Abstract
Atrial fibrillation (AF) is one of the most common types of cardiac arrhythmia, particularly among older adults. AF confers a 5-fold risk for thromboembolic stroke as well as a 2-fold higher risk for congestive heart failure, morbidity, and mortality. Although stroke remains an important and impactful complication of AF, recent studies have shown that AF is independently associated with other neurological disorders, including cognitive impairment and dementia, even after adjusting for prior ischemic stroke. We performed a review of the published literature on the association between AF and cognitive status. Further, we reviewed studies investigating the underlying mechanisms for this association and/or reporting the impact of AF treatment on cognitive function. While most published studies demonstrate associations between AF and impaired cognition, no AF treatment has yet been associated with a reduced incidence of cognitive decline or dementia.
Keywords: Atrial fibrillation, Cognitive decline, Dementia, Alzheimer’s disease, Vascular dementia, Anticoagulants
1. Background
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia. Nearly 2.7 – 6.1 million people in the United States were living with AF in 2010.1 Aging is the single greatest risk factor for AF, with each decade of life after the age of 40 years conferring a 2-fold higher risk.1 AF is a major clinical risk factor for ischemic stroke, thromboembolism, heart failure, myocardial infarction, and death.2 In addition, there is increasing evidence that AF is also a risk factor for cognitive decline and dementia, independent of ischemic stroke.3–13
Dementia is defined as a progressive state of deteriorating cognitive functions and capabilities necessary for independent living ability.14 Multiple risk factors have been identified for dementia, however; age is one of the strongest risk factors for incident dementia. The prevalence of dementia doubles every 5 years after the age of 65.15 The estimated worldwide number of people living with dementia was 36.5 million in 2010; this number is expected to more than triple to reach 115.4 million by 2050. 14 Vascular dementia is a subset of cognitive decline secondary to cerebrovascular events and represents nearly 20% of all dementia cases.16 Interestingly, cerebrovascular risk factors have been shown to play a role not only in vascular dementia but also in other types of dementia such as Alzheimer’s disease (AD) perhaps through promoting vascular oxidative stress and inflammation, which leads to altered cerebral blood flow (CBF) regulation, disruption of the blood brain barrier, and ultimately neuronal damage and worsening of coexisting neurodegenerative processes.17, 18
Similar to AF, hypertension, diabetes mellitus, smoking, and coronary heart disease are vascular risk factors associated with dementia independent of stroke.15, 19 In light of the fact that AF can be paroxysmal and minimally symptomatic, yet contribute to cerebral hypoperfusion and cerebral thromboembolism, it is plausible that AF contributes to subclinical events that lead to cognitive decline and dementia in older individuals and that treatment of AF might prevent or abrogate cognitive decline. Alternatively, because both AF and dementia are strongly associated with advancing age and share common risk factors, they may not have a direct pathophysiological connection. In this review, we searched for published studies examining: 1) independent associations between AF and cognitive decline or dementia, 2) mechanisms linking AF and cognitive decline or dementia, and 3) impact of AF treatments on cognitive decline or dementia.
2. Association of Atrial Fibrillation and Dementia
In a population-based prospective cohort study of over 6000 participants, Ott et al. reported a significant association of AF with all types of dementia (odds ratio, OR: 2.3) and cognitive impairment (OR: 1.7).10 This association was independent of a prior history of strokes, which was rigorously adjudicated. Since Ott et al.’s seminal work, small observational studies have demonstrated independent associations between AF and incident dementia in patients with acute stroke.20 Meielke et al., in a cohort study of 135 patients with incident dementia, reported that the participants with AF had a more rapid decline in cognition as measured by clinical dementia rating and mini-mental state examinations (MMSE) scores over a period of 3 years when compared to patients without AF.21 These studies have prompted a closer assessment of the association between AF and dementia in larger prospective studies.
One such study, a large prospective observational study including over 37000 participants receiving care in a large community health care system (Intermountain Healthcare), Bunch et al. reported that a diagnosis AF was associated with 36% increased risk of incident dementia (as identified by International Classification of Diseases, Ninth Revision, codes from the electronic medical records) over a 5-year period after adjusting for age, sex, hypertension, hyperlipidemia, diabetes, renal failure, smoking, family history of dementia, myocardial infarction, previous stroke, heart failure, statin use, and antihypertensive mediation use.3 Marzona et al. assessed the cognitive status in over 31000 participants of the ONTARGET (ONgoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) and TRANSCEND (Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects with Cardiovascular Disease) through MMSE scores at baseline, penultimate, and 2-year follow up visits.22 Cognitive decline was defined as a drop of ≥ 3 points in the MMSE scores between visits, and incident dementia was defined as a score ≤ 23 points on follow up visits.22 The study found a similar association between history of AF and incident dementia (hazard ratio, HR: 1.4) after adjusting for multiple covariates.
