Abstract
Endoscopic ultrasound (EUS) is a well-established tool for the evaluation of pancreatic lesions. Due to the closer proximity of EUS to the pancreas, EUS offers a high sensitivity for detection of small pancreatic mass and is the preferred modality for obtaining tissue for diagnosis of pancreatic mass. Contrast-enhanced EUS and/or elastography provide additional information to the fundamental B—mode ultrasound images, leading to more accurate diagnosis. The aim of this video-article is to show the different steps in performing EUS on pancreatic lesions and to provide some tips and tricks to improve and facilitate the execution of EUS on pancreatic lesions.
Keywords: Endoscopic ultrasound (EUS), endoscopic ultrasonography, fine needle aspiration (FNA), pancreas
Introduction
Since its first introduction into clinical practice in 1980, endoscopic ultrasound (EUS) has clearly established itself as an important diagnostic tool for a wide range of pancreatic lesions, from solid to cystic lesions (1). One of the earliest successful applications was the detection of small pancreatic neoplasms, where the performance of EUS was shown to be superior than endoscopic retrograde cholangiopancreatography (ERCP), computed tomography (CT) and transabdominal ultrasound (2,3).
Echoendoscopes can be categorized into radial and linear types. Radial echoendoscope produces ultrasound images in a plane that transects the axis of the scope (Figure 1). It was the first to be developed and is used for diagnostic imaging. Linear echoendoscope produces ultrasound images in the plane that lies along the axis of the scope (Figure 2). It is used to facilitate image guided tissue sampling and intervention.
Figure 1.
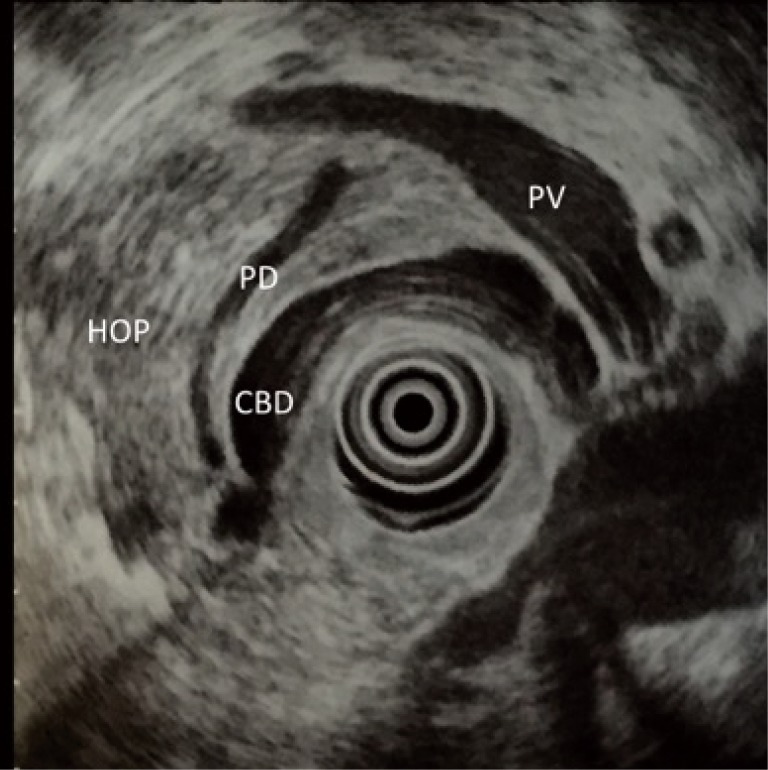
Radial endoscopic ultrasound (EUS) image of pancreatic head. PV, portal vein; CBD, common bile duct; PD, pancreatic duct; HOP, head of pancreas.
Figure 2.
