Abstract
Objective
Hepatic steatosis (HS) is common in HIV-infected individuals. Magnetic resonance spectroscopy (MRS) is the preferred non-invasive method for HS measurement but is expensive. Controlled attenuation parameter (CAP) also assesses HS and is conveniently performed concomitantly with transient elastography. We aimed to assess the accuracy of CAP in the setting of HIV infection.
Design
Cross-sectional study
Methods
CAP and MRS were performed in 82 subjects (39 HIV-monoinfected;7 HCV-monoinfected;21 HIV/HCV-coinfected; 15 with neither infection). We used concordance correlation coefficients to compare log-transformed and standardized CAP and MRS values and linear regression to examine factors associated with CAP and MRS-measured HS. The accuracy of CAP to detect ≥mild HS, defined as MRS-liver fat fraction ≥0.05, and the factors associated with discordance between CAP and MRS were evaluated.
Results
Overall, CAP-HS and MRS-HS correlated moderately well (r=0.60, p<0.001), and correlation was strongest in the HIV-monoinfected group (r=0.67, p<0.001). Body composition factors (higher BMI, waist circumference, visceral and abdominal subcutaneous adipose tissue) and insulin resistance were significantly associated with both greater CAP-HS and MRS-HS. Using a validated CAP cut-off ≥238 decibels/meter, sensitivity and specificity for ≥mild HS were 84% and 75% in the entire cohort; 89% and 80% in the HIV-monoinfected group. Higher body composition parameters were more likely to be misclassified as having HS by CAP.
Conclusions
Our findings suggest CAP is an acceptable non-invasive surrogate for HS in HIV-infected individuals but may overestimate HS prevalence, especially in individuals with high BMI. Evaluation of factors that improve CAP accuracy and determination of optimal cut-offs are warranted.
Keywords: HIV, HCV, hepatic steatosis, fatty liver, CAP, MRS
INTRODUCTION
Hepatic steatosis (HS) is common in HIV-infected individuals, and its prevalence is expected to increase with the rise of obesity and metabolic syndrome and the aging of the HIV-infected population(1–3). Although liver biopsy is the gold standard for detecting and staging HS, it is infrequently performed in HIV-infected patients without viral hepatitis coinfection. Magnetic resonance spectroscopy (MRS) is the preferred non-invasive modality to detect and quantify HS but is costly and not readily available in resource-limited settings(4).
Fibroscan®-measured transient elastography (TE) is increasingly utilized to estimate liver fibrosis. Controlled attenuation parameter (CAP) quantifies HS by measuring the attenuation of ultrasound waves traveling through the liver at the radiofrequency of the Fibroscan® probe. Because it is conveniently performed simultaneously with Fibroscan®-TE and is relatively inexpensive, CAP is an attractive method to measure HS. Indeed, CAP has been evaluated as a surrogate for histologic HS among patients with a variety of liver diseases (5–7) and is increasingly being used to screen for HS in HIV-infected persons(8–12). However, there are no published data on the accuracy of CAP in the setting of HIV infection. Therefore, the objectives of this study were to examine the correlation of CAP and MRS-measured HS in a cohort of patients with and without HIV infection and to determine whether HIV altered this correlation.
PATIENTS AND METHODS
Participants were recruited from the Northern California site of the Women’s Interagency HIV Study (WIHS) and the Study of Visceral Adiposity, HIV, and HCV: Biologic Mediators of Hepatic Steatosis (VAHH). Both WIHS and VAHH enrolled participants with HIV monoinfection, HCV monoinfection, HIV/HCV coinfection and those with neither infection. Recruitment and study design details of both studies have previously been described (13, 14).
