Abstract
Objective
To examine associations between interpregnancy interval, the duration from the preceding birth to the conception of the next-born index child, and adverse birth outcomes using designs that adjust for measured and unmeasured factors.
Methods
In this prospective cohort study we used population-based Swedish registries from 1973 to 2009 to estimate the associations between interpregnancy interval (referent 18-23 months), and adverse birth outcomes [i.e., preterm birth (PTB, < 37 weeks), low birth weight (LBW, <2,500 g), small for gestational age (SGA, > 2 standard deviations below average weight for gestational age)]. Analyses included cousin- and sibling-comparisons, and postbirth intervals (ie, the interval between second- and third-born offspring predicting second-born outcomes), to address unmeasured familial confounding.
Results
Traditional cohort-wide analyses showed higher odds of PTB [adjusted odds ratio (aOR)=1.51, 99% CI=1.39-1.63, 5.99% PTBs)] and LBW (aOR=1.25, 99% CI=1.13-1.39, 3.32% LBW) after short interpregnancy interval (0-5 months) compared to offspring born after an interpregnancy interval of 18-23 months (3.21% PTBs, 1.92% LBW). For pregnancy intervals of 60 months or more, odds of PTB (aOR=1.51, 99% CI=1.43-1.60, 5.07% PTBs), LBW (a OR=1.61, 99% CI=1.50-1.73, 3.43% LBW births), and SGA (aOR=1.54, 99% CI=1.42-1.66, 2.49% SGA births) were also higher when compared with the reference interval (1.53% SGA). Except for PTB (aOR=1.72, 99% CI=1.26-2.35), associations were attenuated in cousin-comparisons. A small association between short interpregnancy interval and PTB remained in sibling-comparisons (aOR=1.22, 99% CI=1.11-1.35), but associations with LBW (aOR=0.83, 99% CI=0.74-0.94) and SGA (aOR=0.74, 99% CI=0.64-0.85) reversed direction. Associations between long interpregnancy interval and adverse birth outcomes remained through cousin- and sibling-comparisons. Post birth interval analyses showed familial confounding is present for short interpregnancy intervals, but supported independent associations for long interpregnancy intervals.
Conclusion
Familial confounding explains most of the association between short interpregnancy interval and adverse birth outcomes while associations with long interpregnancy intervals were independent of measured and unmeasured factors.
Introduction
Interpregnancy interval is the duration between the birth of an earlier born sibling and the conception of the next sibling. Research suggests that deviation from an average interpregnancy interval length of one to three years is associated with adverse offspring outcomes. For example, both short and long interpregnancy intervals are associated with risk for the offspring to be born preterm (PTB, < 37 weeks of gestation), to be of low birth weight (LBW, <2500 grams), and to be small for gestational age (SGA, >2 standard deviations below the mean weight for gestational age)(1-6).
Causal, mechanistic hypotheses linking interpregnancy interval with adverse offspring outcomes have been proposed (for review see 9). Though these causal hypotheses exist, small sample sizes, limited control over important covariates, and skewed measurement of interpregnancy interval length (i.e., birth to birth rather than birth to conception), have hampered previous research (6, 14). Researchers also have suggested that much, if not all, of the association between short interpregnancy interval and adverse birth outcomes may be due to confounding factors (6, 14-17), as there are a multitude of parental demographic, physical, and mental health factors associated with both interpregnancy interval and adverse offspring outcomes (18-20). Determining if associations between interpregnancy interval and adverse birth outcomes are independent of confounding factors, and thus, consistent with causal claims, has important public health implications. Interpregnancy interval is a modifiable risk factor (21) and, when public health recommendations are based on studies that rigorously evaluate causal claims, change in adverse outcomes can occur (1, 22, 23). Therefore, the current study was designed to provide a rigorous examination of the influence of confounding on the associations between interpregnancy interval and adverse birth outcomes.
We estimated the associations between interpregnancy interval and PTB, LBW, and SGA applying rigorous control for potential confounding by adjusting for several measured covariates and comparing differentially exposed cousins and siblings. Cousin-and sibling-comparison designs rule out influence from unmeasured environmental and genetic risks that make relatives similar (24). Previous research has used sibling-comparison designs to investigate these associations (15, 16). We sought to replicate those findings here; we also include a cousin-comparison. Cousin-comparisons address residual confounding due to offspring birth-order and maternal age while also improving the generalizability of the findings. Further, we also performed a negative-control analysis using the post-birth interpregnancy interval. More specifically, we used the interval to the following (next-born) sibling to predict the outcome of the prior-born sibling. Because any association with post-birth interval cannot be due to the pregnancy-related mechanisms through which interpregnancy interval theoretically functions, it may be taken to indicate a role of family confounding (14). In order to apply these methodological advances, we used a nation-wide Swedish population-based sample of families to provide the largest study on this topic to date.
