Conflicting data exist on the requirement for wild-type (WT) MLL1 in MLL-rearranged leukemia. In this issue of Cancer Cell, Chen et al. describe complementary approaches demonstrating that MLL1 is dispensable for MLL-fusion-mediated leukemogenesis. They also observe an unexpected role for MLL2 in MLL-rearranged leukemia cells and identify potential therapeutic targets.
11q23 translocations result in the formation of MLL1 fusion genes that act as potent drivers of acute myeloid and acute lymphoblastic leukemia and confer a poor prognosis. MLL1 fusion proteins, which are formed as a result of these translocations, contribute to leukemogenesis by imposing an aberrant transcription program. The critical consequence of 11q23 chromosomal translocations is the formation of a chimeric oncogenic transcription factor that retains the amino-terminus of MLL1 but replaces carboxyl-terminal domains, including the SET domain, with sequences from its partner proteins (Figure 1). As a result of 11q23 gene rearrangements, MLL fuses in frame with more than 70 different partner proteins. The most common partners are AF4, AF9, ENL, AF10, and ELL, which together account for over 85% of all MLL-rearranged leukemias. AF4, AF5q31, ENL, AF9, and ELL form a super elongation complex (SEC) that recruits the positive regulator of Pol II transcription elongation factor b (P-TEFb) kinase and the histone-3 lysine-79 methyltransferase DOT1L (Lin et al. 2010). The recruitment of the activities of the partner protein complex to MLL1 targets is a key molecular mechanism in MLL1 fusion protein-induced dysregulation of gene expression. Aberrant transcriptional elongation and H3K79 methylation lead to inappropriate activation of certain targets, including the HOXA cluster and MEIS1, that are critical to the transforming properties of MLL fusions.
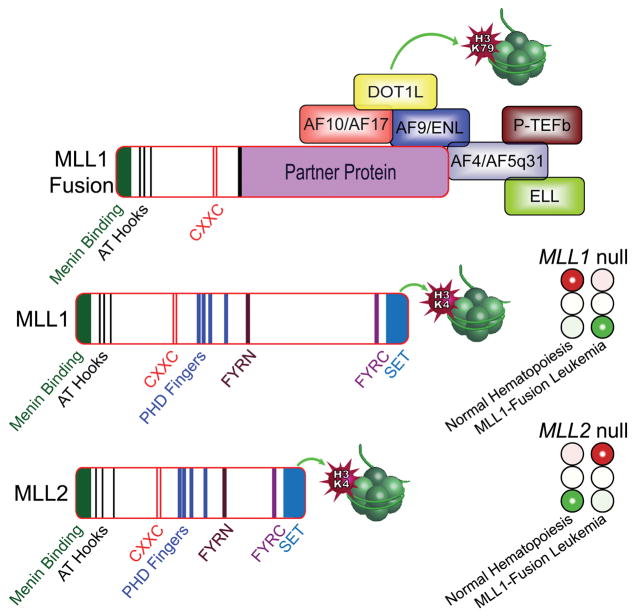
Figure 1. MLL1, MLL2, and MLL1-fusion proteins in MLL1-rearranged leukemia.
MLL1 and MLL2 are highly homologous paralogs that contain a carboxy-terminal SET domain that is a histone-3 lysine-4 methyltransferase. As a result of 11q23 translocations, MLL1 fuses to one of more than 70 partners, including AF4, AF5q31, ENL, AF9, AF10, AF17, and ELL. These MLL partner proteins form a super elongation complex that recruits the positive regulator of Pol II transcription elongation factor b (P-TEFb) kinase and the DOT1L histone-3 lysine-79 methyltransferase. Prior studies have shown that ablation of MLL1 blocks normal hematopoiesis (illustrated by a red stoplight under MLL1 null), but knockout of MLL2 permits normal hematopoietic development to proceed (illustrated by a green light under MLL2 null). Remarkably, Chen et al. show that deletion of MLL1 does not abrogate MLL1-fusion leukemia (green light under MLL1 null), whereas inactivation of MLL2 inhibits MLL1-fusion induced leukemogenesis (red stoplight under MLL2 null).
