Abstract
Biological systems are highly sensitive to changes in their environment. Indeed, the molecular basis of the environmental stress response suggests that the specialized stress responses share more commonalities than previously believed. Here, we used the nematode C. elegans to gain insight into the role of Rho signaling during two common environmental challenges, oxidative and thermal stress. In response to heat shock (HS), wild type (N2) worms demonstrated reduced viability which was rescued by genetic suppression of CDC42 and RHO-1. Visualization of F-actin by phalloidin-rhodamine underscored a strict correlation between the levels of F-actin following GTPase suppression and survival. Additionally, genetic ablation of OSG-1, a Guanine Nucleotide Exchange Factor (GEF) previously implicated in oxidative stress, was associated with constitutively lower levels of F-actin and increased mortality. However, upon an oxidative insult F-actin stability decreased in N2 worms, a rescue of this affect was observed in OSG-1 null worms, consistent with the resistance exhibited by these worms to oxidative stress (OS).
Together these data suggest that during conditions of thermal or oxidative stress Rho signaling promotes vulnerability by altering actin dynamics. Thus, the stability of the actin cytoskeleton, in part through a conserved mechanism mediated by Rho signaling, is a crucial factor for the cell’s survival to environmental challenges.
Keywords: actin, oxidative stress, heat shock, Rho signaling, Guanine nucleotide exchange factors
Introduction
Biological systems are highly tuned and sensitive to changes in their environment, from alterations in the temperature to mechanical damage, osmotic imbalances, hypoxia, toxin exposure, and oxidative stress. Given the diversity of environmental stressors the existence of several specialized molecular responses to different environmental conditions is unsurprising. However, accumulating evidence indicates that these specialized stress signaling pathways and their associated proteins can be multifunctional and implicated in conditions in which they are otherwise not specialized for. For example, the heat shock response (HSR), a program initiated to account for unavoidable alterations in temperature, relies on molecular chaperones of the Heat Shock Protein (HSPs) family for the array of issues arising from exposure to hyperthermic conditions [1]. Though, constitutive expression of certain HSPs, such as HSP70, suggests that these cytoprotective agents play a role outside of the heat shock response. Indeed, evidence indicates that members of HSP family are integral for physiological processes including synaptic transmission [2], apoptosis [3], and more [1]. Additionally, recent evidence supports the notion of HSPs and the HSR are indeed multifunctional as both are involved in the immune [4] and oxidative stress response [5–7]. Moreover, there is a growing body of evidence that there is significant cross-talk between the oxidative stress and heat shock response, further corroborating the concept of multifunctional stress pathways [8]. In addition to HSPs other fundamental cellular components are emerging as key players in the cell’s response to heath shock. Among these is the actin cytoskeleton, a highly conserved and ubiquitous structure integral for a wide array of processes including cell polarity, shape, movement, and more [9]. Indeed, the actin cytoskeleton is an integral cellular component of the heat shock response as it undergoes disorganization under hyperthermic conditions [10]. Maintaining this critical yet complex structure requires a large number of signaling pathways, including Rho signaling. The Rho family is composed of “small” GTPases (~21 kDa) RhoA, Cdc42 and Rac1 which by cycling between a GTP-bound “active” and a GDP-bound “inactive” state, regulate actin dynamics and remodeling. In turn, Rho signaling is critical for cell growth, motility and adhesion, cytokinesis, lipid metabolism, membrane trafficking and transcription [11] thereby underscoring a multifunctional nature of this important signaling modality.
In the present study we report the involvement of Rho signaling in the cell’s response to thermal stress of the nematode Caenorhabditis elegans. Rho signaling has been previously implicated in the response to oxidative stress [12] suggesting that its malfunctioning may represent a general factor of cellular vulnerability. Consistent with this emerging notion we further show the existence of a common mechanism in which Rho GTPase RHO-1 and CDC42 act in concert to decrease the levels of F-actin in the cell increasing susceptibility to stress.