Materials and Methods
The current study used prospectively collected cohort data of individuals living in Sweden from 1973 to 2009. The institutional review board at Indiana University and the Regional Ethical Review Board in Stockholm approved this study. Data for the current study were obtained by linking information available in the several government-maintained, Swedish population-based registries. We first identified offspring and their mothers using the Swedish Medical Birth Register, which provided data on more than 96% of births in Sweden since 1973 (25). After identifying fathers using the Multi-Generation Register (26), we then collected information on several parental characteristics and offspring outcomes from the following registers: (1) the National Crime Register provided information on broad violent and non-violent criminal convictions since 1973, (2) the National Patient Register provided diagnoses for psychopathological and substance-related inpatient hospital admissions since 1973 (27), (3) the Education Register provided information on highest level of completed formal education through 2009; and (5) the Migration Register and the (6) Cause of Death Register provided information important in determining the censoring information.
The initial sample included live birth-related information for 3,403,185 individuals with valid maternal identifiers born between 1973 and 2009 and the final cohort consisted of 1,050,271 second-born and 368,549 third-born offspring (Figure 1). These individuals were born to 1,072,081 distinct biological mothers and 1,083,329 distinct biological fathers. There were 784,640 distinct maternal-side grandmothers represented in the cohort used in cousin-comparison models. Cousin-comparison analyses included 331,283 differentially-exposed cousin pairs. Sibling-comparisons included all second- and third-born siblings, though 554,652 were differentially exposed. Post-birth interval analyses only included the 368,549 individuals who had third-born siblings.
Figure 1.
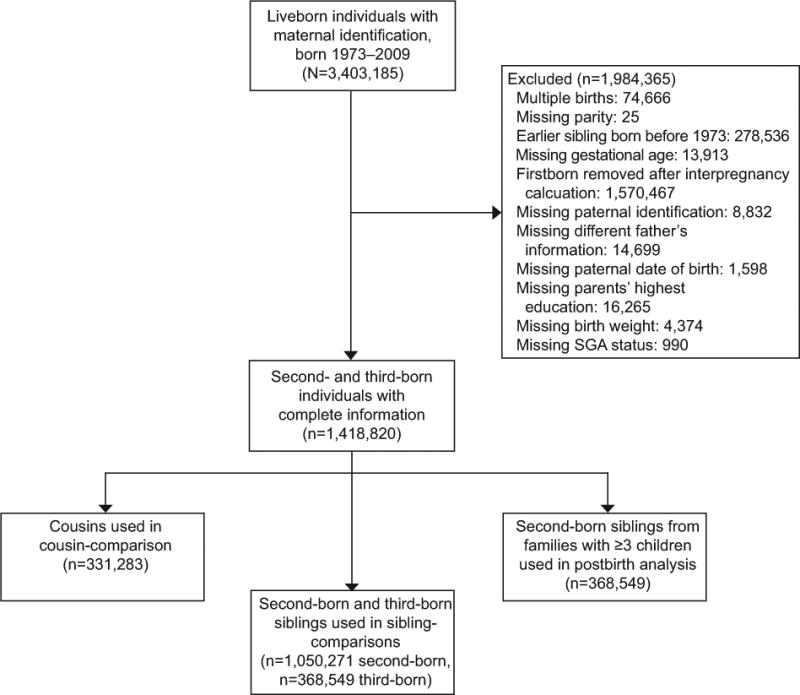
Sample flow chart. SGA, small for gestational age.
We defined interpregnancy interval as the number of completed months between the birth of the preceding (earlier-born) offspring and the date of conception of the index (next-born) offspring. Date of conception was obtained from information on gestational age at birth estimated from last menstrual period or ultrasound. In sibling-comparison analyses, interpregnancy interval was calculated between the first- and second-born, as well as between the second- and third-born offspring. Second- and third-born offspring outcomes were compared. Post-birth intervals were calculated between the birth of the second-born and the conception of the third-born sibling and used to predict second-born outcomes. Interpregnancy intervals were categorized as 0 to 5 months, 6 to 11 months, 12 to 17 months, 18 to 23 months (referent), 24 to 59 months, and 60 or more months in order to allow for comparison across previous studies (15, 16).
We predicted three adverse birth outcomes in the index offspring: PTB (< 37 weeks gestation), LBW (<2500 grams), and SGA (>2 standard deviations below the mean weight for gestational age) . Offspring with birth weights <300 g were excluded from our analyses.
Depending on the model, various measured covariates were included (Table 1). These included maternal and paternal age at the index birth, highest education level, nationality, and if the earlier-born offspring had a different biological father. Some adjusted models also included measured lifetime parental psychopathology. In particular, we included parental criminality as indexed by any criminal conviction under the Swedish Penal code beginning at age 15, the Swedish age of legal responsibility; substance use problem defined as an inpatient hospitalization involving a primary or secondary diagnosis of alcohol- or any other, non-nicotine, substance use disorder; suicide attempt as indicated by an attempt recorded in inpatient care records as the primary or secondary reason for care; and severe mental illness as measured by an inpatient hospitalization for bipolar disorder, broadly defined schizophrenia, or other nonorganic psychotic disorders. Except for criminality, the minimum age for all parental mental health outcomes was 12 years old. All clinical diagnoses were according to ICD versions 8, 9, and 10 (codes available upon request). These factors have been shown to vary with both interpregnancy interval and adverse birth outcomes (28). Some adjusted models also included binary indicators of potential adverse outcomes in the first-born including PTB, LBW, and SGA.
