Abstract
Caffeine is the most widely consumed psychoactive substance worldwide. Intoxication causes central nervous system and haemodynamic complications, which have significant mortality rates. We report the case of a 39-year-old woman who ingested ∼0.5 mol (100 g) of pure caffeine, leading to a peak serum concentration of 2.95 mmol/L (574 mg/L). Three consecutive haemodialysis sessions caused serum caffeine reduction rates of 66, 46 and 45%, indicating that the unbound caffeine fraction is not dose linear in this high serum caffeine concentration range. Aggressive and repeated haemodialysis sessions may be of benefit in cases of severe caffeine intoxication.
Keywords: caffeine, haemodialysis, intoxication
Background
Caffeine (1,3,7-trimethylxanthine, molecular weight 194 g/mol) is the most widely consumed psychoactive compound worldwide. A 150 mL cup of ground-roasted coffee contains 0.44 mmol (85 mg) caffeine on average and the daily caffeine intake of US coffee drinkers has been estimated to be ∼0.02 mmol/kg (4 mg/kg). The lethal dose50 (LD50) of caffeine is 0.8–1.0 mmol/kg (150–200 mg/kg) in rats and presumably in the same range in humans [1]. Serum concentrations of >0.5 mmol/L (100 μg/mL) are considered lethal [2]. Caffeine overdosing often occurs via caffeine tablets, either accidentally or with suicidal intention. Haemodialysis has previously been administered in cases of caffeine intoxication [3].
Case report
A 39-year-old female ingested ∼500 caffeine tablets dissolved in water (200 mg caffeine/tablet, i.e. 0.5 mol = 100 g) in an attempt to commit suicide. One hour after ingestion, a generalized tonic–clonic seizure was successfully treated by the emergency medical service. Subsequently, three episodes of broad complex tachycardia were treated with cardiopulmonary resuscitation, defibrillations, epinephrine and amiodarone. Upon arrival at the hospital, the patient was intubated and was in sinus rhythm with a blood pressure of 62/33 mmHg and a heart rate of 140/min. Activated charcoal (∼60 g) was administered via gastric tube, and multiple ventricular extrasystoles were treated with intravenous magnesium and calcium. To treat hypotension, the patient received 9 L of crystalloids and repeated epinephrine boluses followed by a continuous infusion. Laboratory data are given in Table 1. Lactic acidosis was treated with intravenous sodium bicarbonate. Haemodialysis was initiated when the patient was stabilized ∼5 h after caffeine ingestion, and blood samples were drawn before, at the end of and 1 hour after the end of the first three dialysis sessions for the determination of serum caffeine concentrations. Because of recurrent cardiovascular instability and concomitant lactic acidosis, epinephrine was replaced by dobutamine. Given the development of hypotension- and rhabdomyolysis-induced acute renal failure (creatine kinasemax 69 885 U/L, aspartate transaminasemax 1908 U/L), volume overload and persistent haemodynamic instability, chronic veno-venous haemodiafiltration (CVVHDF) was initiated on Day 2. However, when the pending serum caffeine concentration measurements were reported on Day 3, daily haemodialysis therapy was immediately recommenced.
Table 1.
Serum concentrations of parameters of interest
| Reference | Day 1 | Day 2 | Day 3 | Day 4 | |
| Sodium (mmol/L) | 135–145 | 147 | 137 | 136 | 134 |
| Potassium (mmol/L) | 3.5–4.7 | 3.2 | 3.9 | 4.9 | 4.7 |
| Phosphate (mmol/L) | 0.74–1.55 | 2.53 | 1.13 | 1.54 | 0.69 |
| Glucose (mmol/L) | 3.33–5.55 | 10.6 | 8.7 | 5.1 | 4.6 |
| Creatinine (μmol/L) | 45–84 | 85 | 153 | 126 | 100 |
| AST (U/L) | <32 | 158 | 1353 | 1908 | 1208 |
| ALT (U/L) | <37 | 124 | 374 | 629 | 485 |
| Creatine kinase (U/L) | <170 | 244 | 19 874 | 69 885 | 20 022 |
| pH | 7.35–7.45 | 6.65 | 7.44 | 7.47 | 7.47 |
| Bicarbonate (mmol/L) | 18–29 | 7.5 | 21.8 | 22.8 | 28.4 |
| Lactate (mmol/L) | 0.63–2.44 | 14.9 | 7.3 | 1.5 | 1.4 |
AST, aspartate transaminase; ALT, alanine transaminase. The concentrations of all parameters were determined ‘before’ haemodialysis.