From October 2013 through July 2015, 85 subjects enrolled in the WIHS MRS-Steatosis Substudy and VAHH who had both MRS and CAP measurements available within a median of 14 days (range:0–294) were included in analysis. Magnetic resonance imaging was performed on a 3T whole body scanner (General Electric Healthcare, Waukesha, WI), and MRS was acquired from an 8cc voxel similarly to prior reports, with a 64-acquisition time series of spectra(15). Spectra were automatically phase, frequency, motion and T2 relaxation time corrected(16–18). Quality was visually confirmed by an MR spectroscopist with over 20 years’ experience. We calculated liver fat fraction from the corrected MRS measures of CH2 and CH3 lipids and of water as the total lipids/(total lipids + water). Visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT) volumes were generated based on magnetic resonance slices located at the discs between lumbar vertebrae L2-3, L3-4, and L4-5. Of 85 subjects who underwent CAP assessment (Fibroscan®, Echosens, Paris, France), 82 had valid measurements (39 HIV-monoinfected, 7 HCV-monoinfected, 21 HIV/HCV-coinfected, and 15 uninfected) and were included in the analysis.
Race, ethnicity, alcohol consumption, smoking history, marijuana use and history of injection drug use were obtained through self-report. Alcohol use was categorized as: none; light-moderate (0–12 drinks/week); or heavy (>12 drinks/week). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using 8-hour fasting insulin and glucose values. The aspartate aminotransferase-to-platelet ratio index (APRI) and the FIB-4 score were used to estimate liver fibrosis(19, 20). Probable cirrhosis was defined as APRI>2 or FIB-4>3.25. HIV infection was defined by documentation of a positive HIV enzyme immunoassay confirmed with western blot, and chronic HCV infection was defined as serum HCV antibody and HCV RNA positive.
Both MRS-measured HS (MRS-HS) and CAP-measured HS (CAP-HS) had right-skewed distributions, and therefore results were log-transformed. In order to compare MRS-HS and CAP-HS, we standardized log-transformed values to a mean of 0 and standard deviation (SD) of 1. We used concordance correlation coefficients to compare the standardized measurements and linear regression to examine the factors associated with CAP-HS and MRS-HS. The regression coefficients and 95% confidence intervals (CI) are reported as changes in SD units of the log-transformed MRS or CAP values.
Next, we used receiver operating characteristics (ROC) analysis to evaluate the ability of the CAP cut-off ≥238 decibels/meter (dB/m) to detect ≥mild HS, defined as MRS liver fat fraction ≥0.05. This CAP cut-off was selected because it has been validated in HIV-uninfected patients and has been used in studies of HS in HIV-infected individuals(5, 8, 10, 12). Kappa coefficient was used to measure agreement between HS diagnosed by CAP or MRS. We used the chi-squared and the Wilcoxon rank-sum tests to compare the characteristics of participants with concordant and discordant MRS and CAP values. Specifically, participants were stratified by MRS into no HS and HS groups; within these groups, participants with low CAP (no HS) and high CAP (HS) were compared. Finally, factors associated with false-positive CAP-HS were evaluated using logistic regression. Statistical analyses were performed using SAS system, version 9.4 (Cary, NC) and STATA version 12.1 (College Station, TX).
RESULTS
Table shows characteristics of the study population and factors associated with CAP-HS and MRS-HS. The median age was 56 years, 71% were women; over half were African American; 50% were overweight or obese (median: BMI 25kg/m2), and only 10% reported heavy alcohol use. The majority (98%) were on highly active antiretroviral therapy. The CAP mean was 232 dB/m and standard deviation 51 dB/m, and the MRS mean liver fat fraction was 0.04 with standard deviation 0.05. On univariate analysis, non-white, non-African American race was associated with significantly higher CAP-HS and MRS-HS, as were increasing BMI, waist circumference, VAT, SAT, and HOMA-IR (Table). These factors remained significantly associated with CAP-HS and MRS-HS after adjusting for age and sex. History of clinical AIDS was associated with significantly higher MRS-HS but not CAP-HS, but this was only borderline significant after adjusting for age and sex (p=0.05).
Table.