Table 1. Descriptive characteristics and covariates for the index, second-born offspring.
| Variable | n (%) |
|---|---|
| Second-born offspring | |
| Interpregnancy Interval (months) | |
| 0-5 | 27888 (2.7) |
| 6-11 | 128096 (12.2) |
| 12-17 | 202011 (19.2) |
| 18-23* | 166374 (15.8) |
| 24-59 | 414715 (39.5) |
| 60+ | 111187 (10.6) |
| Maternal Age (yrs) | |
| < 20 | 6618 (0.6) |
| 20-29* | 596101 (56.8) |
| 30-39 | 433634 (41.29) |
| ≥ 40 | 13918 (1.3) |
| Paternal Age (yrs) | |
| < 20 | 1299 (0.1) |
| 20-29* | 390057 (37.1) |
| 30-39 | 576730 (54.9) |
| ≥ 40 | 82185 (7.8) |
| Highest Maternal Education | |
| ≤ 9 years primary and lower secondary* | 17979 (1.7) |
| 9 years primary and lower secondary | 88677 (8.4) |
| 3 years upper secondary | 514757 (49.0) |
| Post-secondary/and or postgraduate | 428858 (40.8) |
| Highest Paternal Education | |
| ≤ 9 years primary and lower secondary* | 43960 (4.2) |
| 9 years primary and lower secondary | 131470(12.5) |
| 3 years upper secondary | 528773 (50.4) |
| Post-secondary/and or postgraduate | 346068 (33.0) |
| Mother of Swedish Nationality | 933506(88.9) |
| Father of Swedish Nationality | 926035 (88.2) |
| First- and Second-born to Different Fathers | 93379 (8.9) |
| Maternal Psychopathology | |
| Criminality | 109882 (10.5) |
| Substance use problem | 23099 (2.2) |
| Attempted suicide | 17300 (1.7) |
| Severe mental illness | 8378(0.8) |
| Paternal Psychopathology | |
| Criminality | 393941(37.5) |
| Substance use problem | 16520(1.6) |
| Attempted suicide | 33079(3.2) |
| Severe mental illness | 6140(0.6) |
| First-born preterm | 56394(5.4) |
| First-born low birth weight | 39238(3.7) |
| First-born small for gestational age | 39297(3.7) |
Note:
reference category
We used logistic regression analyses when predicting the second-born, index offspring's outcomes. We emphasize effect size interpretations, but have also used a 99% confidence interval (CI), due to the number of comparisons we performed. The first model, Model 1, was a baseline model that adjusted only for offspring sex and year of birth. The second model, Model 2, additionally adjusted for the measured covariates of maternal and paternal age, highest education, nationality, and if the fathers were different between the first- and second-born. Model 3 additionally adjusted for parental psychopathology variables, including maternal and paternal criminality, attempted suicide, substance misuse, and severe mental illness. Model 4 additionally adjusted for adverse birth outcomes of the first-born.
Models 5 and 6 were within-family comparisons. Cousins and siblings are more similar on socioeconomic characteristics, familial culture, and genetic factors (i.e., cousins share on average 12.5%, and siblings on average 50% of their genes by descent) than unrelated individuals (29). As such, the increased control of environmental and genetic confounding gained by the within-family designs provides an alternative to traditional methods that compare unrelated individuals. Using both cousin- and sibling-comparisons allows for inferences to be validated across different model assumptions and limitations (e.g., birth order does not confound the cousin-comparison but necessarily limits the sibling-comparison). In Model 5, we limited the sample to maternal cousins and used conditional logistic regression. Model 5 adjusted for all the same measured covariates included in Model 4 as these may have varied between cousins. In Model 6, we compared outcomes across second- and third-born siblings who differed in interpregnancy interval category while also adjusting for measured covariates that may have varied across siblings (i.e., offspring sex, birth year, parity, maternal and paternal age, and different father).
Model 7 explored post-birth intervals as negative control analyses. Post-birth interpregnancy interval, the interval between the second and third-born offspring, was used to predict the second-born's outcomes. Due to timing influences, if we found associations between the post-birth interval and the second-born's birth outcomes, the association suggests that there are genetic or environmental family confounding effects that need to be considered as a post-birth interval cannot have causal influence on the second-born's outcomes.
We re-estimated the models by predicting continuously measured gestational age and birth weight to examine whether results for binary outcomes generalize while also testing for non-linearity. We also explored for cohort effects by stratifying the sample into three birth year ranges: (1) born 1973-1984, (2) 1985-1996 and (3) 1997-2009.