Leukemia cells that harbor 11q23 translocations express one MLL1 fusion gene and one WT MLL1 allele. Prior studies on the importance of the remaining WT allele of MLL1 in MLL1-fusion leukemia have yielded confounding results, leading Chen and colleagues to undertake a rigorous series of experiments to address this issue. MLL-rearranged leukemia cells also express MLL2, a paralog of MLL1 that regulates distinct sets of target genes. Chen and colleagues also analyzed the role of MLL2 in MLL1-rearranged leukemia, which had previously not been examined.
MLL1 (KMT2A) is critical for the maintenance of expression of its target genes, and it mediates chromatin modifications associated with transcriptional activation. A SET domain in the carboxy-terminus of MLL1, conserved with Set1 in S. cerevisiae, acts as a histone-3 lysine-4 (H3K4) methyltransferase. Germline deletion of Mll1 resulted in embryonic lethality, with embryos exhibiting hematopoietic and skeletal defects, and loss of Hoxa-7 and Hoxc-9 expression (Yu et al., 1995). Mll1 heterozygous mice exhibited growth retardation, anemia, thrombocytopenia, segmentation abnormalities, and posterior shift of Hoxa-7 and Hoxc-9 expression, confirming functional conservation of Mll1 with Drosophila trithorax in Hox gene regulation. Using conditional deletion models, Mll1 was found to be essential for the maintenance of adult hematopoietic stem cells. (Jude et al., 2007). Interestingly, mice with a truncated allele of Mll1 lacking its SET domain exhibited altered Hox gene expression but manifested no apparent hematopoietic defects, demonstrating that the histone methyltransferase activity of Mll1 is dispensable for the maintenance of hematopoietic stem cells (Mishra et al., 2014).
MLL2 (KMT2B) was identified by its homology to MLL1, and similar to MLL1, it is expressed broadly (Fitzgerald et al., 1999). In contrast to MLL1, MLL2 has not been found to be involved in chromosome translocations in leukemia. Artificial fusions of MLL2 to MLL partner proteins did not exhibit the capacity to transform hematopoietic cells due to differences in the CXXC domain and adjacent sequences (Bach et al, 2009). The amino-terminus of both MLL1 and MLL2 bind to menin/LEDGF, an interaction that is critical to the normal functions of MLL1 and MLL2 and also to the transforming properties of MLL fusion proteins. Germline deletion of Mll2 resulted in embryonic lethality by E11.5 associated with widespread apoptosis. Loss of Mll2 had no significant effect on expression of several Hoxa genes, but exhibited a strong effect on expression of Hoxb2 and Hoxb5, suggesting that Mll1 and Mll2 regulate different target genes (Glaser et al., 2009). Conditional deletion of Mll2 after E11.5 altered germ cell lineages, but had no apparent effects on adult tissues or hematopoiesis.
In light of the haploinsufficient phenotype observed in Mll1 null mice and the requirement of Mll1 for normal hematopoiesis, multiple investigators have examined whether WT MLL1 might be essential for leukemogenesis mediated by MLL-fusion proteins. Using bone marrow from conditional Mll1f/f mice (Jude et al., 2007) crossed with ubc9-Cre-ER mice and treated with 4-hydroxyl tamoxifen, several experiments demonstrated that Mll1 expression was essential for MLL-AF9 induced leukemogenesis (Thiel et al., 2010). Mll1 excision induced by 4-OHT also significantly reduced the number of colonies from MLL-AF9 transformed Mllf/f;Cre-ER bone marrow. In addition, tamoxifen-induced Mll1 excision in transplanted MLL-AF9 transduced bone marrow in recipient mice increased the survival rate of the mice compared to controls. In line with these data, pharmacologic targeting of MLL1 histone methyltransferase activity was found to inhibit the growth of cells expressing MLL-fusions. Using a small molecule to block MLL1-WDR5 interaction resulted in specific inhibition of MLL1 methyltransferase activity (Cao et al, 2014). Treatment with the MLL1-WDR5 inhibitor specifically reduced proliferation of MLL-fusion expressing bone marrow cells and MLL-rearranged leukemia cell lines by inducing cell-cycle arrest, apoptosis, and myeloid differentiation without toxicity to normal bone marrow cells. In contrast, other experimental data have suggested that WT MLL1 expression is dispensable for MLL-fusion protein mediated leukemia. For example, mice with a truncated allele of Mll1 lacking its SET domain permit leukemogenesis driven by the MLL-AF9 fusion oncoprotein (Mishra et al., 2014). Evidence for a lack of requirement for WT MLL1 can be found in the ML-2 cell line, which harbors two copies of the t(6;11)(q27;q23) encoding MLL-AF6, but lacks a WT MLL1 allele.