Demographic and clinical characteristics of study population and factors associated with CAP-HS and MRS-HS
| Variable | Study Population (N=82)§ |
CAP-HS ΔSD (95%CI)£ |
MRS-HS ΔSD (95%CI)£ |
|---|---|---|---|
| Demographics | |||
|
| |||
| Age | 56 (51, 59) | 0.01¶ (−0.35, 0.33) | 0.22¶ (−0.12, 0.56) |
| Male | 24 (29%) | −0.13 (−0.61, 0.36) | 0.08 (−0.40, 0.57) |
| Race | |||
| African American | 49 (60%) | 0.16 (−0.34, 0.65) | 0.002 (−0.49, 0.50) |
| White | 21 (26%) | Reference | Reference |
| Other | 12 (15%) | 1.03 (0.31, 1.72) | 0.96 (0.27, 1.64) |
| Hispanic | 12 (15%) | 0.17 (−0.45, 0.80) | 0.29 (−0.34, 0.91) |
| Infection status | |||
| Uninfected | 15 (18%) | Reference | Reference |
| HIV-monoinfected | 39 (48%) | −0.26 (−0.87, 0.34) | −0.34 (−0.95, 0.27) |
| HCV-monoinfected | 7 (9%) | −0.29 (−1.20, 0.62) | −0.57 (−1.48, 0.35) |
| HIV/HCV-coinfected | 21 (26%) | −0.60 (−1.27, 0.07) | −0.41 (−1.09 0.27) |
|
| |||
| Lifestyle | |||
|
| |||
| Alcohol | |||
| None | 33 (40%) | Reference | Reference |
| Light-Moderate | 41 (50%) | −0.25 (−0.72, 0.22) | −0.03 (−0.50, 0.44) |
| Heavy | 9 (10%) | −0.42 (−1.21, 0.36) | −0.09 (−0.89, 0.70) |
| Current smoker | 40 (49%) | −0.23 (−0.67, 0.21) | −0.23 (−0.67, 0.21) |
| Current marijuana use | 35 (43%) | −0.24 (−0.68, 0.21) | −0.26 (−0.71, 0.18) |
| Injection drug use, ever | 22 (27%) | −0.25 (−0.74, 0.25) | −0.01 (−0.50, 0.49) |
|
| |||
| Metabolic | |||
|
| |||
| BMI (kg/m2) | 25 (22, 30) | 0.37* (0.21, 0.52) | 0.26* (0.10, 0.43) |
| Waist Circumference (cm) | 91 (83, 103) | 1.95¥ (1.04, 2.87) | 1.54¥ (0.58, 2.49) |
| VAT (cm3) | 163 (114, 204) | 0.71¥ (0.43, 1.00) | 0.74¥ (0.46, 1.02) |
| Abd SAT (cm3) | 254 (161, 379) | 0.46¥ (0.24, 0.69) | 0.33¥ (0.10, 0.57) |
| HOMA-IR | 1.38 (0.73, 2.58) | 0.27¥ (0.09, 0.45) | 0.28¥ (0.11, 0.46) |
|
| |||
| Liver-related | |||
|
| |||
| ALT (U/L) | 19 (14, 31) | −0.01¥ (−0.28, 0.26) | 0.11¥ (−0.16, 0.37) |
| Platelet (109/L) | 232 (194, 280) | 0.06¥ (−0.34, 0.47) | −0.16¥ (−0.57, 0.24) |
| APRI | 0.31 (0.21, 0.51) | −0.06¥ (−0.25, 0.13) | 0.08¥ (−0.11, 0.26) |
| Cirrhosis | 8 (10%) | 0.04 (−0.70, 0.79) | 0.22 (−0.52, 0.97) |
|
| |||
| HIV-related | |||
|
| |||
| Undetectable HIV RNA | 46 (78%) | 0.10 (−0.53, 0.72) | 0.16 (−0.48, 0.80) |
| Current CD4 (cells/mm3) | 658 (429, 828) | −0.01¥ (−0.31, 0.29) | −0.01¥ (−0.32, 0.29) |
| CD4 nadir (cells/mm3) | 214 (124, 327) | −0.05¥ (−0.25, 0.14) | 0.003¥ (−0.20, 0.20) |
| History of clinical AIDS | 21 (36%) | 0.40 (−0.14, 0.93) | 0.59 (0.057, 1.12) |
Bold signifies statistical significance (p<0.05);
Continuous variables are presented as median (interquartile range)
MRS and CAP values were each standardized to a mean of 0 and standard deviation (SD) of 1. Therefore, the regression coefficients and 95% confidence intervals (CI) are reported as changes in SD units of the log-transformed MRS and CAP values. Positive values refer to more steatosis and negative values refer to less steatosis. For example, with each 5 point increase in BMI, logCAP values are 0.37 SD higher and logMRS values are 0.26 SD higher.