Results
In the cohort (Table 1) and across baseline and adjusted analyses, pregnancies that followed short (less than 12 months) or long (greater than 24 months) interpregnancy intervals showed higher odds of adverse birth outcomes (Table 2). For example, in the baseline Model 1 analyses, short (0-5 months) interpregnancy interval was associated with higher odds of PTB (OR=1.88, 99% CI=1.74–2.02), LBW (OR=1.72, 99% CI=1.56- 1.90), and SGA (OR=1.41, 99% CI=1.25- 1.58). Odds of adverse birth outcomes were also higher if interpregnancy intervals were 24 months or greater as compared to the 18-23 months reference category. For example, interpregnancy intervals of 60 or more months were associated with higher odds of PTB (OR=1.75, 99% CI=1.67-1.84), LBW (OR=1.99, 99% CI=1.87- 2.12), and SGA (OR=1.91, 99% CI=1.78- 2.05). Incremental adjustment for measured covariates in Models 2, 3, and 4 attenuated these associations, though across short and long intervals the odds remained significantly higher for all, except for short interpregnancy interval predicting SGA (Table 2).
Table 2. Odds ratios predicting adverse birth outcomes across interpregnancy interval and model.
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Interpregnancy Interval (months) | |||||||||||
|
|
|||||||||||
| 0-5 | 6-11 | 12-17 | 18-23 | 24-59 | 60+ | ||||||
|
| |||||||||||
| Outcome variable and model | OR | 99% CI | OR | 99% CI | OR | 99% CI | OR | 99% CI | OR | 99% CI | |
|
| |||||||||||
| Preterm birth | |||||||||||
| n cases (% by interval) | 2,275 (5.99) | 6,285 (3.83) | 8,102 (3.30) | 6,529 (3.21) | 20,576 (3.63) | 10,179 (5.07) | |||||
| Model 1 | 1.88 | 1.74 – 2.02 | 1.18 | 1.12 – 1.25 | 1.01 | 0.97 – 1.07 | Ref | 1.15 | 1.11 -1.20 | 1.75 | 1.67 – 1.84 |
| Model 2 | 1.76 | 1.63 – 1.90 | 1.17 | 1.11 – 1.23 | 1.02 | 0.98 – 1.07 | Ref | 1.13 | 1.08 – 1.17 | 1.52 | 1.44 – 1.61 |
| Model 3 | 1.72 | 1.59 – 1.86 | 1.16 | 1.10 – 1.22 | 1.02 | 0.97 – 1.07 | Ref | 1.13 | 1.07 – 1.17 | 1.52 | 1.43 – 1.61 |
| Model 4 | 1.51 | 1.39 – 1.63 | 1.16 | 1.10 – 1.22 | 1.03 | 0.98 – 1.08 | Ref | 1.11 | 1.07 – 1.16 | 1.51 | 1.43 – 1.60 |
| Model 5 / Cousin-comparison | 1.72 | 1.26 – 2.35 | 1.26 | 0.97 – 1.63 | 1.07 | 0.83 – 1.38 | Ref | 1.09 | 0.86 – 1.40 | 1.42 | 1.04 – 1.66 |
| Model 6 /Sibling-comparison | 1.23 | 1.11 – 1.36 | 1.15 | 1.07 – 1.24 | 1.10 | 1.02 – 1.19 | Ref | 1.06 | 0.99 – 1.13 | 1.17 | 1.07 – 1.27 |
| Model 7 /Post-birth interval | 2.31 | 2.03 – 2.62 | 1.22 | 1.10 – 1.35 | 1.04 | 0.93 – 1.15 | Ref | 0.96 | 0.88 – 1.05 | 1.00 | 0.92 – 1.10 |
| Low birth weight | |||||||||||
|
| |||||||||||
| n cases (% by interval) | 1,261 (3.32) | 3,372 (2.06) | 4,515 (1.84) | 3,914 (1.92) | 12679 (2.24) | 6,902 (3.43) | |||||
| Model 1 | 1.72 | 1.56 – 1.90 | 1.06 | 0.99 – 1.13 | 0.95 | 0.89 – 1.01 | Ref | 1.19 | 1.12 – 1.25 | 1.99 | 1.87 – 2.12 |
| Model 2 | 1.54 | 1.39 – 1.70 | 1.04 | 0.97 -- 1.11 | 0.96 | 0.90 – 1.02 | Ref | 1.14 | 1.08 – 1.20 | 1.65 | 1.54 – 1.77 |
| Model 3 | 1.48 | 1.34 – 1.64 | 1.02 | 0.96 – 1.10 | 0.95 | 0.90 – 1.02 | Ref | 1.14 | 1.08 – 1.20 | 1.65 | 1.54 – 1.77 |
| Model 4 | 1.25 | 1.13 – 1.39 | 1.01 | 0.94 – 1.08 | 0.96 | 0.90 – 1.03 | Ref | 1.12 | 1.06 – 1.18 | 1.61 | 1.50 – 1.73 |
| Model 5 / Cousin-comparison | 1.42 | 0.96 – 2.10 | 1.13 | 0.81 – 1.56 | 1.13 | 0.82 – 1.55 | Ref | 1.06 | 0.78 – 1.44 | 1.50 | 1.12 – 1.99 |
| Model 6 /Sibling-comparison | 0.86 | 0.76 – 0.97 | 0.93 | 0.85 – 1.03 | 1.00 | 0.91 – 1.09 | Ref | 1.05 | 0.97 – 1.13 | 1.24 | 1.12 – 1.38 |
| Model 7 /Post-birth interval | 2.99 | 2.58 – 3.47 | 1.30 | 1.14 – 1.48 | 1.04 | 0.91 – 1.18 | Ref | 0.92 | 0.83 – 1.03 | 0.98 | 0.88 – 1.11 |
| Small for gestational age | |||||||||||
|
| |||||||||||