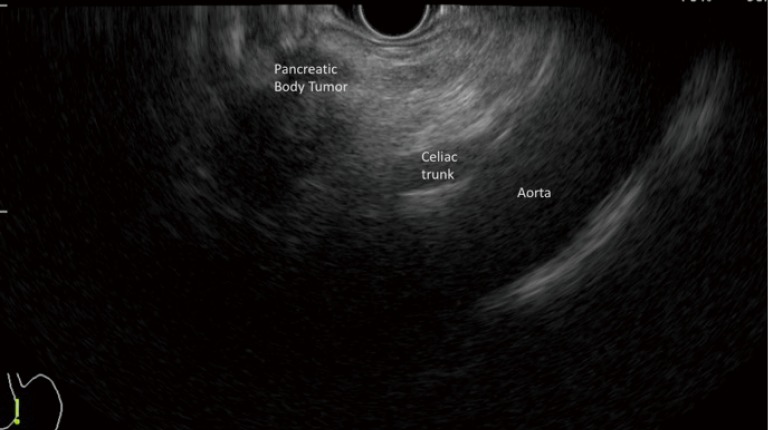
Image of linear endoscopic ultrasound (EUS) showing a pancreatic body tumor and its relationship with aorta and celiac trunk.
EUS is now used as a primary (i.e., initial imaging modality) or secondary (i.e., in the assessment of abnormalities detected by other imaging modalities) diagnostic tool. It is essential in the diagnosis of a wide variety of pancreatic lesions, as well as for tumour staging. This article describes how EUS is performed for pancreatic lesions.
Patient selection and workup
In patients presenting with symptoms concerning for pancreatic neoplasms, non-invasive cross-sectional imaging modalities such as CT or magnetic resonance imaging (MRI) are often the initial step in the evaluation. EUS can add important information to guide clinical management in patients with suspected pancreatic lesions, and can be safely performed in patients without conventional contraindications for endoscopy.
Previous studies have shown that EUS has a higher sensitivity for detecting a pancreatic mass lesion and preoperative tumor staging in patients with suspected non-metastatic pancreatic cancer when compared with CT (3,4). If an obvious pancreatic lesion is observed on cross-sectional imaging, EUS can provide important information for tumor staging and/or tissue diagnosis by fine needle aspiration (FNA). If a pancreatic lesion is highly suspected, but cannot be clearly detected on initial cross-sectional imaging, EUS is particularly valuable in detecting small pancreatic lesions. In a systematic review of 66 studies, EUS has been shown to be the most sensitive and specific investigation technique in identifying pancreatic lesions <2 cm when compared to other imaging modalities (5).
Whether tissue acquisition by FNA is necessary depends upon the stage of the pancreatic tumor and the practice of the individual institution. In patients with inoperable pancreatic cancer, tissue diagnosis by FNA is usually preferred before subjecting the patient to chemotherapy. On the contrary, it is still debatable whether FNA should be performed for potentially resectable small pancreatic tumors in surgically fit patients given concern for tumor seeding along the needle tract of FNA and the fact that a negative FNA may not entirely exclude malignancy. However, if there is a concern for a benign alternative diagnosis such as pseudotumor due to autoimmune pancreatitis, then FNA of such potentially resectable lesion should be considered to clarify the diagnosis.
Pre-operative preparation
Prior to performing EUS, a review of patient symptoms and investigation results should be conducted to optimize procedural results and minimize adverse events. Large pancreatic head lesions may result in gastric and duodenal obstructions, increasing the risk of high gastric residual despite fasting, and aspiration risk during EUS. Pancreatic malignancies may also be associated with splenic vein thrombosis, predisposing to gastric varices development, which maybe complicated by gastrointestinal bleeding. More advanced disease could result in ascites, which may influence patient positioning during EUS.
On bloodwork, thrombocytopenia as a result of splenomegaly from splenic vein thrombosis should be noted if interventional procedures such as FNA, EUS guided biliary access are to be performed. Ideally, cross sectional imaging either CT or MRI is performed prior to EUS. Pre procedural imaging review can help the endoscopist anticipate findings at the various stations during EUS, particularly important when interventional procedures are planned.