The CAP mean (SD) was 232 dB/m (51) and the logCAP mean (SD) was 2.36 (0.10).
The MRS mean (SD) was 0.04 (0.05) and the logMRS mean (SD) was −1.68 (0.49).
CAP mean (SD) by infection status: uninfected 247 dB/m (47), HIV-infected 234 dB/m (48), HCV-monoinfected 236 dB/m (69), HIV/HCV-coinfected 218 dB/m (54)
MRS mean (SD) by infection status: uninfected 0.05 (0.05), HIV-monoinfected 0.04 (0.05), HCV-monoinfected 0.02 (0.02), HIV/HCV-coinfected 0.04 (0.05)
Per decade;
Per 5 point increase;
Per doubling
Correlation of CAP and MRS by HIV and HCV Status
In the entire group, CAP-HS increased with increasing MRS-HS, and we found moderate agreement between the two continuous measurements, with a concordance correlation coefficient(rc)=0.63 (p<0.001). When stratified by disease status, agreement between CAP-HS and MRS-HS was highest among the HIV-monoinfected (rc=0.67;p<0.001) and HIV/HCV-coinfected groups (rc=0.67;p<0.001)(Figure A). CAP detected ≥mild HS, defined as MRS liver fat fraction ≥0.05, with an area under the ROC curve of 0.85(95%CI:0.76–0.95) in the entire cohort and 0.88(95%CI:0.78–0.99) in the HIV-monoinfected group. Using a CAP cut-off ≥238dB/m, sensitivity and specificity for ≥mild HS were 84%(95%CI:60%–97%) and 75%(95%CI:62%–85%), respectively, in the entire cohort and 89%(95%CI:52%–100%) and 80%(95%CI:61%–92%), respectively, in the HIV-monoinfected group.
Figure.

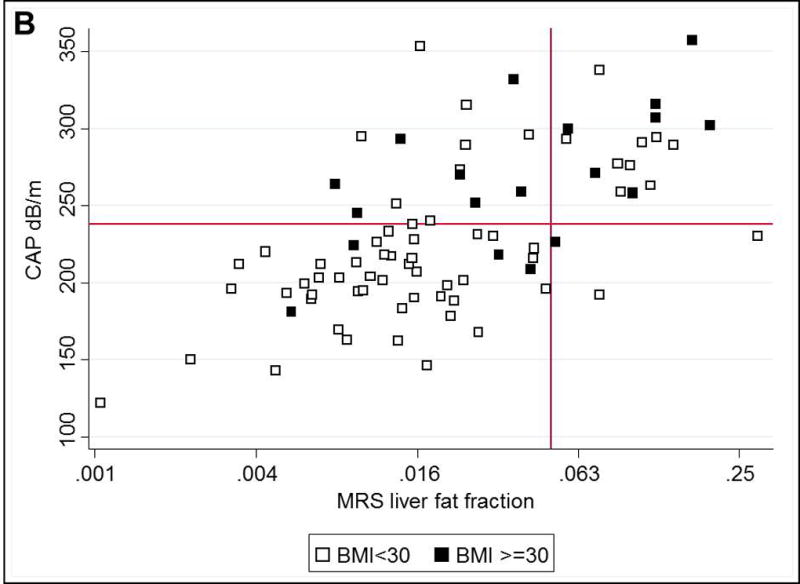
A. Correlation of standardized CAP and MRS-measured steatosis, by HIV and HCV status. Dotted black line indicates identical correlation. B. Concordance and discordance of CAP and MRS-measured steatosis, by BMI. Y-axis reference line indicates CAP 238 dB/m and X-axis reference line indicates MRS liver fat fraction 0.05
CAP-MRS Discordance
The prevalence of HS in our cohort differed depending on the modality used to assess HS: 23% had HS using MRS and 39% had HS using CAP. Among the 63 individuals without HS on MRS, 16 (25%) were categorized as having HS on CAP, whereas among the 19 with HS using MRS, 3 (16%) were identified as not having HS using CAP, yielding a Kappa coefficient of 0.47 (Figure B). Within the group without HS on MRS, the 16 with false-positive CAP values had higher median BMI (30kg/m2 versus 24kg/m2; p=0.002), waist circumference (100cm versus 87cm; p=0.02), VAT volume (176cm3 versus 134cm3; p=0.01), and abdominal SAT volume (285cm3 versus 224cm3; p=0.03) compared to the 47 with true-negative CAP values. After adjusting for age, sex, and race, odds of a false-positive CAP was significantly increased with higher BMI (OR:2.05 per 5 point increase;95%CI:1.15–3.64) and higher VAT (OR:4.18 per doubling;95%CI:1.30–13.42).