| n cases (% by interval) | 819 (2.16) | 2,385 (1.46) | 3,608 (1.47) | 3,109 (1.53) | 10,226 (1.80) | 5,010 (2.49) | |||||
| Model 1 | 1.41 | 1.25 – 1.58 | 0.96 | 0.88 –1.04 | 0.98 | 0.91 – 1.05 | Ref | 1.20 | 1.13 – 1.28 | 1.91 | 1.78 – 2.05 |
| Model 2 | 1.24 | 1.11 – 1.40 | 0.94 | 0.87 – 1.02 | 0.99 | 0.92 – 1.06 | Ref | 1.15 | 1.09 – 1.22 | 1.59 | 1.47 – 1.72 |
| Model 3 | 1.21 | 1.07 – 1.36 | 0.93 | 0.86 – 1.01 | 0.98 | 0.92 – 1.05 | Ref | 1.15 | 1.09 – 1.23 | 1.59 | 1.47 – 1.72 |
| Model 4 | 1.06 | 0.94 – 1.20 | 0.91 | 0.84 – 0.99 | 0.99 | 0.92 – 1.06 | Ref | 1.14 | 1.06 – 1.21 | 1.54 | 1.42 – 1.66 |
| Model 5 / Cousin-comparison | 0.93 | 0.55 – 1.56 | 0.89 | 0.59 – 1.35 | 1.12 | 0.77 – 1.61 | Ref | 1.10 | 0.76 – 1.57 | 1.53 | 1.11 – 2.12 |
| Model 6 /Sibling-comparison | 0.76 | 0.65 – 0.88 | 0.89 | 0.78 – 0.99 | 0.96 | 0.87 – 1.07 | Ref | 1.06 | 0.97 – 1.16 | 1.24 | 1.10 – 1.40 |
| Model 7 / Post-birth interval | 2.14 | 1.79 – 2.56 | 1.20 | 1.03 – 1.39 | 1.11 | 0.96 – 1.29 | Ref | 1.01 | 0.90 – 1.14 | 1.04 | 0.92 – 1.18 |
Models were adjusted for the following measured covariates: Model 1: offspring sex, year of birth Model 2: Model 1 + maternal and paternal age, highest education, nationality, different fathers Model 3: Model 2 + maternal and paternal criminality, attempted suicide, substance misuse, and severe mental illness Model 4: Model 3 + first-born preterm birth, low birth weight, and small for gestational age Model 5 / Cousin-comparison: offspring sex, year of birth, maternal and paternal age, highest education, nationality, different fathers, maternal and paternal criminality, attempted suicide, substance misuse, and severe mental illness, first-born preterm birth, low birth weight, and small for gestational age Model 6 / Sibling-comparison: offspring sex, year of birth, maternal and paternal age, parity, different father Model 7 / Post-birth interval: offspring sex, year of birth, maternal and paternal age, highest education, nationality, different fathers, maternal and paternal criminality, attempted suicide, substance misuse, and severe mental illness, first-born preterm birth, low birth weight, and small for gestational age. Bold font indicates statistical significance with 99% confidence interval.
In fixed-effects cousin-comparisons (Model 5), in which second-born cousins with varying interpregnancy intervals were compared, the association between short interpregnancy interval (i.e., 0-5 months)remained robust when predicting PTB (OR=1.72, 99% CI=1.26–2.35). The effect estimate remained elevated, but not statistically significant when predicting LBW (OR=1.42, 99% CI=0.96–2.10), but was fully attenuated when predicting SGA (OR=0.93, 99% CI=0.55-1.56). For cousin-comparisons of long interpregnancy intervals (i.e., ≥ 60 months), the association was robust when predicting all three adverse birth outcomes: PTB (OR=1.42, 99% CI=1.04 - 1.66), LBW (OR=1.50, 99% CI=1.12 - 1.99), and SGA (OR=1.53, 99% CI=1.11–2.12).
Our sibling-comparison analyses (Table 2, Model 6) compared the odds of outcome between the second- and third-born offspring if their interpregnancy interval categories differed. The association between the shortest interpregnancy interval and PTB remained minimally elevated (OR=1.22, 99% CI=1.11 - 1.35). Associations between the shortest interpregnancy interval and LBW (OR=0.83, 99% CI=0.74-0.94) and SGA (OR=0.74, 99% CI=0.64-0.85), however, reversed direction, suggesting that siblings experiencing the shortest interpregnancy interval in the family were less likely to be born LBW or SGA as compared with their sibling. For long interpregnancy intervals, associations were attenuated, though remained present for PTB (OR=1.18, 99% CI=1.08-1.28), LBW (OR=1.28, 99% CI=1.16-1.43), and SGA (OR=1.32, 99% CI=1.18-1.48).