When FNA is required, lesion location on imaging can help initially decide on size of FNA needle to be used. For celiac plexus/ganglia neurolysis, tumor involvement of the celiac axis on CT or MRI can alert the endoscopist the expected site for neurolysis will be different. For EUS guided biliary interventions, deciding between hepatogastrostomy versus choledochoduodenostomy can be aided by the extent of biliary dilation on imaging.
Equipment preference card
The conventional equipments for EUS include radial and linear echoendoscope, high-end ultrasound platforms, fine needles with different gauges, e.g., 19 G, 22 G and 25 G. For contrast-enhanced EUS, contrast agent, like SonoVue®, will be necessary.
If planned for advanced procedures like pseudocyst drainage or biliary drainage, additional materials, including fluoroscopy, carbon dioxide insufflation, guidewires of different calibers and stents with different shapes and sizes, will be necessary.
Procedure
Conventional EUS (radial and linear)
Procedure will be performed with patient in left lateral position.
Radial EUS (Figure 3)
Figure 3.

Examination of pancreas with radial endoscopic ultrasound (EUS) (6). Available online: http://www.asvide.com/articles/1042
Radial echoendoscope produces images in cross sectional orientation. Techniques involved are usually easier to capture because images are orientated in a similar way as CT which we should be familiar with. Pancreatic body and tail are best examined from the stomach while pancreatic head and uncinate process are best examined from duodenum. For the pancreas examination, we use station approach. Table 1 summarized the basic scanning positions, visualized regions and the landmarks.
Table 1. Stations and landmarks for orientation and scanning in EUS examination of pancreas.
| Station | Visualized regions | Landmarks |
|---|---|---|
| Stomach | Pancreatic body; pancreatic tail | Splenic vessels; left kidney; superior mesenteric vessels; celiac trunk; aorta |
| Duodenal bulb | Pancreatic head; pancreatic body; bile duct; gallbladder | PV; SMV; splenic vein |
| Descending part of duodenum | Pancreatic head; pancreatic genu; major papilla; gallbladder | Aorta; inferior vena cava; superior mesenteric vessels; PV |
EUS, endoscopic ultrasound; PV, portal vein; SMV, superior mesenteric vein.
The first station is from the stomach, at the esophagogastric junction (OGJ). Left lobe of liver readily seen when the probe is placed at OGJ. Rotate the scope clockwise until aorta is seen at the 6 o’clock position. Then advance the scope, follow the aorta until the celiac take off is seen, which will then bifurcate into hepatic artery on the left side of the screen and splenic artery on the right side of the screen. Once the splenic artery is detected, follow it with slightly clockwise turn and pulling out of the scope. This movement allows you to examine the pancreatic body and tail all the way towards the splenic hilum. Main pancreatic duct (PD) can be visualized with back-and-forth movement of the scope. At the splenic hilum, we follow the splenic vein back to the genu of pancreas with counter-clockwise and advance movement. Splenic vein can be traced from splenic hilum back to the splenic vein and portal vein (PV) confluence, which is also called the club-head view.
The second station is from the duodenal bulb. Insert the scope into the duodenal bulb, aspirate air and inflate the balloon. We can start the examination while slowly withdrawing the scope. Liver will usually come into the view from the upper left-hand corner and gallbladder will be visualized between the scope and the liver. PV will be visualized at the lower left hand portion of the screen and pancreatic head is located between the scope and PV. The bile duct is visualized as a tubular structure between the PV and the scope. This area may include image of PD as tubular structures, without Doppler signals.
The third station is from the descending part of duodenum. Advance the tip of the scope to the apex of the duodenal bulb. Then rotate the “right/left” knob to the right and reduce the scope back to the short scope position as in ERCP. With a slightly right and maximum up torque, we can identify the aorta which is usually located at the left side of the screen. Slowly withdrawn the scope at this juncture will show up the uncinate process and head of the pancreas at 6 o’clock position. Here allows a detail examination of pancreatic head and uncinate.