DISCUSSION
The major finding of our study was that CAP-HS and MRS-HS correlated moderately well and that HIV serostatus did not adversely alter the correlation. Furthermore, similar clinical factors were associated with both MRS- and CAP-measured HS, primarily body composition and metabolic factors known to be associated with HS. Although our cohort is small, the findings suggest that CAP is an acceptable noninvasive surrogate for HS in large studies of HIV-infected individuals. However, CAP overestimated HS prevalence, and higher BMI and VAT were associated with increased odds of false-positive CAP.
Nonalcoholic fatty liver disease (NAFLD) refers to HS in the absence of excessive alcohol use. It is the most common cause of liver disease in Western industrialized countries, and prevalence is increasing in parallel to the obesity epidemic(21). Given the scope of the disease, affecting an estimated 30% of the US population(22), a safe, inexpensive, reliable method of HS screening is critical. This is especially important in the setting of HIV infection: several studies indicate HS is common in HIV-infected individuals, ranging from 28–54% in HIV-monoinfected groups(8, 12, 14, 23–25), but the pathophysiology and implications of HS in this population are not well understood.
Although CAP is used to screen for HS in HIV-infected populations, it has not been validated for this purpose. Multiple studies have compared CAP-HS to histologic HS in patients with a variety of underlying liver diseases– in a meta-analysis including 2,735 patients from 19 studies, CAP demonstrated good performance in detecting HS (defined as ≥5% of hepatocytes affected on histology) with an area under the ROC of 0.82(26). However, HIV-infected patients were not included. Moreover, the authors found that patients with NAFLD had higher CAP values than patients with other causes of liver disease such as hepatitis C or B virus, independent of histologic HS. Therefore, validation of CAP within a cohort of patients with HIV infection is important.
Our results that CAP-HS correlated moderately well with MRS-HS are similar to other studies comparing CAP-HS and MRS-HS in HIV-uninfected populations, in which the correlation coefficients ranged from 0.50–0.69(27–29). There was no significant correlation between CAP and MRS in the HCV-monoinfected group, likely due to the very small sample size in this subgroup. Our finding that CAP-MRS was able to detect ≥mild HS with an area under the ROC of 0.85 in the whole cohort and 0.88 in the HIV-monoinfected group is also consistent with published literature, with area under the ROC for detection of ≥mild HS ranging from 0.80–0.88 using histology as the reference standard(27, 30–32) and 0.83–0.90 using MRS as the reference standard(33).
Notably, we found that CAP was more likely to overestimate the presence of HS as BMI increased. Others have similarly reported this; the recent meta-analysis found increasing BMI was associated with significantly higher odds of a discrepancy between CAP and histologic HS(26). This highlights a key limitation of CAP and the importance of exercising caution when comparing HS prevalence across studies and patient populations, especially when varying modalities are used to assess HS. A limitation to our study was lack of liver biopsy to correlate CAP results with histology. However, we used MRI as our reference standard, which is considered the most reliable non-invasive modality for detecting HS(4), and similar factors were associated with both CAP-HS and MRS-HS. Another limitation is that the CAP and MRS measures may not have been taken from the same location in the liver– heterogeneity of steatosis may have led to some of the discordance observed(15).