Using the post-birth interval (i.e., the interval between the second- and third-born offspring) we predicted the second-born's outcomes. Results are presented in Table 2, Model 7. Short post-birth intervals were associated with higher odds of PTB (OR=2.31, 99% CI=2.03-2.62), LBW (OR=2.99, 99% CI=2.58-3.47), and SGA (OR=2.14, 99% CI=1.79-2.56) in the second-born. Thus, the length of interval after the birth of the second-born offspring to the next sibling's conception significantly predicted the outcomes of the second-born offspring, suggesting familial confounding. Long post-birth intervals, on the other hand, were not associated with PTB (OR=1.00, 99% CI=0.92-1.10), LBW (OR=0.98, 99% CI=0.88-1.11), or SGA (OR=0.92, 99% CI=0.92-1.18).
Models predicting continuously measured gestational age and birth weight controlling for gestational age (Appendix 1, available online at http://links.lww.com/xxx), are consistent with our results predicting binary outcomes. Analyses stratified by year of birth [(1) born 1973-1984, (2) 1985-1996 and (3) 1997-2009] were comparable to the main results that spanned over 35 years (Appendix 2, available online at http://links.lww.com/xxx). However, adjusted analyses of short interpregnancy interval predicting adverse birth outcomes were slightly higher for the oldest birth cohort (born 1973-1984) as compared with the younger birth cohorts.
Discussion
Our study indicated that observed associations between short interpregnancy interval and LBW and SGA were likely due to factors that remain constant for a woman throughout her successive pregnancies (e.g., prenatal care practices, genetic vulnerability). The association between short interpregnancy interval and PTB, on the other hand, was robust, but small in magnitude.
Our short interpregnancy interval findings are in agreement with other studies that reported elevated associations with adverse birth outcomes in cross-sectional cohort data (1-6). However, with our more rigorous control of unmeasured confounding factors in sibling-, cousin-, and post-birth interval analyses, our interpretation is different from the causal assumptions made by previous studies. Our findings are in agreement with recent research showing that sibling-comparisons attenuate the association between short interpregnancy intervals and adverse birth outcomes (15, 16). Our findings are also in agreement with another paper that used post-birth interpregnancy intervals (14). In addition, one study also found that the directionality of association switches for LBW in the sibling-comparison model (16) which may suggest a suppression effect.
We found a different pattern of results when examining long-interpregnancy intervals. For long interpregnancy interval, measured and unmeasured factors explain much, but not all, of the observed associations with PTB, LBW, and SGA thereby suggesting an independent association of long interpregnancy interval with elevated risk of adverse birth outcomes. Thus, we cannot exclude a causal explanation to the observed associations, as they were robust even when controlling for genetic and environmental factors shared by cousins and siblings. It may be that unmeasured unique (i.e., non-shared between siblings or cousins) confounders exist in association with long interpregnancy intervals that may still be confounding the association observed. Further, the lack of association between long post-birth interpregnancy interval and adverse birth outcomes indicates that within-family confounding for long interpregnancy interval is minimal, and there is no indication of an earlier-born's birth outcome influencing the odds of future long interpregnancy intervals. This supports and extends previous sibling-comparison studies showing elevated risk for SGA (15) and LBW (16) following long-interpregnancy interval by introducing results from cousin- and post-birth interval analyses. A large meta-analysis (1) has also previously shown increased risk for PTB, LBW, and SGA following a long interpregnancy interval. Though more research is needed, findings suggest that future studies and clinicians need to consider long interpregnancy interval as an independent risk factor for adverse birth outcomes.
The limited independent association between short interpregnancy interval and elevated odds of preterm birth may be due to maternal nutritional depletion (10) or sub-optimal implantation of the placenta (11). An additional possible contributing mechanism to the independent association between short interpregnancy interval and preterm birth could be a failure of contraction-related proteins to return to pre-pregnancy levels (30). While more research is also needed on potential mechanisms linking long interpregnancy interval and adverse birth outcomes, it has been suggested that there is a gradual decline in reproductive capacity following a birth (13). The gradual physiological regression contributes to the parous woman presenting a similar birth outcome profile to a primigravid woman (13). Alternatively, or perhaps in conjunction, infections may contribute to fertility issues, thereby lengthening the interpregnancy interval, as well as increasing adverse pregnancy outcomes for the exposed offspring (13). Sibling-comparisons do not have the ability to control for factors that differentially influence each pregnancy and also influence the outcome, such as infection. However, a sibling-comparison is able to control for underlying medical conditions, such as hypertension, that remain consistently present in the mother in each successive pregnancy. Future research would benefit from exploring the role of breastfeeding in this complex association, as breastfeeding has been shown to elongate the interpregnancy interval and deplete maternal nutrient stores (8).