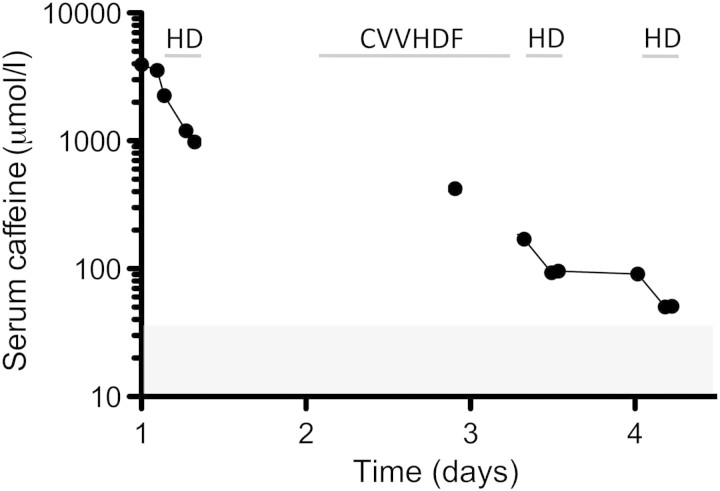
The time course of serum caffeine concentrations is shown in Figure 1. Dialysis sessions with polysulfone dialyser F80S (Fresenius FMC, Bad Homburg, Germany), blood and dialysis flow rates of 300 mL/min and 600 mL/min, respectively, lasted 4 h each. Serum caffeine reduction rates of 66, 46 and 45% were observed during the first three dialysis sessions. This corresponds to total caffeine clearance values of 159, 89 and 87 mL/min assuming a distribution volume of 0.6–0.8 L/kg (∼35 L in our patient). With no rebound observed after dialysis sessions, a one-compartment distribution of caffeine can be assumed.
Fig. 1.
Serum caffeine concentrations. Time course of serum caffeine concentrations as measured by high-performance liquid chromatography. The shaded area indicates serum caffeine concentrations typically measured after intake of 1.3 mmol (250 mg) caffeine, i.e. approximately three cups of regular coffee.
Following a complicated course of treatment, in which pulmonary failure and tricuspid endocarditis likely resulting from line sepsis occurred, renal function improved weeks later and haemodialysis therapy was stopped after a total of 14 haemodialysis sessions.
Discussion
The consumption of ∼0.5 mol (100 g) of pure caffeine is equivalent to ∼1200 cups of regular coffee and resulted in a caffeine serum concentration of 2.95 mmol/L (574 mg/L). It is the highest caffeine overdose which has reportedly been survived to date. By contrast, in another reported case, the ingestion of 10 g caffeine proved fatal [4]. Peak caffeine serum concentrations after drinking three cups of coffee (1.29 mmol, 250 mg caffeine) are in the range of 0.04 mmol/L (7 mg/L) [5].
The toxic effects of caffeine are mainly related to the functional deteriorations of the central nervous and adrenergic systems. The structural similarity between caffeine and adenosine leads to a non-selective antagonism of the adenosine receptor subtypes A1 and A2 by caffeine resulting in seizures [6]. The adrenergic effects are caused by catecholamine release from the adrenals and inhibition of the enzyme phosphodiesterase. Clinical consequences are tachyarrhythmias (β1-stimulation), vasodilation and hypotension, hypokalaemia, leucocytosis and glycogenolysis (all β2-stimulation) and lipolysis (β3-stimulation). Although beta-blocker therapy has been advocated [7], caution is warranted because beta-blockers may unmask massive alpha-receptor stimulation and trigger malignant hypertension in analogy to pheochromocytoma [3].
Lactic acidosis and rhabdomyolysis have been reported in caffeine overdosing. Lactic acidosis is caused by shock-induced tissue hypoxia. Rhabdomyolysis is presumably triggered by a combination of ischaemia, seizures and tetany mediated by intracellular calcium release from the endoplasmic reticulum [8].