Due to the variability in reports from various studies investigating the association of AF and cognitive decline or dementia, multiple meta-analyses have been performed to increase statistical power and resolve uncertainty. In 2011, Kwok et al meta-analyzed the data from 15 prospective studies studying the association between AF and incident dementia (established through rigorous adjudication) with a total of 46637 participants, and found that prevalent AF was associated with a significantly higher risk of dementia (OR: 2.0).23 In another meta-analysis, Santangeli et al. reviewed 8 prospective observational studies with a total of 77668 participants with no acute stroke and normal cognitive function at baseline who carried a diagnosis of AF at the time of enrollment.24 They reported that AF was significantly and independently associated with increased rates of incident dementia (HR: 1.4, diagnosis of dementia made by either diagnosis codes or rigorous adjudication).24 Similarly, Kalantarian et al performed a meta-analysis of 14 studies (5 cross-sectional, 9 prospective cohorts) with a total of 85,414 participants.9 They reported that AF was associated with a higher risk of incident dementia (diagnosis of dementia established by either diagnosis codes or rigorous adjudication) in participants with history of prior strokes (Relative Risk, RR: 2.7), as well as those without prior history of stroke (RR: 1.4).9 When viewed in aggregate, meta-analyses examining relations between AF and dementia suggest a strong and reproducible association between the two diseases, independent of history of stroke, and raise important questions about the potential mechanisms underlying this association.
3. Potential Mechanisms Underlying the Association between Atrial Fibrillation and Dementia
A. Cerebral Hypo-perfusion
Cerebral hypo-perfusion may explain the association between AF and dementia through two potential mechanisms: 1) beat-to-beat variability from AF resulting in frequent episodes of cerebral hypoperfusion;25 and 2) overall reduced cardiac output from AF due to the lack of atrioventricular synchrony.26–28
Due to the irregularly irregular nature of the cardiac rhythm in AF, it is plausible that beat-to-beat variability in cerebral perfusion might coexist during AF as compared to regular sinus rhythm. Given the lack of standardized methods for non-invasively evaluating the high frequency variation in CBF in vivo, Anselmino et al. evaluated the effect of rhythm irregularity in AF on proximal (e.g. middle cerebral artery flow) and distal cerebrovascular flow (e.g. arteriolar and capillary flow) dynamics using two coupled lumped-parameter in vitro models of the general cardiovascular and cerebrovascular circulation.25 Although the mean flow rate in vessels was similar in both AF and sinus rhythm, there was a significantly greater flow variability in AF, especially in the distal circulation. Moreover, over 300 hypo-perfusion events were observed per 5000 cardiac cycles evaluated. The findings from this in vitro model suggest that cerebral hypo-perfusion might occur independent of cardiac output in patients with AF due to beat-to-beat variability in the cerebral perfusion; however whether these observations translate fully to the cognitive decline and/or dementia in humans remains to be determined.25
In 1997, Upshaw et al. systemically reviewed the published literature to evaluate the impact of cardioversion from AF to sinus rhythm on cardiac output, and reported that the cardiac output gradually improved by over 50% at 4 weeks in the participants who underwent a successful cardioversion to sinus rhythm. This was accompanied by simultaneous recovery of left atrial function as detected by Doppler evaluation of the peak atrial wave velocity, supporting the role of atrioventricular synchrony in improving cardiac output after cardioversion.26 Similarly, Peterson et al. measured CBF in 9 patients with history of AF of less than 3 months duration who underwent successful electrical cardioversion to sinus rhythm. CBF was measured using radionucleotide imaging prior to, immediately after, and 30 days following cardioversion. The mean CBF improved from 35.8 ml/100gram/min to 40.3 ml/100gram/min on day 1, and to 46.2 ml/100gram/min on day 30 following cardioversion.27 Using bedside near-infrared spectroscopy, Wutzler et al. reported that cerebral tissue oxygen saturation improved immediately after successful cardioversion from AF to sinus rhythm.28 Their findings confirmed the observations made by Peterson et al. and support the hypothesis that reduced cardiac output and relative cerebral hypo-perfusion exist during AF. Effimova et al., in a prospective analysis of 19 participants with medically refractory AF with rapid ventricular rate, reported that the brain perfusion (as assessed by single-photon emission computed tomography), and cognitive function (as assessed by a battery of seven neuropsychological tests) improved significantly 3 months after atrioventricular node ablation and permanent pacemaker implantation.29
To study the effect of ventricular rate response in AF and cognitive impairment, Cacciatore et al. prospectively followed 358 participants with mild cognitive impairment (MMSE score of less than 24) over 10 years. Of these, 44 participants had AF at baseline. Participants with AF were stratified into low/high ventricular rate (average ventricular rate <50 or >90 beats per minute, as detected by 24-hour Holter monitoring) and moderate ventricular rate (average ventricular rate 50 – 90 beats per minute) groups. The rate of cognitive decline at 10 years of follow-up was significantly higher in the participants with AF in low/high ventricular rate group when compared to those in the moderate ventricular rate group. Although this study did not assess CBF, authors speculated that CBF was lower in low/high ventricular rate group compared to the moderate ventricular rate group, and contributed towards increased risk of dementia. Their observation suggests that even slight deviation in the average ventricular rate among patients with AF could contribute toward cognitive decline and/or dementia.30
Reduced cardiac output has independently been associated with an increased risk of incident dementia. In a prospective assessment of 1,039 participants enrolled in the Framingham Offspring Study without prevalent stroke, cognitive decline and dementia, Jefferson et al. reported an independent association between cardiac output (measured using cardiac magnetic resonance imaging) and incident dementia (adjusted-HR 1.66) and AD (adjusted-HR 1.65) at nearly 8 years of follow-up.31 Of note, this analysis was not restricted to the participants with AF and included a population-based sample. The mechanisms linking reduced cardiac output and CBF to cognitive decline and AD have not been deciphered completely. Bell et al proposed that cerebral hypo-perfusion could contribute to the development of AD by impairing the clearance of amyloid-beta peptides across the brain blood barrier thus promoting its accumulation.32 Yet, proof for this hypothesis is lacking, and it is important to note that human trials testing strategies aimed at reducing the brain amyloid burden to improve cognitive outcome have all failed calling for a better understanding of the impact of amyloid on cognitive outcome.33,34
Although these studies support the role of cerebral hypo-perfusion mechanism for the observed association of AF and dementia, a sub-study of the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) trial argued against this mechanism. The study tested the cognitive status in 245 participants at baseline, at 2 months, and yearly after that.35 No significant difference in the MMSE scores was observed between the rhythm-control and rate-control arms.35 However, this study had a small sample size and these results have not been replicated in other studies. While cerebral hypoperfusion may be present, the mechanisms through which it exacts its effect on cognition remain to be elucidated. Furthermore, because of the conflicting nature of the results, alterative CBF unrelated mechanisms might play a more important role in affecting cognition.