From a research perspective, our study advances the field for several reasons. First, we include a sibling-comparison model, a cousin-comparison model, and a post-birth interval analyses, thereby making methodological advances from previous work (15, 16). These methodological advances improve the clinical relevance of our study because several different approaches are supporting similar conclusions. With the inclusion of a cousin-comparison we were able to eliminate the potential residual influence of birth order and maternal age and strengthen the external validity of the findings. Although a sibling-comparison accounts for a larger extent of genetic and environmental factors than a cousin-comparison, using sibling-comparisons alone to examine interpregnancy interval is problematic because of potential carry-over effects among siblings, possible differences in families with more than two siblings, and potential confounding by birth order and maternal age (29). Second, we were also able to include several important individual, parent, and family-structure confounds including if the father was biologically the same between pregnancies. Third, sensitivity analyses indicated that our results were not due to bias introduced in creating binary outcomes and also not influenced by cohort effects. While we did identify a trend appearing to show stronger associations between short interpregnancy interval and adverse birth outcome in older cohorts, this may have been driven by a general trend of reduction in adverse birth outcomes and improved preconception care. Finally, this is the largest study to date examining the association between interpregnancy intervals and adverse birth outcomes providing more statistical power to examine these rare outcomes.
Our findings can be applied to both family planning and disease prevention efforts. For example, providers may use these findings to support families in making informed decisions about birth spacing. Given that we are only showing a minimal risk between short interpregnancy interval and preterm birth, families desiring closer birth spacing with low risk for preterm birth may opt for birth spacing shorter than the traditionally recommended two years. On the other hand, families should be aware of increased risk for adverse birth outcomes if birth spacing is longer than five years. Our findings also suggest that rather than intervening to modify interpregnancy interval, families at-risk for adverse birth outcomes my benefit from interventions aimed at changing other modifiable family-level risk factors.
Limitations, however, must also be considered. For example, various factors may influence the generalizability of our findings. Due to the relative ethnic homogeneity of the Swedish population, future research should perform within-family analyses across ethnic and racial groups (19, 20). Similarly, prenatal care is advanced and comprehensive in Sweden. This may have influenced both interpregnancy interval length and birth outcomes (23), and replication in different populations is needed. The selection of exposure-discordant pairs in cousin- and sibling-comparisons may also have increased bias due to measurement error and non-shared confounding factors (29). Unfortunately, we were unable to control for miscarriage and spontaneous or induced abortions. These factors may be especially important to consider in the long interpregnancy interval relations. Additionally, cousin- (and sibling-) comparisons are not randomized controlled studies; therefore, the design cannot rule out all possible confounding factors (29).
In conclusion, our findings suggest that modification to increase the interpregnancy interval (i.e., reducing short interpregnancy interval) would only have a minimal independent effect on reducing the likelihood of PTB and no independent effect on reducing LBW or SGA. Rather than intervening to lengthen short interpregnancy intervals, at-risk families may benefit more from interventions that alter other modifiable risk factors for adverse birth outcomes. On the other hand, our findings also suggest that an unusually long interpregnancy interval is associated with a moderate independent effect of elevated odds of PTB, LBW, and SGA. More research into the mechanisms driving these associations is necessary to direct intervention and prevention efforts for this risk factor following replication of these findings.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Child Health and Development (HD061817) to B.M.D., the National Institute of Mental Health (T32MH094011, T32MH103213) to Q.A.C. and A.S. respectively, the Indiana University Mabel LaDuke Lauder Fund to Q.A.C., the National Science Foundation Graduate Research Fellowship (1342962) to A.S., the Swedish Council for Working Life and Social Research to P.L., and the Swedish Research Council through the Swedish Initiative for Research on Microdata in the Social and Medical Sciences (SIMSAM) framework grant (340-2013-5867 to C.A.) and International Postdoc grant (350-2012-340 to A.S.O).
Footnotes
Financial Disclosure: Henrik Larsson has served as a speaker for Eli-Lilly and Shire and has received research grants from Shire. The other authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal's requirements for authorship.