Materials and Methods
Age synchronization
Was previously described [13]. Briefly, nematodes were grown in standard 10 cm NGM plates until a large population of gravid adults was reached. The animals were collected in 1.5 ml Eppendorf tubes, washed in M9 buffer (22 mM KH2PO4, 22 mM NaH2PO4, 85 mM NaCl, 1 mM MgSO4), and lysed with a solution of 0.25 M NaOH and 1% hypochlorite freshly mixed. The eggs were collected by centrifugation at 400g for 1 min and washed in H2O 3 times. Then the eggs were incubated overnight in M9 buffer (22 mM KH2PO4, 22 mM NaH2PO4, 85 mM NaCl, 1 mM MgSO4) and seeded on standard NMG plates (17 g/liter agar, 3 g/liter NaCl, 2.5 g/liter bactopeptone, 1 mM CaCl2, 1 mM MgSO4, 5 mg/liter cholesterol, 25 mM potassium phosphate buffer (132 mM K2HPO4, 868 mM KH2PO4)) at a density of 20–25 worms/plate.
Heat shock
Five day-old worms maintained at 20 °C, were incubated at 37 °C for 4 hours and then incubated at 20 °C for additional 24 hours. Then worms were scored for survival by prodding with a platinum wire. The probability of survival was calculated as the fraction of alive worms over the total number of worms. In a typical experiment, ~75 worms/genotype, divided into 3 plates were used. Experiments were repeated at least 5 times or more.
Bacterial RNAi
Age-synchronized worms were grown in NGM plates containing 25 μg/ml carbenicillin and 1.0 mM IPTG seeded with HT115 double-stranded RNAi synthetizing E. coli.
Phalloidin-rhodamine staining
Examination of F-actin stability was achieved using phalloidin staining in accordance with previous work in the field [14]. Age synchronized worms were washed and collected in 1.5 mL centrifuge tubes with M9 buffer. Upon collection, worms were washed with H2O to remove excess bacteria and subsequently placed in liquid nitrogen for cuticle fracturing. Worms were then placed in a speedvac to lyophilize and shortly after exposed to cold acetone stored and briefly stored at −20 °C. Acetone was aspirated out and residual acetone was removed via speedvac. Excess methanol from reconstitution of the Rhodamine conjugated Phalloidin (Cytoskeleton Inc., Denver, CO) was removed via speedvac and resuspended in S mix (0.2 mM Na-phosphate buffer: 8.1 ml 1M Na2HPO4, 1.9 ml 1M NaH2PO4, 2.5 ml dH20; 1 mM MgCl2; 0.004% SDS; 745 ml dH2O). Worms were stained with Rhodamine-Phalloidin and S-mix solution in the absence of light for 30 minutes at room temperature. Worms were then washed with PBBT (PBS, 0.5% BSA, 0.5% Tween-20) and mounted on agar pads with mounting solution (90% glycerol, 10% PBS, 1 mg/ml phenlyenediamine) prior to visualization. Worms were analyzed and photographed with an Olympus BX61 microscope equipped with a digital camera.
POSG-1::ABDmoe::mCherry worms
To create POSG-1::ABDmoe::mCherry a ~2.3 kb genomic fragment upstream the OSG-1 gene containing the open reading frame was amplified by PCR and subcloned into ABDmoe::mCherry (a gift of Dr. David Sherwood). The reporter construct was co-injected with the transformation marker rol-6, into the syncytial gonad of adult hermaphrodite nematodes [15] at the concentration of 25 ng/μl, and 100 ng/μl respectively. Worms were analyzed and photographed with an Olympus BX61 microscope equipped with a digital camera.
Statistical analysis
Quantitative data are presented as mean ± standard error of the mean (SEM). The level of significance, assumed at the 95% confidence limit or greater (P<0.05), was calculated using Student’s t-test (http://studentsttest.com) or one-way ANOVA with a Tukey post hoc test (http://astatsa.com/OneWay_Anova_with_TukeyHSD).