B. Vascular Inflammation
Dementia and AF have both been linked to pathological vascular remodeling and are both pro-inflammatory states.36–38, 40 As such, vascular inflammation has been proposed as a potential pathophysiological mechanism linking AF to dementia. 36–38, 40
In the Honolulu-Asia Aging Study, participants with an elevated C-reactive protein (CRP) had a 3-fold higher risk for dementia.36 Similar associations with incident dementia have been observed with CRP and another marker of systemic inflammation, interleukin (IL)-6 in the Rotterdam Study.37 It has been suggested that Amyloid-β plaques and neurofibrillary tangles in AD patients result from inflammation and damage to the blood-brain-barrier. AD has been strongly linked to up-regulation in inflammatory markers such as IL-1, IL-6, and Tumor Necrotizing Factor-α (TNF-α).38
Framingham Heart Study investigators did not find an independent association between circulating CRP or IL-6 and incident dementia over a mean follow-up of 7 years in 1016 Framingham Heart Study Participants.39 However, in the same study, participants with higher spontaneous production of TNF-α by peripheral blood mononuclear cells was associated with a risk of incident dementia. Thus, data are conflicting and do not definitely establish or refute the association of circulating inflammatory cytokines with dementia. However, majority of studies do support the association of inflammation (circulating markers as such as CRP and IL-6, other biomarkers (amyloid- beta peptides, Leptin, Clusterin, and apoliporotein), and/or spontaneous production of TNF-α) with an increased risk of dementia.40
Circulating inflammatory cytokines such as CRP and IL-6 have also been associated with incident AF.41 In participants in the Cardiovascular Health Study, elevated CRP was independently associated with 24% increased risk of incident AF per standard deviation.42 Likewise, in participants in the Framingham Heart Study, elevated Osteoprotegerin (a TNF-receptor family member) was associated with an increased risk of incident AF.43 It is still unclear whether inflammation is a cause or a consequence of AF. Frustaci et al. performed endomyocardial biopsies of the atrial septum in 12 patients and showed evidence of histopathological inflammation and fibrosis in patients with lone AF.44 Thus, it is plausible that inflammation perpetuates the adverse atrial electrical and structural remodeling, and facilitates chronicity of AF.45 Marcus et al. measured the gradient of CRP and IL-6 between the left atrium and coronary sinus in 46 patients with symptomatic AF undergoing catheter ablation. They reported that the median left atrial to coronary sinus gradient for CRP was significantly higher in patients who were in AF (positive number) at the time of blood collection as compared to those who were in sinus rhythm (negative number). Similar trend was observed in the levels of IL-6. Their observations suggest that presence of AF might stimulate inflammation rather than AF being a consequence of inflammation.46
If AF is associated with the creation of a pro-inflammatory state, it appears possible that patients with AF are more susceptible to injury to the blood-brain-barrier and deposition of amyloid, resulting in cognitive decline and progression of dementia.47 These observations provide the impetus for further studies to determine the role of inflammation in mediating the association of AF with dementia, and test anti-inflammatory treatment strategies to reduce incidence and progression of cognitive decline in AF patients.
C. Cerebral Small Vessel Disease
Although cerebral infarction manifesting as a stroke is one of the most feared sequela of AF, subclinical cerebral ischemic lesions (as detected by magnetic resonance imaging [MRI]) have been reported in up to 90% of participants with AF. Although such subclinical lesions are often presumed to be caused by cerebral small vessel disease, the pathogenesis is not well understood and is likely multifactorial.48, 49 Importantly, cerebral small vessel disease related lesions have been consistently associated with an increased rate of dementia and decline in global cognitive function.50–52 Accordingly, small vessel disease associated brain pathology (such as lacunar infarcts, MRI-defined white matter hyperintensities, and cerebral micro-bleeds) may be a key mechanism linking AF to cognitive decline, even in the absence of overt strokes. Indeed, in a prospective study, Gaita et al. compared 180 subjects with history of AF (paroxysmal and persistent), but without history of stroke with 90 individuals in normal sinus rhythm.53 Both groups underwent cognitive function testing and brain magnetic resonance imaging to determine the presence of MRI-defined white matter hyperintensities.53 The study reported that 89% of individuals with paroxysmal AF and 92% of individuals with persistent AF had at least 1 area of WMH seen on MRI.53 Chen et al. prospectively monitored cognitive status of 935 participants of the Atherosclerosis Risk Communities Study with no prior history of strokes or cognitive impairment.4 They reported that over 10 years of follow-up, incident AF was associated with a greater annual average decline in digit symbol substitution and word fluency in patients with evidence of lacunar infarcts on MRI.4 In contrast, incident AF was not associated with cognitive decline in patients without evidence of subclinical cerebral ischemia on MRI.