Linear EUS
Figure 4 demonstrated the linear EUS examination in cases with carcinoma of pancreas. In linear echoendoscope, the ultrasound signals are transmitted out in a linear manner. For pancreatic examination, we usually use aorta as a starting point which is readily located by positioning the scope at the OGJ. Similar to radial examination, pancreatic examination with linear scope is mainly carried out in three positions: the stomach, duodenal bulb and the second part of duodenum. Once the scope entered OGJ, the left lobe of liver is readily visible. We then rotate the scope clockwise and we can see the hepatic vein, IVC and subsequently the abdominal aorta. From here, we move the scope in and out to locate the celiac take off. Follow the celiac trunk and advance the scope slightly to identify splenic artery. Once splenic artery is identified, rotate the scope clockwise and slightly withdraw to follow it to the splenic hilum. From these positions, we can have a close and detail examination of pancreatic neck, body and tail. After that, we trace the splenic vein back by anti-clockwise rotation and slight scope advancement. Then we will see the splenic vein joining superior mesenteric vein (SMV) to form PV. Further anti-clockwise rotation will see the PD and common bile duct (CBD), as well as the surrounding pancreatic head.
Figure 4.

Examination of pancreas with linear endoscopic ultrasound (EUS) (7). Available online: http://www.asvide.com/articles/1043
Inserting the scope into duodenal bulb can produce better image on pancreatic head. After entering the duodenal bulb, rotate the scope clockwise to see three luminal structures, i.e., PV, bile duct and common hepatic artery. Trace along the PV to the confluence of SMV allows a detail examination of the pancreatic head. In case of carcinoma of head of pancreas (HOP), we also look for any vascular invasion from this position.
At the second part of duodenum, we use the same maneuver as in ERCP to reduce the scope and rotate clockwise to see aorta and inferior vena cava. Then, follow the aorta; we slowly withdraw the scope to observe the lower part of pancreatic head and uncinate process, which is located between the aorta and the transducer.
EUS-guided FNA
Figure 5 demonstrated the steps in performing EUS-guided FNA on pancreatic body tumour. Linear echoendoscope is used for FNA. After the target lesion is endosonographically visualized, the region would be scanned for intervening vessels. Check the ultrasound image or endoscopic image to make sure that the sheath of the aspiration needle is projecting from the instrument channel. This is to protect the endoscope channel from damage from the FNA needle. If significant resistance is encountered upon insertion of the FNA needle through the channel, adjust the scope angulation until the FNA needle can be inserted smoothly without resistance. Then, check the insertion angle based on the EUS image of the sheath and measure the distance from the site of needle entry to the puncture target so that the needle would not overshoot beyond the puncture target (Figure 6). Once the lesion is penetrated, the stylet is pushed in and removed completely. The FNA needle would pass through the largest diameter possible in each lesion. Moreover, aspiration of lesion should be targeted to the periphery of the lesion or at multiple areas since the center of a cancerous mass is usually more necrotic, which may sample non-diagnostic tissue. The needle would be moved to and fro within the lesion to a total of 10–15 times. Some endosonographers use fanning technique, in which the needle is positioned at different areas within the lesion and then moved back and forth multiple times in each area to procure tissue (9). The trajectory of the needle can be altered not only by using the elevator, but also the “up/down” endoscope dial. The use of suction at EUS-guided FNA remains a hot issue of debate. In general, the use of suction at EUS-guided FNA yields specimens that are more bloody but may not have any improvement in diagnostic yield. For this reason, FNA of solid lesions can be initiated without suction. If the aspirate obtained is scant, then suction can be used to procure a better aspirate.
Figure 5.

Video showing endoscopic ultrasound (EUS) fine needle aspiration (FNA) of a pancreatic body tumor (8). Available online: http://www.asvide.com/articles/1044
Figure 6.