Results
Rho signaling is implicated in the heat shock response of C. elegans
Six day-old Bristol strain (N2) worms were subjected to a heat shock (HS, 4 hours at 37 °C) and scored for survival 24 hours later. Under those conditions roughly 50% of worms survived the thermal insult (Fig. 1A). To determine whether Rho signaling plays a role in the HS response of C. elegans we knocked down RHO-1, CDC42 and RAC-2/CED-10/MIG-2 (“mix” in Fig. 1A. These GTPases function redundantly [16]) homologues of respectively RhoA, Cdc42 and Rac1 [17] by bacterial RNAi [12]. Notably, knock down of RHO-1 and CDC42 improved thermotolerance whereas RAC-2/CED-10/MIG-2 knock down did not affect survival.
Figure 1. Rho signaling is involved in the heat shock response of C. elegans.
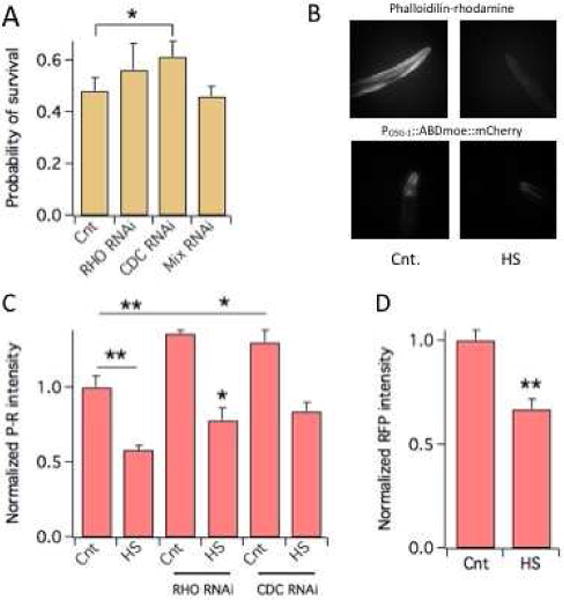
A) Probability of survival of N2 worms to a HS (number of worms surviving the HS divided by the total number of worms) in control or subjected to RNAi of the indicated Rho genes. Mix indicates RAC-2/CED-10/MIG-2 RNAi. N ≥ 5 experiments/group.
B) F-actin visualization in the heads of N2 nematodes stained with P-R and of POSG-1::ABDmoe::mCherry worms in control or after a HS. N ≥ 30 worms. *P<0.05; **P<0.01.
C) Normalized P-R fluorescence in the heads of N2 worms in control or after a HS. Worms subjected to RHO-1 or CDC42 RNAi are indicated. N = 3 experiments/group.
D) Normalized RFP fluorescence in the heads of POSG-1::ABDmoe::mCherry worms in control or after a HS. N ≥ 3 experiments/group. *P<0.05; **P<0.01.
Loss of F-actin lowers thermotolerance
Rho signaling regulates actin dynamics and maintenance and the actin cytoskeleton has been implicated in the response of C. elegans to HS [9, 11]. Therefore, we sought to determine whether Rho signaling contributes to the HS injury through its regulation of actin dynamics. Toward this end, we quantified F-actin by fluorimetry and densitometry in worms stained with phalloidin-rhodamine- (P-R, Figs 1B–1C) or in transgenic worms named POSG-1::ABDmoe::mCherry (Figs. 1B–1D), that expressed F-actin binding protein moesin (moe) driven by the OSG-1 [12] promoter (POSG-1). Both approaches revealed significant decreases in the levels of F-actin following a HS, thereby corroborating the notion that the integrity of the actin cytoskeleton is important for thermotolerance. In what follows we primarily used P-R staining to quantify F-actin, first, to avoid possible transcriptional effects in the POSG-1::ABDmoe::mCherry worm and second, because this method stained more actin and thus improved the signal to noise ratio. Notably, knock down of RHO-1 or CDC42 was associated with a significant increase in the levels of F-actin (~30% Fig. 1C) whereas, RAC-2/CED-10/MIG-2 knock down did not significantly change basal F-actin levels (data not shown). A decrease of F-actin was a general effect of the HS in all conditions tested. Though, in the case of worms subjected to RHO-1 or CDC42 knock down actin stability was maintained at higher levels relative to control worms of F-actin, primarily due to higher cytoskeletal integrity at baseline.