Similar to white matter hyperintensities and lacunar infarcts, cerebral microbleeds could also contribute towards the association of AF and dementia or cognitive decline. Cerebral microbleeds are small perivascular hemosiderin deposits that are best seen in the T2 gradient-recall echo and susceptibility-weighted MRI sequences as hypointensities (less than 10 mm in size).48 Microbleeds have been observed in the elderly population, and in individuals with history of AD and prior strokes.54 In the population-based Rotterdam scan study, the prevalence of microbleeds increased with age, from 6.5% in patients aged 45–50 years to 37.5% in patients over 80 years of aged and approximately 15.3% of participants had at least 1 cerebral microbleed.55 In addition, the presence of cardiovascular risk factors (hypertension, and smoking) in this study was also associated with increased risk of microbleeds. Although AF has not been independently associated with microbleeds, the two conditions share risk factors.56 Furthermore, microbleeds create a great deal of controversy for treatment with anticoagulants in patients with AF because microbleeds have been associated with increased risk of incident intracranial hemorrhage (the most feared and devastating complication of the anticoagulation treatment).57 Interestingly, the number of microbleeds in AF is directly related to future ischemic stroke risk. In a cross-sectional analysis of 550 participants with history of AF and ischemic stroke, Song et al. reported that increasing number of cerebral microbleeds was associated with higher CHADS2 and CHA2DS2-Vasc scores.54 Furthermore, Von Norden et al. reported that the presence and number of cerebral microbleeds is associated with cognitive impairment in subjects with no history of dementia, independent of other coexisting small vessel disease related lesions. 58
Taken in sum, the literature supports the hypothesis that cerebral small vessel disease, and cerebrovascular ischemia or injury secondary to AF may contribute to the pathophysiology of dementia and cognitive decline.
D. Brain Atrophy
Multiple studies have reported the association of AF with loss of brain volume and cognitive impairment. In a cross-sectional study, Knecht et al. evaluated 122 subjects with history of AF but with no history of stroke in the German Competence Network on AF and compared them to 563 individuals with no prior history of AF or stroke in the same community.59 The study found that hippocampal volume by MRI was significantly lower in the subjects with AF compared to participants without AF. Total brain volume and white matter hyperintensities were not significantly different between participants with AF and no AF.59 Furthermore, Similarly, Stefansdottir et al. evaluated 4,251 participants of the population-based Age, Gene/Environment Susceptibility-Reykjavik Study with no history of dementia in a cross-sectional analysis (330 participants had AF). They reported that AF was associated with a lower total cerebral volume on MRI and the association was significantly stronger with persistent AF when compared to the paroxysmal AF.60 In 2,144 participants of the Framingham Offspring Study, Piers et al. recently reported the inverse association of frontal lobe volume loss with AF despite adjusting for vascular risk factors and ApoE4 in the multivariable regression models.61 Global cerebral volume has been associated with worse cognitive function in patients in dementia.62 Thus, current evidence supports the role of global or regional brain volume loss as one of the potential mediators for the association of AF and dementia. The mechanism by which AF leads to cerebral volume loss, however, remains to be explored.
E. Shared Risk Factors between Atrial Fibrillation and Cognitive Decline or Dementia
Associations between cognitive decline, dementia and AF may relate to the fact that these processes share several cardiovascular risk factors that increase in prevalence with advancing age and include: hypertension, heart failure, diabetes mellitus, excessive alcohol intake, sleep disorders and other risk factors. 63–65 AF may represent a more sensitive marker of intensity and/or duration of exposure to shared risk factors and a predictor of dementia. Saliba et al. recently reported that CHADS2 score could predict incident AF.66 Similarly, Chou et al. found that every 1-point increase in the CHADS2 score was associated with 54% increased hazards of vascular dementia and 40% increased hazards of AD.67 Shared risk factors may confer a risk that persists even when clinically manifest disease is adjusted for.
