Abstract
When endoscopic surgery is indicated for myasthenia gravis and thymomas, most institutions use a lateral thoracic approach that includes robot-assisted surgery. However, with the unilateral thoracic approach, it can be difficult to ensure the operative field in the neck and difficult to identify the location of the contralateral phrenic nerve. In 2015, we reported on a robotic subxiphoid thymectomy (RST) in which the camera is inserted from the subxiphoid incision and robotic forceps are inserted from the bilateral intercostal spaces. With this approach, a camera is inserted into a subxiphoid incision which is the midline of the body and a surgical field comparable to that in a median sternotomy can be achieved. This makes it easier to identify the location of the bilateral phrenic nerves and offer the good visualization in the neck area. Here we report on our RST techniques. For a thymectomy without suturing, a subxiphoid, single-port thymectomy is performed because it is minimally invasive. In patients who require suturing, such as with a pericardial patch closure, RST is selected. The RST has excellent operability when performed with a robot, making it suitable for more difficult procedures. In the future, we believe that a robot-assisted thymectomy might become the standard method.
Keywords: Thymectomy, subxiphoid, thoracoscopy/VATS, robot
Introduction
When endoscopic surgery is indicated for myasthenia gravis and thymomas, most institutions use a lateral thoracic approach that includes robot-assisted surgery (1-3). However, with the unilateral thoracic approach, it can be difficult to ensure the operative field in the neck and difficult to identify the location of the contralateral phrenic nerve. Previously, we reported on a single-port thymectomy using a subxiphoid approach to extract the thymus from a single subxiphoid incision (4). The operative field from the camera inserted through the midline of the body made it easy to verify the neck area and identify the location of the bilateral phrenic nerves. However, a shortcoming of this approach is operability. With surgery through a single incision, there is interference between the camera scope and the forceps in the surgeon’s hands, making it difficult to perform more complex procedures such as suturing operations.
In 2003, a robot-assisted surgery for myasthenia gravis and anterior mediastinal tumors was reported (5), and in recent years good outcomes with such surgery have been reported (6). However, these robot-assisted surgical techniques use a lateral thoracic approach (7). Even with the use of a robot, the lateral thoracic approach makes it difficult to gain a good operative field of the neck and to identify the location of the contralateral phrenic nerve. Furthermore, to adequately exert the performance of the robot system, the target, i.e., the thymus, should be between the left and right arms of the robot; however, with the lateral thoracic approach, the neck portion of the thymus is not between the left and right arms. In 2015, we reported on a robotic subxiphoid thymectomy (RST) (Figure 1) (8). With this approach, a camera is inserted into a subxiphoid incision which is the midline of the body and a surgical field comparable to that in a median sternotomy can be achieved. This makes it easier to identify the location of the bilateral phrenic nerves and offer the good visualization in the neck area. Furthermore, with this approach, the left and right robot arms are inserted in the 6th intercostal space of the bilateral precordium and the entire target/thymus lies between the left and right arms, thereby enabling maximum robot performance (Figure 2) (9). In the event of suspected invasion into the left brachiocephalic vein, to perform the surgery safely, taping of the left brachiocephalic vein distal and proximal to the tumor is needed. However, with robot-assisted surgery using a lateral thoracic approach, the presence of a tumor makes it difficult to identify the contralateral left brachiocephalic vein. In contrast, with RST, the entire left brachiocephalic vein can be observed through a midline incision, and the left brachiocephalic vein may be taped distally and proximally to the tumor (Figure 3). Furthermore, in the event of tumor invasion into the pericardium, an endoscopic pericardial incision and patch closure with a pericardial sheet is a highly difficult procedure when performed endoscopically with human hands; however, if the robot-assisted system with articulated forceps is used, it is very easy (9). In cases where the left brachiocephalic vein is taped distally and proximally to the tumor, or a concurrent pericardial resection and reconstruction is required, it is generally highly likely that endoscopic surgery will not be indicated; however, if a robot is used they can become minimally invasive procedures. As robot-assisted surgery is expensive, the benefits to the patient should balance the high cost. In general, we indicate robot-assisted surgery for highly complicated procedures that are difficult for human hands to perform endoscopically. RST helps secure the operative field of the neck and facilitates the identification of the location of the bilateral phrenic nerves. Furthermore, it has excellent operability when performed with a robot, making it suitable for more difficult procedures. In the future, we believe that a robot-assisted thymectomy might become the standard method. Here we report on our RST technique.