Thermal preconditioning increases resilience through F-actin
Evidence shows that short exposure to high temperatures protect C. elegans against a subsequent full thermal insult and we therefore sought to determine whether the actin cytoskeleton plays a role in this protective mechanism. In agreement with a previous report [18], the probability of survival to a HS increased in worms that had been pre-exposed to 37 °C for 45 min, compared to worms that had not undergone this treatment (Fig. 2A). Thermal preconditioning was associated with a transient decrease of F-actin (Fig. 2B. Compare the levels of F-actin in those worms after the preconditioning thermal insult (“pre”), and 48 hours later (“rec”), prior the HS), as expected. However, after those worms underwent a standard HS, they exhibited significantly higher cytoskeletal integrity compared to control worms. This indicates that thermal preconditioning acts to stimulate F-actin stability which results in increased thermotolerance.
Figure 2. Thermal preconditioning increases actin stability.
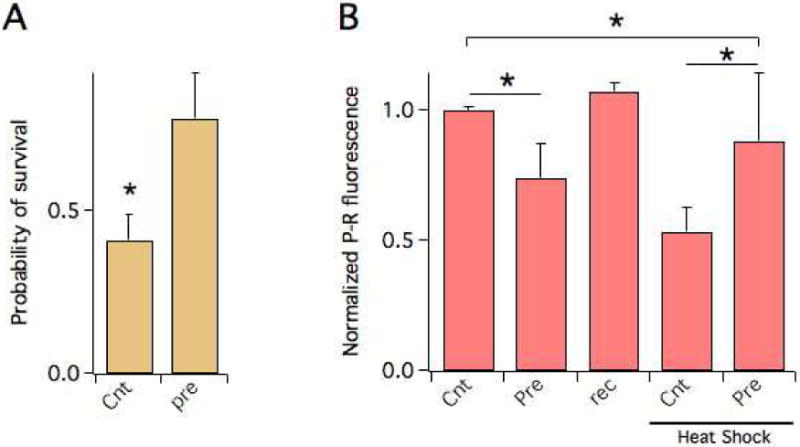
A) Probability of survival to a HS of N2 worms in control or subjected to a pre HS (“pre”. Worms were maintained at 37 ° C for 45 minutes) 48 hours before the HS. N ≥ 5 experiments/group.
C) Normalized P-R fluorescence in the heads of N2 worms in control or subjected to a pre-HS, before and after the HS. The P-R fluorescence of worms subjected to the pre HS measured before the HS is shown (“rec”) is shown for comparison. N ≥ 2 experiments/group. *P<0.05; **P<0.01.
Convergent responses of Rho signaling to heat shock and oxidative stress
RHO-1 has been previously implicated in the response to oxidative stress of C. elegans, where, likewise the HS, its knock down was protective [12]. Indeed, exposure to 1.0 mM hydrogen peroxide (H2O2) for 20 min [12] led to a 25% decrease in the amounts of F-actin in N2 worms (Fig. 3), corroborating the notion that also during an oxidative challenge RHO-1 acts to alter actin dynamics.
Figure 3. Increased F-actin in OSG-1 KO worms following an oxidative insult.