Caffeine is normally absorbed within 30 min [5], but it is not known whether a dose of 0.5 mol (100 g) is as rapidly and completely absorbed. Active charcoal may thus be effective even when given with a time lag after caffeine ingestion. Systemically, caffeine is demethylated to paraxanthine (80%), theobromine (11%) and theophylline (4%) [9] by the hepatic cytochrome P450 CYP1A2 system [10]. Demethylation occurs with a half-time of 4 h [5] and the toxicity of the four methylxanthines ascends in the order paraxanthine < theobromine = caffeine < theophylline [11]. The pharmacodynamic effects of paraxanthine and theobromine have not been elucidated in detail but likely resemble those of caffeine. Plasma protein binding of caffeine is 36%; together with its volume of distribution of 0.6–0.8 L/kg, this implies that caffeine can be removed from the bloodstream by haemodialysis. Haemodialysis has previously been administered in the treatment of caffeine intoxication [3]. In our case, a significantly higher proportion of caffeine was eliminated during the first dialysis session than during the next two. Assuming some ongoing absorption during the first round of dialysis, the caffeine reduction rate during the initial dialysis should have been, if anything, lower than by the sessions after conclusion of the absorption. We therefore assume that the unbound fraction is no longer dose linear in this high serum caffeine concentration range and that a significantly higher amount of unbound drug was available for dialysis. As there is no danger of disequilibrium syndrome in acute intoxication of patients with previously normal renal function, haemodialysis was started with a blood flow as high as the haemodynamic situation permitted. CVVHDF is a widely available modality of renal replacement therapy in intensive care units. While this method is an adequate choice for the removal of excess fluid and uraemic toxins, its clearing capacity in severe cases of caffeine intoxications does not suffice. Accordingly, CVVHDF was clearly less effective than haemodialysis for the elimination of caffeine (Figure 1). However, we do not have sufficient data to estimate any clearance values during CVVHDF in our patient.
Toxin removal is a basic treatment principle of intoxication. The risk of line sepsis and ensuing tricuspid endocarditis is related to catheter insertion and haemodialysis treatment. In weighing this risk against the risk of not removing the toxin, we advocate haemodialysis treatment for severe caffeine intoxication. The therapeutic approach needs to be based on the clinical picture at presentation (haemodynamic instability, ingested caffeine amount more than ∼10 g) and re-evaluated in the further course of treatment based on clinical course, serum caffeine concentrations (LD50 0.5 mmol/L) [2] and renal function.
In summary, we report on the clinical course and complications of severe caffeine intoxication in a 39-year-old woman. Based on longitudinally determined serum caffeine concentrations and pharmacokinetic modelling, aggressive and repeated haemodialysis sessions may be of benefit in cases of severe caffeine intoxication.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Peters J. Factors affecting caffeine toxicity: a review of the literature. J Clin Pharmacol. 1967;7:131–141. [Google Scholar]
- 2.Winek CL, Wahba WW, Winek CL, Jr, et al. Drug and chemical blood-level data 2001. Forensic Sci Int. 2001;122:107–123. doi: 10.1016/s0379-0738(01)00483-2. [DOI] [PubMed] [Google Scholar]
- 3.Kapur R, Smith MD. Treatment of cardiovascular collapse from caffeine overdose with lidocaine, phenylephrine, and hemodialysis. Am J Emerg Med. 2009;27:253.e3–253.e6. doi: 10.1016/j.ajem.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph T, Knudsen K. A case of fatal caffeine poisoning. Acta Anaesthesiol Scand. 2010;54:521–523. doi: 10.1111/j.1399-6576.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan GB, Greenblatt DJ, Ehrenberg BL, et al. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37:693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 6.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt A, Karlson-Stiber C. Caffeine poisoning and lactate rise: an overlooked toxic effect? Acta Anaesthesiol Scand. 2008;52:1012–1014. doi: 10.1111/j.1399-6576.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 8.Huddart H, Abram RG. Modification of excitation-contraction coupling in locust skeletal muscle induced by caffeine. J Exp Zool. 1969;171:49–58. doi: 10.1002/jez.1401710108. [DOI] [PubMed] [Google Scholar]
- 9.Lelo A, Miners JO, Robson RA, et al. Quantitative assessment of caffeine partial clearances in man. Br J Clin Pharmacol. 1986;22:183–186. doi: 10.1111/j.1365-2125.1986.tb05247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tassaneeyakul W, Birkett DJ, McManus ME, et al. Caffeine metabolism by human hepatic cytochromes P450: contributions of 1A2, 2E1 and 3A isoforms. Biochem Pharmacol. 1994;47:1767–1776. doi: 10.1016/0006-2952(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 11.Berkowitz BA, Spector S. Effect of caffeine and theophylline on peripheral catecholamines. Eur J Pharmacol. 1971;13:193–196. doi: 10.1016/0014-2999(71)90150-6. [DOI] [PubMed] [Google Scholar]