Figure 1.
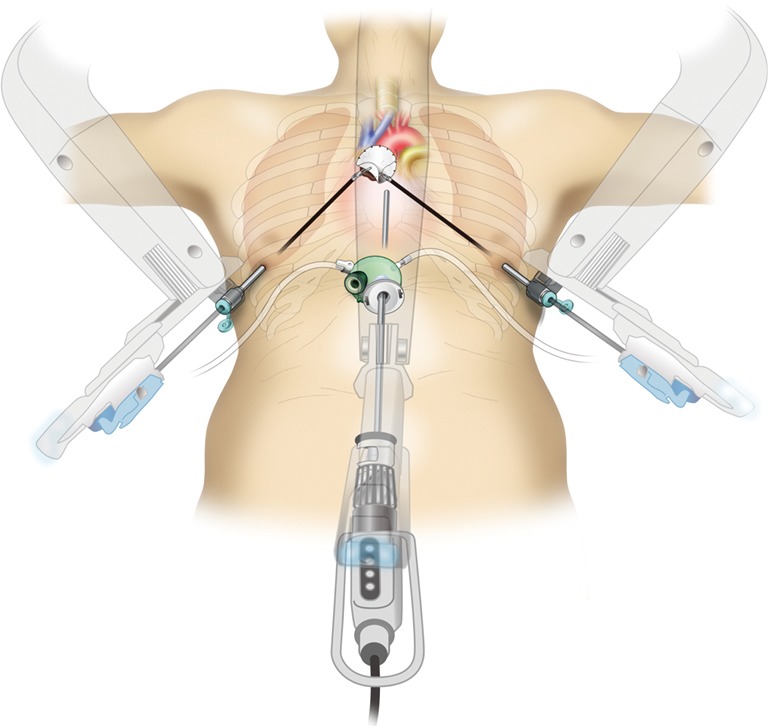
Robotic subxiphoid thymectomy (RST). A pericardial resection and substitution with an artificial pericardial sheet are very easy with an articulated robotic system.
Figure 2.
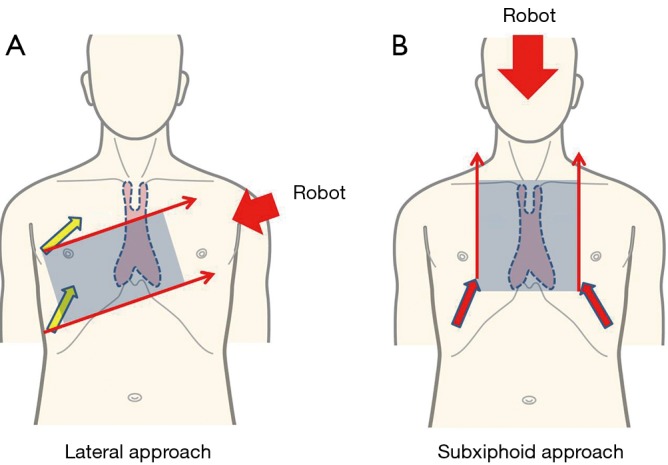
With the approach from the lateral thoracic side, the neck portion of the thymus is not between the left and right arms (A). In contrast, with the subxiphoid approach, the entire thymus is between the left and right arms, thereby enabling good robotic operability (B) (9).
Figure 3.