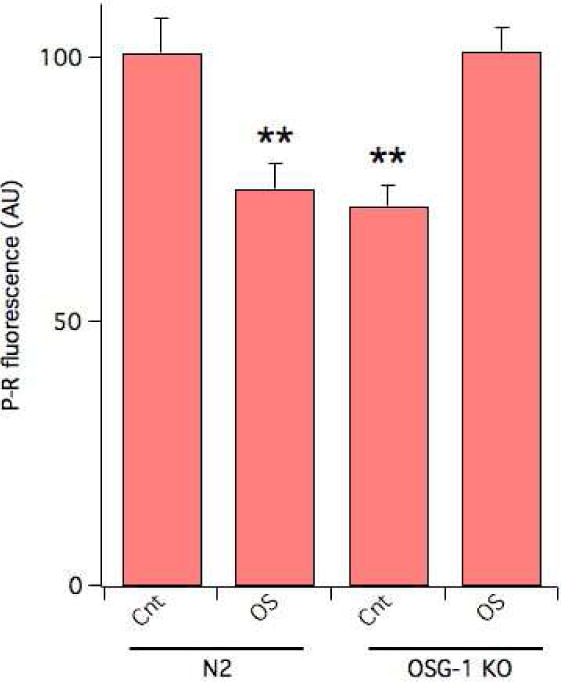
P-R fluorescence in control or following 20 minutes exposure to 1.0 mM H2O2 in N2 or OSG-1 KO worms. N ≥ 60 worms **P<0.01.
Divergent triggers of Rho signaling during heat shock and oxidative stress
The response of RHO-1 to conditions of OS, is controlled by a guanine nucleotide exchange factor of the Dbl family, named OSG-1 (oxidative stress susceptible GEF 1) [12]. Genetic ablation of OSG-1 increases resistance of the animal to oxidative stress suggesting that this might correlate with altered actin dynamics. Notably, OS reduced F-actin stability by ~25% in N2 worms, as assessed by P-R staining (Fig. 3). However, following an oxidative insult a significant increase in cytoskeletal integrity was observed in OSG-1 KO worms compared to control.
OSG-1 is not implicated in the HS response
Consistent with their constitutive lower levels of F-actin, OSG-1 KO worms survived a HS roughly 3-fold less efficiently compared to N2 worms (N2 = 0.48±0.05; OSG-1 0.18±0.04; P<0.021; compare Figs 1A and 4A). RHO-1 or CDC42 knock down was associated with significantly increased probability of survival which correlated with increased F-actin levels (Figs. 4A–4B). In contrast RAC-2/CED-10/MIG-2 knock down did not affect OSG-1 KO worms’ resiliency to HS.
Figure 4. Constitutive low F-actin in OSG-1 KO worms.
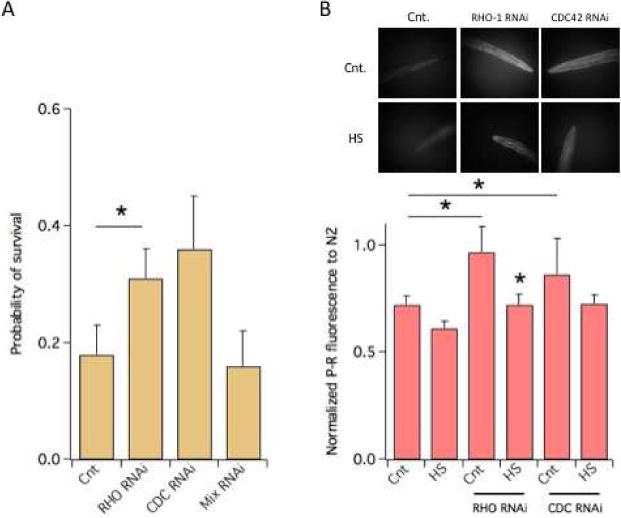
A) Probability of survival of OSG-1 KO worms to a HS in control or subjected to the RNAi of the indicated Rho genes. Mix indicates RAC-2/CED-10/MIG-2 RNAi. N ≥ 5 experiments/group.
B) P-R visualization of F-actin in the heads of the OSG-1 KO nematodes in the absence/presence of RHO-1 or CDC42 RNAi treatment, in control or after a HS.
C) Normalized P-R fluorescence in the heads of the OSG-1 KO nematodes in the absence/presence of RHO-1 or CDC42 RNAi treatment, in control or after a HS. Data are scaled to the fluorescence of N2 worms in control which is = 1. N ≥ 3 experiments/group. *P<0.05; **P<0.01.
