, Niaz Q, et al. Massive and disproportionate elevation of blood urea nitrogen in acute azotemia. Int Urol Nephrol. 2002;34:143–145. doi: 10.1023/a:1021346401701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.