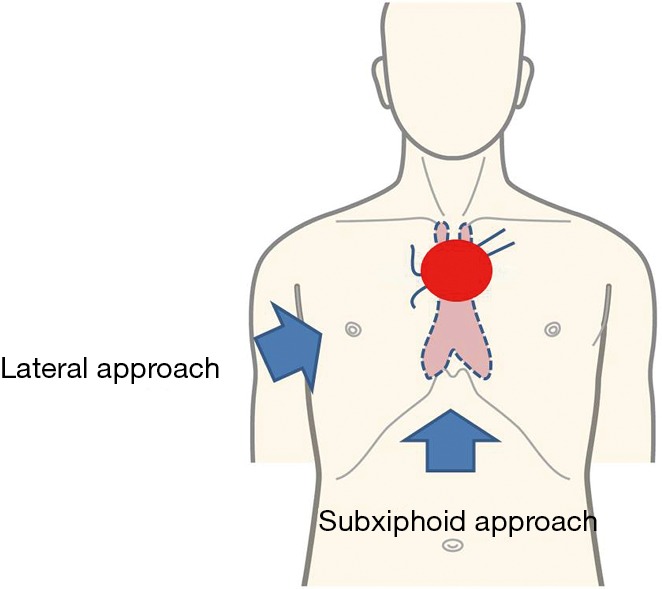
When the tumor is close to the brachiocephalic vein, the approach from the lateral chest cannot identify the contralateral brachiocephalic vein beyond the tumor (Lateral approach). In contrast, with the subxiphoid approach, the left brachiocephalic vein may be identified at proximal and distal ends to the tumor (Subxiphoid approach).
Patient selection
For a thymectomy without suturing, a subxiphoid, single-port thymectomy is performed because it is minimally invasive. In patients who require suturing, such as with a pericardial patch closure, dual-port thymectomy with an additional port placed in the right 5th intercostal space along with a subxiphoid single-port thymectomy (10) or RST is selected.
Pre-operative preparation
The patient is placed in the supine position. The arms are open so as not to be hit by the robot arms. The configuration of the equipment when using the da Vinci SI surgical system (Intuitive Surgical, Sunnyvale, CA, USA) is shown in Figure 4.
Figure 4.
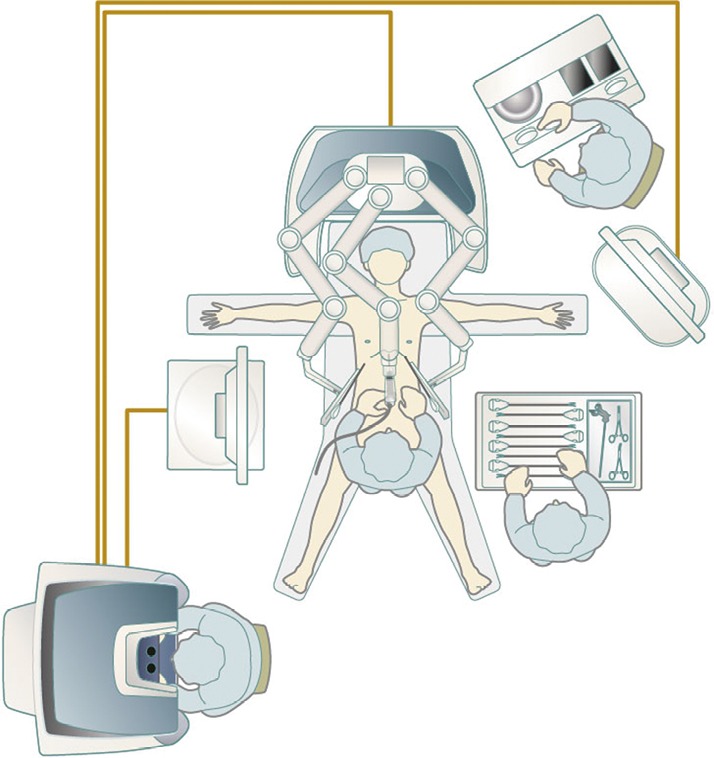
Equipment configuration with the da Vinci SI surgical system.
Equipment preference card
We use the da Vinci SI surgical systems, which are capable of using vessel sealers as the vessel-sealing system. We use the GelPOINT Mini (Applied Medical, Rancho Santa Margarita, CA, USA) as the port for a single-port surgery. The GelPOINT Mini has a gel seal on the port platform, which prevents over-fixation of the mini-ports and decreases interference of the instruments once inserted. It also enables of CO2 insufflation. The surgeon performs a pre-docking procedure using the vessel-sealing device. There are various types of vessel-sealing devices; however the LigaSureTM Maryland Jaw 37 cm (Covidien, Mansfield, MA, USA) device with a dissecting shaped tip is suitable for this surgery.