4. Atrial Fibrillation Treatments, Cognitive Function, and Dementia
A. Anticoagulation Therapy
Several studies have investigated the impact of various anticoagulation strategies for AF treatment on dementia. Warfarin is the most commonly prescribed anticoagulation treatment for AF treatment. Bunch et al. recently evaluated the medical records of 10537 patients on warfarin therapy (target international normalized ratio, INR 2–3) in the Intermountain Healthcare system.68 About 42% of the participants had a history of AF while the rest had non-AF indications for warfarin use (e.g. venous thromboembolism).68 Similar to prior studies, the risk of all types of incident dementia (AD, vascular and senile dementia; captured by searching for the International Classification of Diseases, Ninth Revision codes in electronic medical records) was significantly higher in subjects with AF when compared to those without AF, after adjusting for multiple covariates.68 Furthermore, they reported that the rates of incident dementia were nearly 2-fold higher in the subjects with the poorest anticoagulation maintenance compared to those with the highest anticoagulation time in therapeutic range. In another recent study from the Intermountain Healthcare system, Jacobs et al. reported significantly lower rates of incident dementia associated with use of direct oral anticoagulant (55.3% of participants were on rivaroxaban, 22.5% on apixaban, and 22.2% on dabigatran) for AF when compared to warfarin use for AF (0.3% with direct oral anticoagulants vs. 0.7% with warfarin, p=0.03) during a median follow-up of 243 days.69 Since the median duration of follow-up for the diagnosis of dementia was short in this study, the differential association of warfarin and direct oral anticoagulants with dementia could merely be a reflection of the differences in the prescription patterns. For example, direct oral anticoagulants have a less favorable safety profile for the elderly, frail, and those with kidney disease, all factors that independently relate to choice of anticoagulant, dementia and cognitive impairment. Taken together, published studies suggest that appropriate anticoagulation with vitamin K antagonists or direct oral anticoagulation may be associated with a lower risk of incident dementia.
B. Statin Therapy
HMG-CoA reductase inhibitor use (or statin therapy) decreases vascular inflammation and has been examined as a potential therapy to protect against cognitive impairment and rates of dementia.70 Recent pooled analyses have reported significant reduction in incident dementia (HR 0.71, n=23443) and AF (OR 0.69, n=71005) with statin therapy.71, 72 In a recent analysis from the National Health Insurance Research Database of Taiwan, Chao et al. reported significantly lower rates of incident non-vascular dementia (adjusted-HR 0.83) in 51253 patients with AF who were prescribed statin therapy compared to those who were not prescribed statin therapy.73 Lappegard et al. studied the effect of statin therapy on circulating inflammatory cytokines and cognitive status in 34 participants with AF with no indication for cholesterol lowering therapy. Participants were assigned to either an atorvastatin (40 mg oral daily) and ezetimibe (10 mg daily) group or to a double placebo group in double blind 1:1 randomized manner.74 Serum cytokines, and inflammatory markers were measured at 1, 3, 6, 9 and 12 months after randomization. In addition, participants underwent neurocognitive assessment at baseline and 1 year after enrollment in the study.74 The authors reported that the levels of inflammatory markers (including CRP) and cytokines were significantly reduced in the atorvastatin/ezetimibe group, compared to the placebo group. Furthermore, the reduction in some of the inflammatory cytokines was also associated with improved neurocognitive function and lower volume loss of the left amygdala.74 Although the data are sparse in this area, further study appears warranted to examine the potential benefits of statins to reduce vascular inflammation and cognitive decline among AF patients.
C. Catheter Ablation
Bunch et al. reviewed the medical records of 4212 patients with AF who underwent catheter ablation in the Intermountain Healthcare hospitals and compared them to 16848 age- and sex-matched patients with AF and no history of ablation, and 16484 age- and sex-matched control patients without history of AF. The study found significantly lower rates of incident dementia among patients treated with catheter ablation when compared to no ablation over a 3-year follow up.75 The results from this study stand in contrast to the findings of Chung et al. using data from a sub-study of the AFFIRM trial, which showed no significant difference in mini mental state examination scores between rate- and rhythm-control arms over a 3-year follow up.35 Future studies are warranted to understand the impact of rhythm restoration by catheter ablation or pharmacological approach on incidence and progression of dementia.
5. Conclusions and Future Goals
Both AF and dementia share many risk factors. Although the prevalence of AF continues to increase likely due to ever-increasing aging population,76 the prevalence of dementia has declined in the United States over the last decade.77 Multiple studies have proven the association of AF and dementia, independent of the history of stroke. A variety of mechanisms have been proposed to explain this relationship, however, larger studies investigating the impact of targeting the individual mechanisms have not been performed yet. Furthermore, to improve the rates of dementia detection in AF patients seen in the cardiovascular clinics, efficient risk stratifying and screening tools for dementia should be utilized. For example, since CHADS2 score is already utilized to estimate the risk of thromboembolism in AF, its association with dementia makes it a potential tool to risk stratify these patients for developing dementia.78 Hui et al. proposed a quick screening tool (Rapid Cognitive Screen), which focuses on skills required for five objects recall, drawing a clock with a set time, and insight.79 Such screening tools have potential for widespread applicability especially in AF patients with new diagnosis of dementia, as it will influence the course of their medical management (rate control vs. rhythm control, medications vs. catheter ablation). Perhaps, a higher CHADS2 score in AF patients should automatically trigger utilization of tools such as Rapid Cognitive Screen to detect dementia.