In summary, we found that CAP-HS and MRS-HS correlated moderately well in our cohort of patients with and without HIV and HCV infection. Importantly, HIV infection did not adversely impact CAP performance in detecting HS, but participants with higher BMI were more likely to be falsely identified as having HS using CAP. Our results support the use of CAP for initial HS screening in large cohorts of HIV-infected individuals. However, optimal CAP cut-offs are needed, especially if CAP results are used to make individual patient decisions. Further evaluations of factors that could improve CAP accuracy and interpretation of longitudinal changes in CAP are warranted.
Acknowledgments
Funding/Support: This work was supported by an ACG Junior Faculty Development Award from the American College of Gastroenterology and the Gilead Research Scholars Program in HIV. The Women’s Interagency HIV Study (WIHS) is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) (U01-AI-103401, U01-AI-103408, UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, U01-AI-103397, U01-AI-103390, UO1-AI-34989, and UO1-AI-42590), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). This study was also supported by the UCSF Liver Center National Institute of Health [P30 DK026743], by the National Institute of Allergy and Infectious Diseases [K24 AI 108516 and R01 AI 087176, which was administered by the Northern California Institute for Research and Education and with resources of the Veterans Affairs Medical Center, San Francisco, CA], and by a grant from the National Institutes of Health, University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research, P30-AI027763. Data in this manuscript were collected by the WIHS. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Footnotes
Conflicts of interest: There are no conflicts of interest.
Author contributions:
Price: study concept and design, analysis and interpretation of data, drafting of manuscript, obtained funding
Dodge: analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Ma: analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Scherzer: analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Korn: acquisition of data, technical support, critical revision of the manuscript for important intellectual content
Tillinghast: acquisition of data, technical support, critical revision of the manuscript for important intellectual content
Peters: analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Noworolski: analysis and interpretation of data, acquisition of data, technical support, critical revision of the manuscript for important intellectual content
Tien: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtained funding
References
- 1.Crum-Cianflone N, Roediger MP, Eberly L, Headd M, Marconi V, Ganesan A, Weintrob A, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5:e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan S, Schouten JT, Atkinson B, Brown T, Wohl D, McComsey GA, Glesby MJ, et al. Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-infected individuals. J Acquir Immune Defic Syndr. 2012;61:381–389. doi: 10.1097/QAI.0b013e3182690e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13–20. doi: 10.1016/j.clinre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 6.de Ledinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918. doi: 10.1111/j.1478-3231.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 7.Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Macias J, Gonzalez J, Tural C, Ortega-Gonzalez E, Pulido F, Rubio R, Cifuentes C, et al. Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV-infected patients. AIDS. 2014;28:1279–1287. doi: 10.1097/QAD.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 9.Macias J, Real LM, Rivero-Juarez A, Merchante N, Camacho A, Neukam K, Rivero A, et al. Changes in liver steatosis evaluated by transient elastography with the controlled attenuation parameter in HIV-infected patients. HIV Med. 2016 doi: 10.1111/hiv.12384. [DOI] [PubMed] [Google Scholar]
- 10.Sulyok M, Makara M, Rupnik Z, Ferenci T, Ujhelyi E, Kormos L, Gerlei Z, et al. Hepatic steatosis in individuals living with HIV measured by controlled attenuation parameter: a cross-sectional study. Eur J Gastroenterol Hepatol. 2015;27:679–685. doi: 10.1097/MEG.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 11.Scheiner B, Mandorfer M, Schwabl P, Payer BA, Bucsics T, Bota S, Aichelburg MC, et al. The Impact of PNPLA3 rs738409 SNP on Liver Fibrosis Progression, Portal Hypertension and Hepatic Steatosis in HIV/HCV Coinfection. PLoS One. 2015;10:e0143429. doi: 10.1371/journal.pone.0143429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vuille-Lessard E, Lebouche B, Lennox L, Routy JP, Costiniuk CT, Pexos C, Giannakis A, et al. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS. 2016;30:2635–2643. doi: 10.1097/QAD.0000000000001241. [DOI] [PubMed] [Google Scholar]