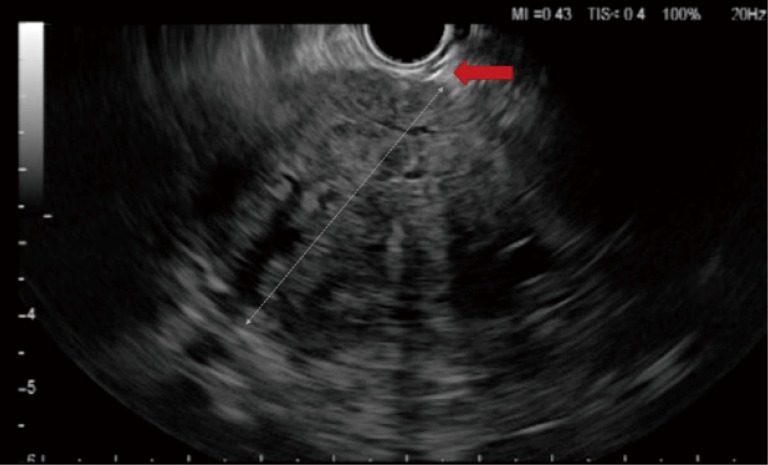
Image of linear endoscopic ultrasound (EUS) showing the sheath of fine needle aspiration (FNA) needle protruding out from the channel, indicated by the red arrow.
When FNA completed, pull the needle tube back insider the sheath and remove the FNA needle from the scope. The specimen can be pushed by reinserting the stylet into the needle. Then submit the specimen for cytology and cell black preparation for histology.
Role of team members
Diagnostic pancreatobiliary EUS with or without FNA can be performed by gastroenterologists or surgical endoscopists who are familiar with the anatomy of pancreatobiliary systems, their surrounding vasculatures, as well as the current pancreatobiliary cancer staging/treatment guidelines. An endoscopy nurse with experience in echoendoscope and equipment setup, and FNA specimen handing is also key to a smooth and successful procedure. While diagnostic pancreatobiliary EUS can usually be performed with standard conscious sedation with benzodiazepine and/or opioid analgesic administered by the endoscopist or endoscopy nurse, more complex interventional EUS procedures would benefit from sedation by an anesthetist, e.g., monitored anesthetic care (MAC).
EUS guided FNA cytology specimen should be evaluated by a dedicated cytopathologist during or after the EUS procedure. The presence of an on-site cytopathologist has been shown to improve diagnostic sensitivity, and reduce the number of FNA passes needed to obtain a diagnostic specimen (10).
Post-operative management
Post-procedure management mainly focuses on the monitoring for potential complications. Despite an increasing range of indications, complications of EUS have remained low. Complications of EUS include perforation, bleeding and bacteremia. The reported complication rate of pancreatic EUS is 0.03% and the reported complication rate of EUS-guided FNA is 1–2% (11,12).
For diagnostic EUS, procedure can be done on outpatient basis. Patients are usually kept nil by mouth for 1 hour and kept closely monitored in the recovery area. They can be discharged if no adverse event happens.
For interventional EUS, post-procedure management should be individualized depends on the interventions performed. Patient should be closely monitored for the presence of bleeding, perforation, leakage and sepsis especially when advanced interventions like EUS-guided pseudocyst drainage or biliary drainage have been done.
Tips, tricks and pitfalls
Although EUS has been shown to be superior to CT in detecting small pancreatic tumors in general, there are circumstances in which false-negative EUS examinations can result in patients with suspected pancreatic malignancy. In a multi-center study involving nine experienced endosonographers, the presence of chronic pancreatitis, a diffusely infiltrating carcinoma, a prominent ventral/dorsal split, and a recent episode of acute pancreatitis (<4 weeks) were associated with missed pancreatic cancer on the initial EUS examination (13). A follow up EUS should be arranged in 2 to 3 months if clinical suspicion for pancreatic tumor remains high despite an initial unrevealing EUS examination.