Discussion
This study was undertaken to gain insight into the role of Rho signaling in the response to environmental stressors. Behavioral and fluorimetry data underscored a causative relationship between the physiological resistance of C. elegans nematodes to thermal and oxidative stress and the regulation of actin dynamics by Rho signaling. Specifically, a decrease in F-actin, mediated by RHO-1, appears to be a shared mechanism of vulnerability to both oxidative and thermal stress. Overall, these data corroborate the emerging notion that actin stability plays a crucial role in the cell’s response to stress. Previous studies in yeast have linked the formation of aggregates of F-actin to apoptosis. Thus, pharmacological agents that stabilize actin turnover enhance apoptotic death and manipulation of genes that control the formation of actin aggregates is associated with decreased ROS and longer lifespan [19]. The findings reported here offer a more complex picture of the potential effects of F-actin dysregulation, as increased F-actin can also be protective against thermal and oxidative stress. A simplistic explanation for the correlation between the levels of F-actin and resilience rests in the fact that the rapid translocation of proteins across the cell is vital under immediate conditions of stress. In response to a heat shock, the cell activates the conserved transcription factor HSF-1 which triggers rapid upregulation of a network of heat shock proteins (HSPs) that act to stabilize new proteins, refold damaged proteins or tag them for degradation if they are damaged below repair. Oxidative stress, especially when acute, is also characterized by diffuse damage of proteins, lipids and DNA/RNAs and therefore similar considerations apply. These complex responses are coupled with large movement of proteins within the cells and the integrity of the actin cytoskeleton–which provides mechanical support to cells and tracks for trafficking routes for signal transduction– may represent a crucial asset for the survival of the organism under stress. Thermal preconditioning, a mechanism that may resemble ischemic preconditioning in mammalian heart [20], further underscores the importance of the actin cytoskeleton for resistance to stress. C. elegans appear to be capable of adapting to thermal stress through acute exposure to non-lethal thermal stress, which leads to increased F-actin stability following the full thermal insult. The molecular mechanisms that trigger temperature-induced actin remodeling are poorly understood but the conservation of the actin cytoskeleton argues that this may be a mechanism of adaption to environmental stressors that is conserved in biological organisms, including Homo sapiens.
Rho signaling plays a crucial role for the regulation of actin dynamics, indeed OSG-1 KO worms exhibit constitutively low levels of F-actin. However under conditions of stress the activity of Rho signaling results in less F-actin thereby increasing susceptibility to stress. This pleiotropic nature of Rho is emerging as an intrinsic property of biological networks. Examples include, just to mention a few, defective proteolytic cleavage of Amyloid Precursor protein that generates toxic beta-amyloid in Alzheimer’s disease, the fact that alpha-synuclein [21], whose function in the healthy brain is not known, is a major constituent of Lewy bodies in Parkinson’s disease and Focal Adhesion kinase (FAK), which can either protect or induce apoptosis depending on the specific cellular conditions [22, 23]. Rho signaling specificity and spatio-temporal control are under the command of multiple regulators including GEFs, Guanine nucleotide dissociation inhibitors (GDIs), and GTPase activating proteins (GAPs), that regulate individual GTPases [24]. This further increases complexity, as the regulation of Rho GTPases during a HS or OS may likely depend on distinct molecular events and competing regulators. The case of OSG-1, which controls RHO-1 during oxidative stress and not under a heat shock, is an example in this sense. Thus, while the action of Rho GTPases in decreasing F-actin during thermal and oxidative stresses is a shared mechanism of susceptibility, its regulators are not. This may guide pharmacological strategies in the future, which should be able to intervene on specific signaling modalities while leaving the others unaffected, by targeting individual GTPase modulatory proteins.
Highlights.