Forceps mounted on the robotic arms include Cadiere forceps or fenestrated grasping forceps attached to the bipolar vessel-sealing device on the left hand arm, and Maryland forceps attached to the bipolar vessel-sealing device or spatula attached to a monopolar vessel-sealing device on the right hand arm. A needle holder is used when suturing. The da Vinci surgical system can use four arms; however, to decrease the number of incisions, we do not use the 4th arm.
Procedure
Pre-docking procedure
Under general anesthesia, artificial ventilation is performed using a double-lumen endotracheal intubation tube. The surgeon performs the surgery standing between the legs of the patient, while the scopist stands to the right of the patient to operate the thoracoscope. To begin with, a 3-cm transverse incision is made 1 cm below the xiphoid process, and the rectus abdominis is dissected at its attachment to the xiphoid process. The posterior aspect of the sternum is blindly detached using the finger. A 5-mm vertical incision is made on the fascia of the rectus abdominis, the GelPOINT Mini-port for a single-port surgery is inserted into the subxiphoid incision and CO2 insufflation is performed at 8 mmHg. Using a rigid scope of 5 mm in diameter with 30° oblique view and using a LigaSureTM Maryland type device, the thymus is detached from the posterior aspect of the sternum. An incision is made into the bilateral mediastinal pleura and the thoracic cavity is exposed bilaterally. Next, 1-cm skin incisions are made on either side in the 6th intercostal space along the anterior axillary line of the precordium, and a port used for the da Vinci robotic surgery is inserted (Figure 5).
Figure 5.

Pre-docking procedure (11). Available online: http://www.asvide.com/articles/1039
RST
When using the da Vinci SI surgical system, the system is docked from the cranial side (Figure 4). A port used for a 12-mm camera is inserted into the subxiphoid port for a single-port surgery and attached to the da Vinci camera scope. The da Vinci arms are then attached to the bilateral ports in the 6th intercostal space along the anterior axillary line of the precordium. The camera scope is used changing opportunely from direct-forward viewing to a 30° oblique viewing. At times, the assistant will expand the surgical field by pulling the thymus using an Autonomy Grasper 45 cm (Cambridge Endo, Framingham, MA, USA), which are forceps for single-port surgery, or a SILSTM Hand Instrument SILS Clinch 36 cm (Covidien, Mansfield, MA, USA). The thymic vein is dissected using an EndoWrist Vessel Sealer (Intuitive Surgical, Sunnyvale, CA, USA). The thymus and thymoma are placed in a pouch inside the mediastinum and extracted via the subxiphoid incision. A 20-Fr drain is inserted through the subxiphoid incision into the mediastinum (Figure 6).
Figure 6.

Robotic subxiphoid thymectomy (RST) (12). Available online: http://www.asvide.com/articles/1040
In the event of a suspected tumor infiltration of the lungs, a stapler is inserted through the subxiphoid port or the bilateral lateral thoracic ports, and a partial lung resection is performed. In recent years, it has become possible to attach a stapler to the da Vinci surgical system. An articulated stapler will facilitate surgery in a narrow mediastinum and thoracic cavity. In the event that pericardial invasion is suspected and a pericardial patch closure is performed, the incision is performed after the pericardium has been adequately detached from the tumor, and the pericardium is dissected using the EndoWrist Vessel Sealer. The defective portion of the pericardium is closed by a patch using a Gor-tex pericardial sheet with 3–0 Vicryl interrupted sutures (Figure 1). The interrupted sutures can be easily performed using the articulated robotic forceps (Figure 7).
Figure 7.

Robotic pericardial patch closure by the subxiphoid approach (13). Available online: http://www.asvide.com/articles/1041
Role of team members
For a safe robotic surgery, procedures must be performed by a surgical team consisting of a surgeon, anesthesiologist, engineer, and nurse, all of whom must be well-skilled in robotic surgery. When performing a new surgery, the entire team must meet in advance to perform a simulation.