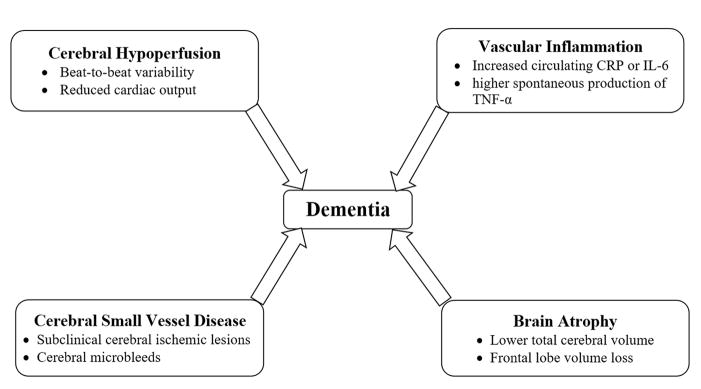
Figure 1.
Proposed mechanisms for the association of atrial fibrillation and dementia. Both conditions share risk factors such as hypertension, coronary artery disease, congestive heart failure, diabetes mellitus, age. Potential mechanisms include linking atrial fibrillation and cognitive impaurment/dementia include cerebral hypoperfusion, vascular inflammation, cerebral small vessel disease, and brain atrophy.
Footnotes
Disclosures: NH was supported by 5K08NS091499-01 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health and is an advisory board member for Omniox, Inc. JSS was supported by K01AG033643 from the National Institute on Aging. JSS and DDM were supported by 5R01HL126911-02 the National Heart, Lung and Blood Institute of the National Institutes of Health. DDM was supported by KL2RR031981, 1R15HL121761-01A1, and 1UH2TR000921-02 from the National Heart, Lung and Blood Institute of the National Institutes of Health. DDM has also been supported by grants from Biotronik, Philips, Bristol Meyers Squibb, and Otsuka Pharmaceuticals. He is also an equity holder in Mobile Sense, LLC and has received consulting fees from Pfizer and Biotronik, LLC.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 2.Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH, Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. European heart journal. 2013:eht483. doi: 10.1093/eurheartj/eht483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm. 2010;7:433–437. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Chen LY, Lopez FL, Gottesman RF, Huxley RR, Agarwal SK, Loehr L, Mosley T, Alonso A. Atrial fibrillation and cognitive decline-the role of subclinical cerebral infarcts: the atherosclerosis risk in communities study. Stroke. 2014;45:2568–2574. doi: 10.1161/STROKEAHA.114.005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Association Between Atrial Fibrillation and Dementia in the General Population. JAMA Neurol. 2015;72:1288–1294. doi: 10.1001/jamaneurol.2015.2161. [DOI] [PubMed] [Google Scholar]
- 6.Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JC, McCormick W, Bowen JD, Teri L, McCurry SM, Larson EB. Atrial fibrillation and risk of dementia: a prospective cohort study. J Am Geriatr Soc. 2011;59:1369–1375. doi: 10.1111/j.1532-5415.2011.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias MF, Sullivan LM, Elias PK, Vasan RS, D’Agostino RB, Sr, Seshadri S, Au R, Wolf PA, Benjamin EJ. Atrial fibrillation is associated with lower cognitive performance in the Framingham offspring men. J Stroke Cerebrovasc Dis. 2006;15:214–222. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Forti P, Maioli F, Pisacane N, Rietti E, Montesi F, Ravaglia G. Atrial fibrillation and risk of dementia in non-demented elderly subjects with and without mild cognitive impairment (MCI) Archives of gerontology and geriatrics. 2007;44:155–165. doi: 10.1016/j.archger.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Annals of internal medicine. 2013;158:338–346. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study the Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 11.Thacker EL, McKnight B, Psaty BM, Longstreth W, Sitlani CM, Dublin S, Arnold AM, Fitzpatrick AL, Gottesman RF, Heckbert SR. Atrial fibrillation and cognitive decline A longitudinal cohort study. Neurology. 2013;81:119–125. doi: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udompanich S, Lip GY, Apostolakis S, Lane DA. Atrial fibrillation as a risk factor for cognitive impairment: a semi-systematic review. QJM. 2013;106:795–802. doi: 10.1093/qjmed/hct129. [DOI] [PubMed] [Google Scholar]
- 13.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 14.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s & Dementia. 2013;9:63–75. e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clinics in geriatric medicine. 2014;30:421–442. doi: 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta neuropathologica. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. International Journal of Stroke. 2012;7:61–73. doi: 10.1111/j.1747-4949.2011.00731.x. [DOI] [PubMed] [Google Scholar]
- 20.Tatemichi TK, Foulkes MA, Mohr JP, Hewitt JR, Hier DB, Price TR, Wolf PA. Dementia in stroke survivors in the Stroke Data Bank cohort. Prevalence, incidence, risk factors, and computed tomographic findings. Stroke. 1990;21:858–866. doi: 10.1161/01.str.21.6.858. [DOI] [PubMed] [Google Scholar]
- 21.Mielke MM, Rosenberg PB, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, Breitner JC, Munger R, Lyketsos CG. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69:1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- 22.Marzona I, O’Donnell M, Teo K, Gao P, Anderson C, Bosch J, Yusuf S. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. Canadian Medical Association Journal. 2012 doi: 10.1503/cmaj.111173. cmaj.111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwok C, Loke Y, Hale R, Potter J, Myint P. Atrial fibrillation and incidence of dementia A systematic review and meta-analysis. Neurology. 2011;76:914–922. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- 24.Santangeli P, Di Biase L, Bai R, Mohanty S, Pump A, Brantes MC, Horton R, Burkhardt JD, Lakkireddy D, Reddy YM. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 2012;9:1761–1768. e1762. doi: 10.1016/j.hrthm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Anselmino M, Scarsoglio S, Saglietto A, Gaita F, Ridolfi L. Transient cerebral hypoperfusion and hypertensive events during atrial fibrillation: a plausible mechanism for cognitive impairment. Sci Rep. 2016;6:28635. doi: 10.1038/srep28635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upshaw CB. Hemodynamic changes after cardioversion of chronic atrial fibrillation. Archives of internal medicine. 1997;157:1070–1076. [PubMed] [Google Scholar]