Using two distinct conditional Mll1 null alleles along with CRISPR/Cas9 editing of MLL1 in human MLL-AF9 leukemia cells, Chen et al. observed that Mll1 is dispensable for MLL-rearranged leukemia. To examine the potential for redundancy with Mll2, the authors also examined deletion of Mll2 using conditional Mll2 null mice and CRISPR/Cas9 editing of MLL2 in human cells. Strikingly, they observed significant effects of Mll2 deletion in MLL-AF9 transformed cells. Similarly, deletion of MLL2 inhibited the proliferation of MLL-rearranged leukemia cell lines. CRISPR/Cas9-mediated deletion of both MLL1 and MLL2 exhibited a greater effect on survival than deletion of MLL2 alone in human MLL-fusion protein leukemia cell lines. Taken together, these data are surprising in light of the requirement of Mll1, but not of Mll2, for normal hematopoietic development.
The work of Chen and colleagues highlights the importance of using multiple experimental approaches and model systems to resolve critical questions. This strategy minimizes the possibility that off-target effects and unexpected confounding factors unduly impact the interpretation of data.
The surprising observation of a role for MLL2 in MLL-fusion protein induced leukemia raises a number of important questions. Chen and colleagues identify MLL2-regulated pathways that may be targets for therapy, confirmation of which requires further study. Although deletion of MLL2 inhibited the viability of MLL-rearranged leukemia cells, it is also not clear whether these effects are restricted to MLL fusions or whether MLL2 target genes might be critical in other genetic subtypes of leukemia, a question that also warrants further investigation.
The surprising observation of a role for MLL2 in MLL-fusion protein induced leukemia raises a number of important questions. Whether the MLL2-regulated pathways identified by Chen and colleagues are suitable targets for therapy requires further study. Although deletion of MLL2 inhibited the viability of MLL-rearranged leukemia cells, it is not clear whether these effects are restricted to MLL fusions or whether MLL2 target genes might be critical in other genetic subtypes of leukemia, a question that also warrants further investigation.
References
- Bach C, Mueller D, Buhl S, Garcia-Cuellar MP, Slany RK. Oncogene. 2009;28:815–823. doi: 10.1038/onc.2008.443. [DOI] [PubMed] [Google Scholar]
- Cao F, Townsend EC, Karatas H, Xu J, Li L, Lee S, Liu L, Chen Y, Ouillette P, Zhu J, Hess JL, Atadja P, Lei M, Qin ZS, Malek S, Wang S, Dou Y. Mol Cell. 2014;53:247–261. doi: 10.1016/j.molcel.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Anastassiadis K, Kranz A, Stewart AF, Arndt K, Waskow C, Yokoyama A, Jones K, Neff T, Lee Y, Ernst P. Cancer Cell. 2017 doi: 10.1016/j.ccell.2017.05.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald KT, Diaz MO. Genomics. 1999;59:187–192. doi: 10.1006/geno.1999.5860. [DOI] [PubMed] [Google Scholar]
- Glaser S, Lubitz S, Loveland KL, Ohbo K, Robb L, Schwenk F, Seibler J, Roellig D, Kranz A, Anastassiadis K, Stewart AF. Epigenetics Chromatin. 2009;2:5. doi: 10.1186/1756-8935-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jude CD, Climer L, Xu D, Artinger E, Fisher JK, Ernst P. Cell Stem Cell. 2007;1:324–337. doi: 10.1016/j.stem.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, Washburn MP, Conaway JW, Conaway RC, Shilatifard A. Mol Cell. 2010;37:429–437. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BP, Zaffuto KM, Artinger EL, Org T, Mikkola HK, Cheng C, Djabali M, Ernst P. Cell Rep. 2014;7:1239–1247. doi: 10.1016/j.celrep.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel AT, Blessington P, Zou T, Feather D, Wu X, Yan J, Zhang H, Liu Z, Ernst P, Koretzky GA, Hua X. Cancer Cell. 2010;17:148–159. doi: 10.1016/j.ccr.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]