Detection of small pancreatic tumors may still be challenging at times despite the use of conventional EUS imaging. Novel diagnostic EUS imaging techniques such as contrast enhanced harmonic EUS (CEH-EUS) and elastography can further improve detection and characterization of small pancreatic lesions (14). In CEH-EUS, an ultrasound contrast agent composed of microbubbles is injected intravenously to highlight the slow-flowing intra-tumoral vessels. Pancreatic cancer is most commonly depicted as a hypo-enhanced lesion on CEH-EUS (14). Elastography allows real-time assessment of the stiffness of a suspected lesion. In general, malignant tumors are noted to be stiff, while normal tissue or a benign lesion is generally noted to be soft on elastography (14). Figure 7 illustrates the detection and characterization of a small pancreatic cancer that was not clearly seen on initial CT by a combination of conventional EUS, CEH-EUS and elastography.
Figure 7.

Video illustration on the detection and characterization of a small pancreatic cancer by a combination of conventional endoscopic ultrasound (EUS), contrast enhanced harmonic EUS (CEH-EUS) and elastography (15). Available online: http://www.asvide.com/articles/1045
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet 1980;1:629-31. 10.1016/S0140-6736(80)91122-8 [DOI] [PubMed] [Google Scholar]
- 2.Yasuda K, Tanaka Y, Fujimoto S, et al. Use of endoscopic ultrasonography in small pancreatic cancer. Scand J Gastroenterol Suppl 1984;102:9-17. [PubMed] [Google Scholar]
- 3.DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med 2004;141:753-63. 10.7326/0003-4819-141-10-200411160-00006 [DOI] [PubMed] [Google Scholar]
- 4.Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc 1999;50:786-91. 10.1016/S0016-5107(99)70159-8 [DOI] [PubMed] [Google Scholar]
- 5.Shrikhande SV, Barreto SG, Goel M, et al. Multimodality imaging of pancreatic ductal adenocarcinoma: a review of the literature. HPB (Oxford) 2012;14:658-68. 10.1111/j.1477-2574.2012.00508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong CC, Tang RS, Wong JC, et al. Examination of pancreas with radial endoscopic ultrasound (EUS). Asvide 2016;3:280. Available online: http://www.asvide.com/articles/1042
- 7.Chong CC, Tang RS, Wong JC, et al. Examination of pancreas with linear endoscopic ultrasound (EUS). Asvide 2016;3:281. Available online: http://www.asvide.com/articles/1043
- 8.Chong CC, Tang RS, Wong JC, et al. Video showing endoscopic ultrasound (EUS) fine needle aspiration (FNA) of a pancreatic body tumor. Asvide 2016;3:282. Available online: http://www.asvide.com/articles/1044
- 9.Bang JY, Magee SH, Ramesh J, et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy 2013;45:445-50. 10.1055/s-0032-1326268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varadarajulu S, Fockens P, Hawes RH. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol 2012;10:697-703. 10.1016/j.cgh.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 11.Jenssen C, Alvarez-Sánchez MV, Napoléon B, et al. Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol 2012;18:4659-76. 10.3748/wjg.v18.i34.4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc 2011;73:283-90. 10.1016/j.gie.2010.10.045 [DOI] [PubMed] [Google Scholar]
- 13.Bhutani MS, Gress FG, Giovannini M, et al. The No Endosonographic Detection of Tumor (NEST) Study: a case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy 2004;36:385-9. 10.1055/s-2004-814320 [DOI] [PubMed] [Google Scholar]
- 14.Teoh AY, Tang RS. Clinical evaluation of new diagnostic modalities of endoscopic ultrasound for pancreaticobiliary diseases. Dig Endosc 2015;27 Suppl 1:55-9. 10.1111/den.12444 [DOI] [PubMed] [Google Scholar]
- 15.Chong CC, Tang RS, Wong JC, et al. Video illustration on the detection and characterization of a small pancreatic cancer by a combination of conventional endoscopic ultrasound (EUS), contrast enhanced harmonic EUS (CEH-EUS) and elastography. Asvide 2016;3:283. Available online: http://www.asvide.com/articles/1045