Identification of stress specific recruitment of Rho GTPases during oxidative and thermal stress
Thermal preconditioning enhances cytoskeletal resilience
Multifunctional GEF, OSG-1, differentially modulates thermal and oxidative stress responses
Acknowledgments
We thank Dr. David Sherwood for the ABDmoe::mCherry construct, Dr. Zhibing Duan for help constructing the POSG-1::ABDmoe::mCherry worm and Dr. Shuang Liu for critical reading of the manuscript. This work was supported by a NSF grant (1456675) and a NIH grant (1R21NS096619) to Federico Sesti and two Aresty Research Fellowships to Rahul Patel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they do not have conflict of interests
References
- 1.Li Z, Srivastava P. Heat-shock proteins. Curr Protoc Immunol, Appendix. 2004;1 doi: 10.1002/0471142735.ima01ts58. Appendix 1T. [DOI] [PubMed] [Google Scholar]
- 2.Ohtsuka K, Suzuki T. Roles of molecular chaperones in the nervous system. Brain Res Bull. 2000;53:141–146. doi: 10.1016/s0361-9230(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 3.Beere HM. The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann SH. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 5.Liu CP, Fu J, Xu FP, Wang XS, Li S. The role of heat shock proteins in oxidative stress damage induced by Se deficiency in chicken livers. Biometals. 2015;28:163–173. doi: 10.1007/s10534-014-9812-x. [DOI] [PubMed] [Google Scholar]
- 6.Pappolla MA, Sos M, Omar RA, Sambamurti K. The heat shock/oxidative stress connection. Relevance to Alzheimer disease. Mol Chem Neuropathol. 1996;28:21–34. doi: 10.1007/BF02815201. [DOI] [PubMed] [Google Scholar]
- 7.Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Morano KA, Grant CM, Moye-Rowley WS. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics. 2012;190:1157–1195. doi: 10.1534/genetics.111.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird NA, Douglas PM, Simic MS, Grant AR, Moresco JJ, Wolff SC, Yates JR, 3rd, Manning G, Dillin A. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science. 2014;346:360–363. doi: 10.1126/science.1253168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch WJ, Suhan JP. Morphological study of the mammalian stress response: characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblasts after heat-shock treatment. J Cell Biol. 1985;101:1198–1211. doi: 10.1083/jcb.101.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. BioEssays : news and reviews in molecular, cellular and developmental biology. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan Z, Sesti F. Guanine nucleotide exchange factor OSG-1 confers functional aging via dysregulated Rho signaling in Caenorhabditis elegans neurons. Genetics. 2015;199:487–496. doi: 10.1534/genetics.114.173500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotella D, Hernandez-Enriquez B, Duan Z, Wu X, Gazula VR, Brown MR, Kaczmarek LK, Sesti F. An evolutionarily conserved mode of modulation of Shaw-like K(+) channels. FASEB J. 2013;27:1381–1393. doi: 10.1096/fj.12-222778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strome S. Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode Caenorhabditis elegans. J Cell Biol. 1986;103:2241–2252. doi: 10.1083/jcb.103.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park KH, Hernandez L, Cai SQ, Wang Y, Sesti F. A Family of K+ Channel Ancillary Subunits Regulate Taste Sensitivity in Caenorhabditis elegans. J Biol Chem. 2005;280:21893–21899. doi: 10.1074/jbc.M502732200. [DOI] [PubMed] [Google Scholar]
- 16.Lundquist EA, Reddien PW, Hartwieg E, Horvitz HR, Bargmann CI. Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development. 2001;128:4475–4488. doi: 10.1242/dev.128.22.4475. [DOI] [PubMed] [Google Scholar]
- 17.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Kourtis N, Nikoletopoulou V, Tavernarakis N. Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature. 2012;490:213–218. doi: 10.1038/nature11417. [DOI] [PubMed] [Google Scholar]
- 19.Gourlay CW, Ayscough KR. The actin cytoskeleton in ageing and apoptosis. FEMS Yeast Res. 2005;5:1193–1198. doi: 10.1016/j.femsyr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 21.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 22.Yu W, Gowda M, Sharad Y, Singh SA, Sesti F. Oxidation of KCNB1 potassium channels triggers apoptotic integrin signaling in the brain. Cell Death Dis. 2017;8:e2737. doi: 10.1038/cddis.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes & development. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]