- 27.Petersen P, Kastrup J, Videbæk R, Boysen G. Cerebral blood flow before and after cardioversion of atrial fibrillation. Journal of Cerebral Blood Flow & Metabolism. 1989;9:422–425. doi: 10.1038/jcbfm.1989.62. [DOI] [PubMed] [Google Scholar]
- 28.Wutzler A, Nee J, Boldt LH, Kuhnle Y, Graser S, Schroder T, Haverkamp W, Storm C. Improvement of cerebral oxygen saturation after successful electrical cardioversion of atrial fibrillation. Europace. 2014;16:189–194. doi: 10.1093/europace/eut246. [DOI] [PubMed] [Google Scholar]
- 29.Efimova I, Efimova N, Chernov V, Popov S, Lishmanov Y. Ablation and pacing: improving brain perfusion and cognitive function in patients with atrial fibrillation and uncontrolled ventricular rates. Pacing Clin Electrophysiol. 2012;35:320–326. doi: 10.1111/j.1540-8159.2011.03277.x. [DOI] [PubMed] [Google Scholar]
- 30.Cacciatore F, Testa G, Langellotto A, Galizia G, Della-Morte D, Gargiulo G, Bevilacqua A, Del Genio MT, Canonico V, Rengo F. Role of ventricular rate response on dementia in cognitively impaired elderly subjects with atrial fibrillation: a 10-year study. Dementia and geriatric cognitive disorders. 2012;34:143–148. doi: 10.1159/000342195. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson AL, Beiser AS, Himali JJ, Seshadri S, O’Donnell CJ, Manning WJ, Wolf PA, Au R, Benjamin EJ. Low cardiac index is associated with incident dementia and Alzheimer’s Disease: the Framingham Heart Study. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.012438. CIRCULATIONAHA. 114.012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease. Acta neuropathologica. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. New England Journal of Medicine. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. New England Journal of Medicine. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 35.Chung MK, Shemanski L, Sherman DG, Greene HL, Hogan DB, Kellen JC, Kim SG, Martin LW, Rosenberg Y, Wyse DG Investigators A. Functional status in rate- versus rhythm-control strategies for atrial fibrillation: results of the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Functional Status Substudy. J Am Coll Cardiol. 2005;46:1891–1899. doi: 10.1016/j.jacc.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 37.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 38.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL. Inflammation and Alzheimer’s disease. Neurobiology of aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 40.Weinstein G, Seshadri S. Circulating biomarkers that predict incident dementia. Alzheimers Res Ther. 2014;6:6. doi: 10.1186/alzrt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korantzopoulos P, Kalantzi K, Siogas K, Goudevenos JA. Long-Term Prognostic Value of Baseline C-Reactive Protein in Predicting Recurrence of Atrial Fibrillation after Electrical Cardioversion. Pacing and clinical electrophysiology. 2008;31:1272–1276. doi: 10.1111/j.1540-8159.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- 42.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 43.Schnabel RB, Larson MG, Yamamoto JF, Kathiresan S, Rong J, Levy D, Keaney JF, Jr, Wang TJ, Vasan RS, Benjamin EJ. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104:92–96. doi: 10.1016/j.amjcard.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 45.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. Journal of the American College of Cardiology. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 46.Marcus GM, Smith LM, Ordovas K, Scheinman MM, Kim AM, Badhwar N, Lee RJ, Tseng ZH, Lee BK, Olgin JE. Intracardiac and extracardiac markers of inflammation during atrial fibrillation. Heart Rhythm. 2010;7:149–154. doi: 10.1016/j.hrthm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Frontiers in aging neuroscience. 2014;6:171. doi: 10.3389/fnagi.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M nEuroimaging STfRVco. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. The Lancet Neurology. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 50.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. New England Journal of Medicine. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 51.Debette S, Markus H. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. Bmj. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Current opinion in neurology. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- 53.Gaita F, Corsinovi L, Anselmino M, Raimondo C, Pianelli M, Toso E, Bergamasco L, Boffano C, Valentini MC, Cesarani F, Scaglione M. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J Am Coll Cardiol. 2013;62:1990–1997. doi: 10.1016/j.jacc.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 54.Song TJ, Kim J, Lee H, Nam C, Nam H, Heo J, Kim Y. The frequency of cerebral microbleeds increases with CHADS2 scores in stroke patients with non-valvular atrial fibrillation. European journal of neurology. 2013;20:502–508. doi: 10.1111/ene.12003. [DOI] [PubMed] [Google Scholar]