References
- 1.Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA: The Journal of the American Medical Association. 2006;295(15):1809–23. doi: 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- 2.Fuentes-Afflick E, Hessol NA. Interpregnancy interval and the risk of premature infants. Obstetrics and Gynecology. 2000;95(3):383–90. doi: 10.1016/s0029-7844(99)00583-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. New England Journal of Medicine. 1999;340:589–94. doi: 10.1056/NEJM199902253400801. [DOI] [PubMed] [Google Scholar]
- 4.Khoshnood B, Lee KS, Wall S, Hsieh HL, Mittendorf R. Short interpregnancy intervals and the risk of adverse birth outcomes among five racial/ethnic groups in the United States. American Journal of Epidemiology. 1998;148(8):798–805. doi: 10.1093/oxfordjournals.aje.a009701. [DOI] [PubMed] [Google Scholar]
- 5.Klerman LV, Cliver SP, Glodenberg RL. The impact of short interpregnancy intervals on pregnancy outcomes in a low-income population. American Journal of Public Health. 1998;88(8):1182–5. doi: 10.2105/ajph.88.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith GCS, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study. British Medical Journal. 2003;327:1–6. doi: 10.1136/bmj.327.7410.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephansson O, Dickman PW, Cnattingius S. The influence of interpregnancy interval on the subsequent risk of stillbirth and early neonatal death. Obstetrics and Gynecology. 2003;102(1):101–8. doi: 10.1016/s0029-7844(03)00366-1. [DOI] [PubMed] [Google Scholar]
- 8.Smits L, Essed GGM. Short interpregnancy intervals and unfavorable pregnancy outcome: role of folate depletion. Lancet. 2001;358:2074–7. doi: 10.1016/S0140-6736(01)07105-7. [DOI] [PubMed] [Google Scholar]
- 9.Conde-Agudelo A, Rosas-Bermudez A, Castano F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Studies in Family Planning. 2012;43(2):93–114. doi: 10.1111/j.1728-4465.2012.00308.x. [DOI] [PubMed] [Google Scholar]
- 10.Winkvist A, Rasmussen KM, Habicht JP. A new definition of maternal depletion syndrome. American Journal of Public Health. 1992;82:691–4. doi: 10.2105/ajph.82.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Getahun D, Oyelese Y, Salihu HM, Anath CV. Previous cesarean delivery and risks of placenta previa and placental abruption. Obstetrics and Gynecology. 2006;107:771–8. doi: 10.1097/01.AOG.0000206182.63788.80. [DOI] [PubMed] [Google Scholar]
- 12.Jacob JA. Another frontier in microbiome research: Preterm birth. JAMA. 2015;314(15):1–2. doi: 10.1001/jama.2015.11563. [DOI] [PubMed] [Google Scholar]
- 13.Zhu B, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. New England Journal of Medicine. 1999;340(8):589–94. doi: 10.1056/NEJM199902253400801. [DOI] [PubMed] [Google Scholar]
- 14.Erickson JD, Bjerkedal T. Interpregnancy interval. Journal of Epidemiology and Community Health. 1978;32:124–30. doi: 10.1136/jech.32.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball SJ, Pereira G, Jacoby P, de Klerk N, Stanley FJ. Re-evaluation of the link between interpregnancy interval and adverse birth outcomes: retrospective cohort study matching two intervals per mother. British Medical Journal. 2014;349:g4333. doi: 10.1136/bmj.g4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanley GE, Hutcheon JA, Kinniburgh B, Lee L. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstetrics & Gynecology. 2017;129(3):408–15. doi: 10.1097/AOG.0000000000001891. [DOI] [PubMed] [Google Scholar]
- 17.Klebanoff MA. Interpregnancy interval and pregnancy outcomes: Causal or Not? Obstetrics & Gynecology. 2017;129(3):405–7. doi: 10.1097/AOG.0000000000001913. [DOI] [PubMed] [Google Scholar]
- 18.Crittenden CP, Boris NW, Rice JC, Taylor CA, Olds DL. The role of maternal health factors, behavioral factors, and past experiences in the prediction of rapid repeat pregnancy in adolescence. Journal of Adolescent Health. 2009;44:25–32. doi: 10.1016/j.jadohealth.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schelar E, Franzetta K, Manlove J. Child trends research brief. Washington, DC: Child Trends; 2007. Repeat teen childbearing: differences across states and by race and ethnicity. [Google Scholar]
- 20.Khoshnood B, Lee K, Wall S, Hsieh H, Mittendorf R. Short interpregnancy intervals and the risk of adverse birth outcomes among five racial/ethnic groups in the United States. American Journal of Epidemiology. 1998;148(8):798–805. doi: 10.1093/oxfordjournals.aje.a009701. [DOI] [PubMed] [Google Scholar]
- 21.Schachar BZ, Lyell DJ. Interpregnancy interval and obstetric complications. Obstetrical & Gynecological Survey. 2012;67(9):584–96. doi: 10.1097/OGX.0b013e31826b2c3e. [DOI] [PubMed] [Google Scholar]
- 22.Healthy People 2020. FP-5: Reduce the proportion of pregnancies conceived within 18 months of a previous birth. 2010 [cited 2014 4-15-2014]; Available from: http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=13.
- 23.Teitler JO, Das D, Kruse L, Reichman NE. Prenatal care and subsequent birth intervals. Perspectives on sexual health and reproductive health. 2012;44(1):13–21. doi: 10.1363/4401312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutter M. Proceeding from observed correlation to causal inference:The use of natural experiments. Perspectives on psychological science. 2007;2(4):377–95. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 25.Centre for Epidemiology. The Swedish Medical Birth Register - A summary of content and quality. 2003 [Google Scholar]
- 26.Statistics Sweden. Multi-generation register 2005 - A description of contents and quality. Orebro; Statistics Sweden: 2006. [Google Scholar]
- 27.Centre for Epidemiology. The Swedish hospital discharge register. 2005 http:www.socialstyrelsen.se/en/statistics/statsbysubject/the+swedish+hospital+discharge+register.htm.
- 28.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Onofrio BM, Lahey B, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. American Journal of Public Health. 2013;103:S46–S55. doi: 10.2105/AJPH.2013.301252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norwitz ER, Robinson JN, Challis JRG. The control of labor. New England Journal of Medicine. 1999;341(9):660–6. doi: 10.1056/NEJM199908263410906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