Tips, tricks, and pitfalls
When using the da Vinci SI surgical system, the robot is docked from the cranial side. At this point in time, the authors have no experience in this approach using the daVinci Xi system. However, with the da Vinci Xi system the robotic arms are mounted on the ceiling and therefore docking can be performed from the lateral side of the patient. If docking from the lateral side of the patient can be performed, the robot does not lie over the patient’s head, ensuring that a space is available near the head of the patient for the anesthesiologist.
For the endotracheal intubation tube, if there is no lung invasion, a single-lumen tube can be used. However, if there is suspected tumor invasion of the lungs, a double-lumen tube is chosen to enable differential lung ventilation. When establishing artificial ventilation, pressure control ventilation delivered at the minimal intratracheal pressure is used to ensure sufficient ventilation. PEEP is not used as it inflates the lungs and disturbs the operative field. CO2 insufflation in the mediastinum at 8 mmHg provides a good operative field by maintaining lung ventilation and eliminating pressure moderately on the bilateral lungs.
In CO2 insufflation, when using suction, the supplied CO2 is aspired, which inflates the lungs and worsens the surgical operative field. As an alternative to suction, a gauze roll should be placed in the mediastinum to wipe up blood as required. The AirSeal system (SurgiQuest, Milford, CT, USA) is a CO2 insufflation system with which suction can be used, and thus might be useful for robotic surgery.
In the event that pulling the thymus is desired to enlarge the operative field, the assistant does so by inserting forceps with an articulated tip through the subxiphoid port; however, if this is difficult to do, then an additional port can be placed on the lateral chest. Although we do not use this practice, if necessary, a 4th robotic arm may be used.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. 10.1016/S0003-4975(10)62457-5 [DOI] [PubMed] [Google Scholar]
- 2.Landreneau RJ, Dowling RD, Castillo WM, et al. Thoracoscopic resection of an anterior mediastinal tumor. Ann Thorac Surg 1992;54:142-4. 10.1016/0003-4975(92)91162-3 [DOI] [PubMed] [Google Scholar]
- 3.Detterbeck FC, Kim AW, Zielinski M. Looking in from above and up from below: new vistas in thoracic surgery. Innovations (Phila) 2012;7:161-4. 10.1097/IMI.0b013e31826145b1 [DOI] [PubMed] [Google Scholar]
- 4.Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. 10.1016/j.athoracsur.2011.08.047 [DOI] [PubMed] [Google Scholar]
- 5.Ashton RC, Jr, McGinnis KM, Connery CP, et al. Totally endoscopic robotic thymectomy for myasthenia gravis. Ann Thorac Surg 2003;75:569-71. 10.1016/S0003-4975(02)04296-0 [DOI] [PubMed] [Google Scholar]
- 6.Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. 10.1016/j.jtcvs.2010.11.042 [DOI] [PubMed] [Google Scholar]
- 7.Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. 10.1016/j.jtcvs.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 8.Suda T, Tochii D, Tochii S, Takagi Y. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. 10.1093/icvts/ivv001 [DOI] [PubMed] [Google Scholar]
- 9.Suda T. Subxiphoid Robotic Extended Thymectomy With a Pericardial Patch Closure. CTSNet. Available online: http://www.ctsnet.org/article/subxiphoid-robotic-extended-thymectomy-pericardial-patch-closure
- 10.Suda T, Ashikari S, Tochii D, et al. Dual-port thymectomy using subxiphoid approach. Gen Thorac Cardiovasc Surg 2014;62:570-2. 10.1007/s11748-013-0337-y [DOI] [PubMed] [Google Scholar]
- 11.Suda T. Pre-docking procedure. Asvide 2016;3:277. Available online: http://www.asvide.com/articles/1039
- 12.Suda T. Robotic subxiphoid thymectomy (RST). Asvide 2016;3:278. Available online: http://www.asvide.com/articles/1040
- 13.Suda T. Robotic pericardial patch closure by the subxiphoid approach. Asvide 2016;3:279. Available online: http://www.asvide.com/articles/1041