- 55.Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 56.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44:995–1001. doi: 10.1161/STROKEAHA.111.000038. [DOI] [PubMed] [Google Scholar]
- 58.van Norden AG, van den Berg HA, de Laat KF, Gons RA, van Dijk EJ, de Leeuw F-E. Frontal and Temporal Microbleeds Are Related to Cognitive Function The Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke. 2011;42:3382–3386. doi: 10.1161/STROKEAHA.111.629634. [DOI] [PubMed] [Google Scholar]
- 59.Knecht S, Oelschlager C, Duning T, Lohmann H, Albers J, Stehling C, Heindel W, Breithardt G, Berger K, Ringelstein EB, Kirchhof P, Wersching H. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J. 2008;29:2125–2132. doi: 10.1093/eurheartj/ehn341. [DOI] [PubMed] [Google Scholar]
- 60.Stefansdottir H, Arnar DO, Aspelund T, Sigurdsson S, Jonsdottir MK, Hjaltason H, Launer LJ, Gudnason V. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke. 2013;44:1020–1025. doi: 10.1161/STROKEAHA.12.679381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piers RJ, Nishtala A, Preis SR, DeCarli C, Wolf PA, Benjamin EJ, Au R. Association between Atrial Fibrillation and Volumetric MRI Brain Measures: Framingham Offspring Study. Heart Rhythm. 2016 doi: 10.1016/j.hrthm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox N, Scahill R, Crum W, Rossor M. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999;52:1687–1687. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- 63.McCullagh CD, Craig D, McIlroy SP, Passmore AP. Risk factors for dementia. Advances in psychiatric treatment. 2001;7:24–31. [Google Scholar]
- 64.Djoussé L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG, Massaro JM, D’Agostino RB, Wolf PA, Ellison RC. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. The American journal of cardiology. 2004;93:710–713. doi: 10.1016/j.amjcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Benjamin EJ, Levy D, Vaziri SM, D’agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. Jama. 1994;271:840–844. [PubMed] [Google Scholar]
- 66.Saliba W, Gronich N, Barnett-Griness O, Rennert G. Usefulness of CHADS2 and CHA2DS2-VASc Scores in the Prediction of New-Onset Atrial Fibrillation: A Population-Based Study. Am J Med. 2016;129:843–849. doi: 10.1016/j.amjmed.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 67.Chou RH, Chiu CC, Huang CC, Chan WL, Huang PH, Chen YC, Chen TJ, Chung CM, Lin SJ, Chen JW, Leu HB. Prediction of vascular dementia and Alzheimer’s disease in patients with atrial fibrillation or atrial flutter using CHADS2 score. J Chin Med Assoc. 2016 doi: 10.1016/j.jcma.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 68.Bunch TJ, May HT, Bair TL, Crandall BG, Cutler MJ, Day JD, Jacobs V, Mallender C, Osborn JS, Stevens SM, Weiss JP, Woller SC. Atrial Fibrillation Patients Treated With Long-Term Warfarin Anticoagulation Have Higher Rates of All Dementia Types Compared With Patients Receiving Long-Term Warfarin for Other Indications. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobs V, May HT, Bair TL, Crandall BG, Cutler MJ, Day JD, Mallender C, Osborn JS, Stevens SM, Weiss JP, Woller SC, Bunch TJ. Long-Term Population-Based Cerebral Ischemic Event and Cognitive Outcomes of Direct Oral Anticoagulants Compared With Warfarin Among Long-term Anticoagulated Patients for Atrial Fibrillation. Am J Cardiol. 2016;118:210–214. doi: 10.1016/j.amjcard.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 70.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 71.Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc. 2013;88:1213–1221. doi: 10.1016/j.mayocp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 72.Fauchier L, Clementy N, Babuty D. Statin therapy and atrial fibrillation: systematic review and updated meta-analysis of published randomized controlled trials. Curr Opin Cardiol. 2013;28:7–18. doi: 10.1097/HCO.0b013e32835b0956. [DOI] [PubMed] [Google Scholar]
- 73.Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Lip GY, Chiang CE, Chen SA. Statins and the risk of dementia in patients with atrial fibrillation: A nationwide population-based cohort study. Int J Cardiol. 2015;196:91–97. doi: 10.1016/j.ijcard.2015.05.159. [DOI] [PubMed] [Google Scholar]
- 74.Lappegård KT, Pop-Purceleanu M, van Heerde W, Sexton J, Tendolkar I, Pop G. Improved neurocognitive functions correlate with reduced inflammatory burden in atrial fibrillation patients treated with intensive cholesterol lowering therapy. Journal of neuroinflammation. 2013;10:1. doi: 10.1186/1742-2094-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:839–845. doi: 10.1111/j.1540-8167.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 76.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chou R-H, Chiu C-C, Huang C-C, Chan W-L, Huang P-H, Chen Y-C, Chen T-J, Chung C-M, Lin S-J, Chen J-W. Prediction of vascular dementia and Alzheimer’s disease in patients with atrial fibrillation or atrial flutter using CHADS 2 score. Journal of the Chinese Medical Association. 2016 doi: 10.1016/j.jcma.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Hui DS, Morley JE, Mikolajczak PC, Lee R. Atrial fibrillation: A major risk factor for cognitive decline. Am Heart J. 2015;169:448–456. doi: 10.1016/j.ahj.2014.12.015. [DOI] [PubMed] [Google Scholar]