- 13.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 14.Price JC, Ma Y, Scherzer R, Korn N, Tillinghast K, Peters MG, Noworolski SM, et al. HIV-infected and Uninfected Adults with Non-Genotype 3 Hepatitis C Virus Have Less Hepatic Steatosis than Adults with Neither Infection. Hepatology. 2016 doi: 10.1002/hep.28968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noworolski SM, Lam MM, Merriman RB, Ferrell L, Qayyum A. Liver steatosis: concordance of MR imaging and MR spectroscopic data with histologic grade. Radiology. 2012;264:88–96. doi: 10.1148/radiol.12110673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson SJ. Analysis of volume MRI and MR spectroscopic imaging data for the evaluation of patients with brain tumors. Magn Reson Med. 2001;46:228–239. doi: 10.1002/mrm.1183. [DOI] [PubMed] [Google Scholar]
- 17.Noworolski SM, Tien PC, Merriman R, Vigneron DB, Qayyum A. Respiratory motion-corrected proton magnetic resonance spectroscopy of the liver. Magn Reson Imaging. 2009;27:570–576. doi: 10.1016/j.mri.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilman A, Qayyum A, Nystrom M, Noworolski SM. Liver Fat and Water MR T2 Values at 3T: Dependence Upon Steatosis Level. Intn’l Soc of Mag Res in Med. 2011:734. [Google Scholar]
- 19.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 20.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 21.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. e521; quiz e560. [DOI] [PubMed] [Google Scholar]
- 22.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 23.Crum-Cianflone N, Dilay A, Collins G, Asher D, Campin R, Medina S, Goodman Z, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464–473. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guaraldi G, Squillace N, Stentarelli C, Orlando G, D'Amico R, Ligabue G, Fiocchi F, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–257. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 25.Li Vecchi V, Soresi M, Giannitrapani L, Di Carlo P, Mazzola G, Colletti P, Terranova A, et al. Prospective evaluation of hepatic steatosis in HIV-infected patients with or without hepatitis C virus co-infection. Int J Infect Dis. 2012;16:e397–402. doi: 10.1016/j.ijid.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, Kumar M, et al. Individual Patient Data Meta-Analysis of Controlled Attenuation Parameter (CAP) Technology for Assessing Steatosis. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Karlas T, Petroff D, Garnov N, Bohm S, Tenckhoff H, Wittekind C, Wiese M, et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One. 2014;9:e91987. doi: 10.1371/journal.pone.0091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlas T, Berger J, Garnov N, Lindner F, Busse H, Linder N, Schaudinn A, et al. Estimating steatosis and fibrosis: Comparison of acoustic structure quantification with established techniques. World J Gastroenterol. 2015;21:4894–4902. doi: 10.3748/wjg.v21.i16.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Faliva M, Perna S, Perani G, et al. Correlation of the controlled attenuation parameter with indices of liver steatosis in overweight or obese individuals: a pilot study. Eur J Gastroenterol Hepatol. 2015;27:305–312. doi: 10.1097/MEG.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 30.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626–637. doi: 10.1053/j.gastro.2015.11.048. e627. [DOI] [PubMed] [Google Scholar]
- 31.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598–607. doi: 10.1053/j.gastro.2016.10.026. e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, Poupon R, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan((R)): validation in chronic hepatitis C. J Viral Hepat. 2012;19:244–253. doi: 10.1111/j.1365-2893.2011.01534.x. [DOI] [PubMed] [Google Scholar]
- 33.Sasso M, Audiere S, Kemgang A, Gaouar F, Corpechot C, Chazouilleres O, Fournier C, et al. Liver Steatosis Assessed by Controlled Attenuation Parameter (CAP) Measured with the XL Probe of the FibroScan: A Pilot Study Assessing Diagnostic Accuracy. Ultrasound Med Biol. 2016;42:92–103. doi: 10.1016/j.ultrasmedbio.2015.08.008. [DOI] [PubMed] [Google Scholar]


